Abstract
Background
Individuals with more copies of CCL3L1 (CCR5 ligand) than their population median have been found to be less susceptible to HIV infection. We investigated whether maternal or infant CCL3L1 gene copy numbers are associated with perinatal HIV transmission when single-dose nevirapine is given for prevention.
Method
A nested case–control study was undertaken combining data from four cohorts including 849 HIV-infected mothers and their infants followed prospectively in Johannesburg, South Africa. Access to antiretroviral drugs for the prevention of perinatal transmission differed across the cohorts. Maternal and infant CCL3L1 gene copy numbers per diploid genome (pdg) were determined by real-time polymerase chain reaction for 79 out of 83 transmitting pairs (~10% transmission rate) and 235 randomly selected non-transmitting pairs.
Results
Higher numbers of infant, but not maternal, CCL3L1 gene copies were associated with reduced HIV transmission (P=0.004) overall, but the association was attenuated if mothers took single-dose nevirapine or if the maternal viral load was low. Maternal nevirapine was also associated with reduced spontaneously released CCL3 (P=0.007) and phytohemagglutinin-stimulated CCL3 (P=0.005) production in cord blood mononuclear cells from uninfected infants.
Conclusion
We observed a strong association between higher infant CCL3L1 gene copies and reduced susceptibility to HIV in the absence of maternal nevirapine. We also observed a reduction in newborn CCL3 production with nevirapine exposure. Taken together, we hypothesize that nevirapine may have direct or indirect effects that partly modify the role of the CCR5 ligand CCL3 in HIV transmission, obscuring the relationship between this genetic marker and perinatal HIV transmission.
Keywords: CCL3/MIP1alpha, chemokines, mother-to-child HIV transmission , nevirapine
Introduction
Genome duplication is a key mechanism in human evolution and genetic adaptation. As segmental duplications overrepresent genes involved in immunity, duplications are thought to benefit host immunity [1]. Direct phenotypic evidence is, however, limited.
CC chemokine ligand 3 like 1 (CCL3L1) provides an excellent example of the benefits of gene duplication [2,3]. CCL3L1 gene duplication increases the production of CCL3L1 protein (formerly known as macrophage inhibitory protein 1 alpha; MIP-1α) [2-4], a potent ligand for CC chemokine receptor 5 (CCR5) and a major HIV-suppressive CC chemokine [4]. In a large study, wide variability was found in the average number of CCL3L1 gene copies across populations, with African individuals having significantly greater numbers than non-African individuals [3]. The study further demonstrated that adults and children with higher numbers of CCL3L1 copies relative to their population average were at lower risk of HIV infection [3].
The association between CCL3L1 gene copies and perinatal HIV transmission was confirmed in a study of infants born to HIV-infected mothers in South Africa [5]. Independent confirmation of epidemiological associations is essential because associations can arise by chance given the extreme heterogeneity of the human genome. In the case of perinatal HIV transmission, it is also necessary to investigate the influence of antiretroviral drugs commonly given to prevent transmission. Antiretroviral drugs are effective, but imperfect, interventions to prevent transmission. Therefore, complementary interventions are needed to eliminate perinatal transmission [6]. Here we investigated whether infants’ and mothers’ CCL3L1 gene copy numbers are associated with perinatal HIV infection in the presence and absence of the maternal dose of single-dose nevirapine, one of the most widely used interventions in sub-Saharan Africa to prevent mother-to-child HIV transmission [7].
Methods
Study design
A nested case–control study was undertaken combining data from four cohorts including 849 HIV-infected mothers and their infants followed prospectively at two hospitals in Johannesburg, South Africa. HIV-infected mothers with HIV-infected children (transmitting cases) were compared with HIV-infected mothers with uninfected children (non-transmitting controls). Within the four cohorts, 83 infants acquired HIV (transmission rate 10%). The CCL3L1 gene copy number was determined by real-time polymerase chain reaction (PCR) for mothers and infants from 79 out of 83 transmitting pairs. For comparison, 235 non-transmitting pairs matched by cohort were randomly selected as controls.
