Abstract
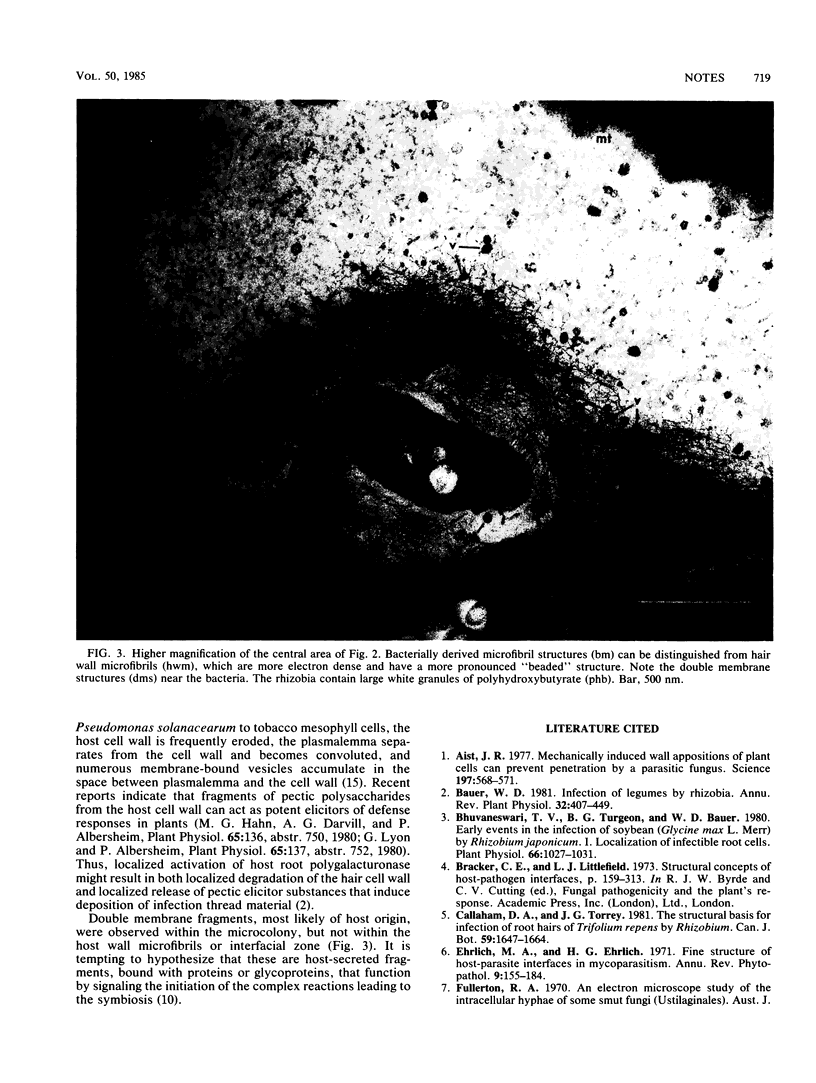
Using a new microinoculation technique, we demonstrated that penetration of Rhizobium sp. into the host root hair cell occurs at 20 to 22 h after inoculation. It did this by dissolving the cell wall maxtrix, leaving a layer of depolymerized wall microfibrils. Colony growth pressure “stretched” the weakened wall, forming a bulge into an interfacial zone between the wall and plasmalemma. At the same time vesicular bodies, similar to plasmalemmasomes, accumulated at the penetration site in a manner which parallels host-pathogen systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aist J. R. Mechanically induced wall appositions of plant cells can prevent penetration by a parasitic fungus. Science. 1977 Aug 5;197(4303):568–571. doi: 10.1126/science.197.4303.568. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LJUNGGREN H., FAHRAEUS G. Effect of Rhizobium polysaccharide on the formation of polygalacturonase in lucerne and clover. Nature. 1959 Nov 14;184(Suppl 20):1578–1580. doi: 10.1038/1841578a0. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Hau C. Y., Trinick M. J., Shine J., Rolfe B. G. Heat curing of a sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J Bacteriol. 1983 Jan;153(1):527–531. doi: 10.1128/jb.153.1.527-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C. A., Hubbell D. H. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl Microbiol. 1975 Dec;30(6):1003–1009. doi: 10.1128/am.30.6.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHLMAN K. AN ELECTRON MICROSCOPE STUDY OF ROOT-HAIR INFECTION BY RHIZOBIUM. J Gen Microbiol. 1963 Dec;33:425–427. doi: 10.1099/00221287-33-3-425. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]