Study populations
The use of antiretroviral drugs for the prevention of mother-to-child transmission (PMTCT) differed across the cohorts (summarized in Table 1). In cohort 1, undertaken at Chris Hani Baragwanath Hospital, women were recruited postpartum before discharge after delivery as part of screening for a trial of postexposure prophylaxis [8]. HIV-positive women who had not accessed HIV testing antenatally were identified through a postpartum voluntary counselling and testing service that had been set up for the trial. These women had received no antiretroviral drugs before delivery. Their infants received either zidovudine or nevirapine as postexposure prophylaxis. A smaller number of postpartum HIV-positive women who had received single-dose nevirapine as part of a demonstration PMTCT project were also enrolled [9]. Cord blood was collected from all deliveries, and if the woman enrolled the cord blood was retained for research, otherwise it was discarded. Blood was drawn from mothers and infants at enrolment. Infants were scheduled for follow-up at 6 weeks and, if still breast-feeding, for continued follow-up until at least 4 weeks after all breastfeeding had stopped. Infant samples were tested for HIV-1 DNA by PCR to determine their infection status.
Table 1.
Summary of the numbers of mother–child pairs recruited as part of perinatal cohorts in Johannesburg, South Africa and genotyped to be included in the nested case–control studya
| Child infected and presumed timing
|
|||||
|---|---|---|---|---|---|
| Intrauterine | Intrapartum | Unknown | Child uninfected | Total | |
| Cohort 1 [8,9] | 8 (7) | 17 (15) | 0 | 177 (95) | 202 (117) |
| No maternal antiretroviral drugs | 4 (4) | 14 (13) | 0 | 106 (89) | 124 (106) |
| Maternal single-dose nevirapine | 4 (3) | 3 (2) | 0 | 71 (6) | 78 (11) |
| Cohort 2 | 12 (12) | 12 (12) | 2 (2) | 258 (55) | 284 (81) |
| No maternal antiretroviral drugs | 2 (2) | 3 (3) | 1 (1) | 71 (20) | 77 (26) |
| Maternal single-dose nevirapine | 10 (10) | 9 (9) | 1 (1) | 187 (35) | 207 (55) |
| Cohort 3 [10,11] | 1 (1) | 4 (4) | 3 (3) | 23 (22) | 31 (30) |
| No maternal antiretroviral drugs | 0 | 1 (1) | 0 | 6 (6) | 7 (7) |
| Other antiretroviral druss | 1 (1) | 3 (3) | 3 (3) | 17 (16) | 24 (23) |
| Cohort 4 | 0 | 0 | 24 (23) | 308 (63) | 332 (86) |
| Maternal single-dose nevirapine | 0 | 0 | 22 (21) | 269 (57) | 291 (78) |
| Other antiretroviral drugs | 0 | 0 | 2 (2) | 39 (6) | 41 (8) |
| Total all four cohorts | 21 (20) | 33 (31) | 29 (28) | 766 (235) | 849 (314) |
| No maternal antiretroviral drugs | 6 (6) | 18 (17) | 1 (1) | 183 (115) | 208 (139) |
| Maternal single-dose nevirapine | 14 (13) | 12 (11) | 23 (22) | 527 (98) | 576 (144) |
| Other antiretroviral drugs | 1 (1) | 3 (3) | 5 (5) | 56 (22) | 65 (31) |
Shown are the numbers of mother–child pairs recruited in each cohort by maternal antiretroviral drug use before delivery. In parentheses are the numbers of pairs with genotype data available. For the nested case–control study, we genotyped all the infected children (not all could be found or were sufficient for genotyping) as cases and a stratified random sample of the uninfected children as controls.
Cohort 2, also at this site but later, recruited postpartum drug-naive HIV-positive women who had not accessed antenatal PMTCT services as well as HIV-positive women who received single-dose nevirapine now as part of routine services. Infants of mothers who had received no antiretroviral drugs before delivery received nevirapine postexposure prophylaxis. As above, blood was drawn from mothers and infants at enrolment soon after birth, before discharge and at 6 weeks postpartum. Cohort 3 included maternal and infant samples from an earlier trial at the site of short courses of zidovudine and lamivudine versus placebo for the prevention of vertical transmission [10] some of whom were co-enrolled in this study of immunogenetic factors and transmission [11].
Cohort 4 was assembled at Coronation Hospital from among HIV-positive women who were already enrolled in PMTCT services. Women were recruited at 6 weeks postpartum, at which time blood was drawn from mothers and infants. Women either received single-dose nevirapine or triple-drug combination treatment regimens.
All women signed informed consent for participation in this study of immunogenetic factors, which was approved by the institutional review boards of the investigators, as well as consent for other trials or services in which they may have been co-enrolled, if required.
Clinical information
The child’s infection status was determined by HIV-1 DNA PCR tests. If the 6-week sample was positive, the birth sample was tested if available (birth samples were not available for cohort 4). If the birth sample was positive, the child was presumed to have acquired HIV intrauterine. If the birth sample was negative, the child was presumed to have acquired HIV intrapartum or during the early postpartum period if breast-fed. Children with positive PCR results were recalled for confirmatory tests. Maternal CD4 cell counts were performed, and stored plasma samples collected at 6 weeks postpartum were tested for HIV-1 RNA levels using the Roche Amplicor RNA Monitor assay version 1.5 (Roche Diagnostic Systems, Inc., Branchburg, New Jersey, USA) with a lower detection limit of 400 HIV-1 RNA copies/ml. Other clinical data on mothers and children were collected at enrolment.
CCL3L1 gene copy number determinations
Genomic DNA was extracted from peripheral blood mononuclear cells using the Qiagen QIAamp DNA minikit (Qiagen Inc., Valencia, California, USA). A real-time PCR was developed to test for CCL3L1 gene copy numbers per diploid genome (pdg) using betaglobin and CCL3 as controls. The following primers and probes were synthesized (DNA Synthesis Laboratory, Department of Molecular and Cellular Biology, University of Cape Town, South Africa): β-globin gene upstream 5′–ggcaaccctaaggtgaaggc–3′, β-globin gene downstream 5′–ggtgagccaggccatcacta–3′, β-globin gene probe 5′–catggcaagaaagtgctcggtgcct–3′; CCL3L1 gene upstream 5′–tctccacagcttcctaaccaaga–3′, CCL3 and CCL3L1 genes downstream 5′–ctggacccactcctcactgg–3′, CCL3L1 gene probe 5′–aggccggcaggtctgtgctga–3′ [12]. The CCL3 gene upstream 5′–tctccacagcttcctaaccaagc–3′ and CCL3 gene probe 5′–aagccggcaggtctgtgctga–3′ were designed and synthesized. All probes were labeled with 5′ 6-carboxyfluorescein (FAM) and a 3′ 6-carboxytetramethylrhodamine (TAMRA) quencher.
Real-time PCR was performed using an ABI PRISM 7500 (Applied Biosystems, Nieuwerkerk, Netherlands) according to the protocol supplied. For each sample, the β-globin, CCL3 and CCL3L1 genes were amplified in duplicate, using approximately 20 ng genomic DNA per sample. The CCL3 gene copy number was confirmed at two copies pdg for each sample, calculated using the relative quantification method and using β-globin (present at two copies pdg) as the endogenous control. CCL3 was used as the endogenous control to calculate the CCL3L1 copy number, using the relative quantification method against a known copy control. Samples giving a result of a single CCL3L1 gene copy pdg were confirmed by sequencing to ensure homozygosity.
Measurement of CCL3, CCL4, and CCL5 production
A subset of cord blood samples collected from cohort 1 was tested for CCL3, CCL4, and CCL5 production. Samples were tested from 45 non-transmitting pairs in which the mother had received no antiretroviral drugs before delivery, from 20 non-transmitting pairs in which the mother received single-dose nevirapine and from 20 HIV-seronegative women as controls. Infant cord blood mononuclear cells (CBMC) were isolated by centrifugation on Histopaque Ficoll. Contaminating erythrocytes were lysed using a solution of 0.15 mol ammonium chloride, 10 mmol potassium bicarbonate, and 1 mmol sodium ethylenediamine tetraacetic acid (pH 7.0). After isolation, the number of viable CBMC was determined by trypan blue exclusion, and the cells were resuspended at 3×106 cells/ml in RPMI containing 1% l-glutamine. CBMC were (a) unstimulated or (b) stimulated with phytohemagglutinin at a final concentration of 12.5 μg/ ml. Human serum (10%) was then added to each well. After 24 h incubation at 37°C in a moist, 5% carbon dioxide atmosphere, culture supernatants were harvested and stored at −70°C. Supernatants were tested for CCL3, CCL4 and CCL5 production using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota, USA), according to the manufacturer’s instructions.
Statistical analysis
Mantel–Haenszel chi-square tests were used to test for differences in proportions between groups; and t-tests and Kruskall–Wallis tests were used to compare normal and non-normal continuous variables, respectively. Spearman rank correlation coefficients were used to describe bivariate relationships. Multiple logistic regression was used for multivariable analysis using SAS 9.1 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Study population
The transmission rate among infants whose mothers received single-dose nevirapine was 8.5% (49/576). The rate among infants who received only postexposure prophylaxis but whose mothers received no antiretroviral drugs was 11.9% (24/201) and among infants whose mothers received triple combination therapy was 4.9% (2/41).
Within the case–control population, transmitting mothers had higher plasma viral loads and lower CD4 cell counts than non-transmitting mothers (Table 2), consistent with known risk factors for transmission. Associations between risk factors and transmission were similar within the case–control population to those observed within the combined cohorts overall (data not shown), except that prematurity and low birth weight were significant risk factors for infection in the whole cohort.
Table 2.
Risk factors for perinatal HIV transmission within the nested case–control population of transmitting and non-transmitting mother–infant pairs
| Na | Transmitting (n=79) | Na | Non-transmitting (n=235) | P value | |
|---|---|---|---|---|---|
| Maternal viral load (copies/ml) | |||||
| Median (IQR) | 70 | 58 100 (5820–219 000) | 213 | 13 100 (1950–55 400) | <0.0001 |
| Maternal CD4 cell count | |||||
| Mean (std) | 70 | 416 (223) | 223 | 502 (264) | 0.01 |
| Maternal age (years) | |||||
| Mean (std) | 78 | 27.5 (5.20) | 235 | 26.9 (5.16) | 0.27 |
| Parity | |||||
| Mean (std) | 77 | 2.25 (1.20) | 233 | 2.16 (1.08) | 0.80 |
| Child sex N (%) | |||||
| Male | 78 | 38 (48.7) | 231 | 100 (43.3) | 0.41 |
| Gestation N (%) | |||||
| Preterm <37 weeks | 69 | 12 (17.4) | 212 | 27 (12.7) | 0.33 |
| Birth weight (g) | |||||
| Mean (std) | 78 | 2903 (446) | 234 | 2984 (454) | 0.16 |
| Breast fed N (%) | |||||
| >3 days | 78 | 11 (14.1) | 235 | 33 (14.0) | 0.99 |
IQR, Interquartile range.
Data on all of these parameters were not available for all members of the cohorts.
CCL3L1 gene copies and transmission
Infant, but not maternal, CCL3L1 gene copies were significantly associated with perinatal HIV transmission (Table 3). Higher CCL3L1 copies were more frequent among uninfected compared with infected infants (31 versus 20% with more than five copies pdg), whereas lower CCL3L1 copies were less frequent among uninfected compared with infected infants (20 versus 33% with less than four copies pdg; P=0.009). There was a slight, non-significant trend towards higher maternal CCL3L1 copies among non-transmitting mothers, presumably because maternal and infant CCL3L1 copy numbers were correlated (rho 0.371; P<0.0001).
Table 3.
Infant and maternal CCL3L1 gene copies per diploid genome among transmitting and nontransmitting mother-child pairs
| Transmitting | Non-transmitting | P value | |
|---|---|---|---|
| Infant CCL3L1 gene copies pdg | |||
| Total N (%) | 79 | 235 | |
| 1 | 1 (1.27) | 3 (1.28) | |
| 2 | 8 (10.13) | 2 (0.85) | |
| 3 | 17 (21.52) | 41 (17.45) | 0.004a |
| 4 | 22 (27.85) | 61 (25.96) | |
| 5 | 15 (18.99) | 55 (23.40) | |
| 6 | 11 (13.92) | 42 (17.87) | |
| 7 | 4 (5.06) | 21 (8.94) | |
| 8 | 0 0 | 8 (3.40) | |
| 9 | 1 (1.27) | 1 (0.43) | |
| 10 | 1 (0.43) | ||
| Mean (std) | 4.24 (1.54) | 4.81 (1.49) | |
| Median (IQR) | 4 (3 to 5) | 5 (4 to 6) | 0.003b |
| Maternal CCL3L1 gene copies pdg | |||
| Total N (%) | 79 | 235 | |
| 1 | 1 (0.43) | 0 | |
| 2 | 13 (5.53) | 1 (1.27) | |
| 3 | 25 (10.64) | 15 (18.99) | 0.11* |
| 4 | 54 (22.98) | 24 (31.65) | |
| 5 | 59 (25.11) | 16 (20.25) | |
| 6 | 44 (18.72) | 13 (17.72) | |
| 7 | 22 (9.36) | 7 (8.86) | |
| 8 | 11 (4.68) | 0 | |
| 9 | 5 (2.13) | 1 (1.27) | |
| 10 | 1 (0.43) | 0 | |
| Mean (std) | 4.67 (1.36) | 5.00 (1.63) | |
| Median (IQR) | 4 (4 to 6) | 5 (4 to 6) | 0.09† |
IQR, Interquartile range; pdg, per diploid genome.
P value from Mantel–Haenszel chi-square test.
P value from Kruskall–Wallis test.
Uninfected infants were more likely than infected infants to have more CCL3L1 copies than their mothers (35 versus 22% among uninfected compared with infected infants, respectively, P=0.03) but were equally as likely to have fewer copies than their mothers (44 versus 41% among uninfected compared with infected infants, P=0.61). The absolute infant copy number rather than the infant-relative-to-mother number was associated with reduced transmission. The infant minus mother difference was in the expected direction (less transmission with higher infant-relative-to-mother number) but was not significant (P=0.326).
Maternal CCL3L1 copies were correlated with maternal viral load (rho −0.14; P=0.017), but differences in maternal viral load did not explain the association between infant CCL3L1 gene copies and transmission. After adjustment for maternal CCL3L1 copies, viral load, and CD4 cell count, there continued to be a significant association between higher numbers of infant CCL3L1 gene copies (continuous scale) and a lower risk of HIV transmission (odds ratio 0.75, 95% confidence interval 0.59–0.95; P=0.018).
Influence of antiretroviral drugs and maternal viral load
We examined whether the association between infant CCL3L1 copies and transmission was consistent across the cohorts, and found that it was strongest within the cohorts with the fewest mothers taking single-dose nevirapine. We therefore examined the CCL3L1–transmission relationship separately among those pairs in which the mother had taken nevirapine before delivery and those pairs in which the mother had taken no antiretroviral drugs, excluding those with other combinations.
The influence of infant CCL3L1 copies on transmission was attenuated if mothers received single-dose nevirapine (n=139), and was strongest if mothers received no antiretroviral drugs (n=144; Table 4).
Table 4.
Associations between infant CCL3L1 gene copy numbers and transmission stratified by maternal antiretroviral drug exposure and maternal viral load
| No maternal antiretroviral drugs
|
Maternal single-dose nevirapine
|
|||||
|---|---|---|---|---|---|---|
| Transmitting | Non-transmitting | P value | Transmitting | Non-transmitting | P value | |
| N (%) Infant CCL3L1 gene copies pdg | ||||||
| ≤3 | 9 (37.5) | 22 (19.1) | 13 (28.3) | 18 (18.4) | ||
| 4–5 | 11 (45.8) | 54 (47.0) | 22 (47.8) | 59 (60.2) | ||
| ≥6 | 4 (16.7) | 39 (33.9) | 0.03 | 11 (23.9) | 21 (21.4) | 0.53 |
| Total | 24 | 115 | 46 | 98 | ||
| Maternal viral load >10 000 copies/ml | ||||||
| ≤3 | 8 (42.1) | 8 (12.7) | 8 (25.8) | 6 (11.5) | ||
| 4–5 | 8 (42.1) | 35 (55.6) | 18 (58.1) | 33 (63.5) | ||
| ≥6 | 3 (15.8) | 20 (31.7) | 0.02 | 5 (16.1) | 13 (25.0) | 0.21 |
| Total | 19 | 63 | 31 | 52 | ||
| Maternal viral load ≤10 000 copies/ml | ||||||
| ≤3 | 0 | 13 (28.3) | 4 (28.6) | 12 (26.1) | ||
| 4–5 | 3 (75.0) | 16 (34.8) | 4 (28.6) | 26 (56.5) | ||
| ≥6 | 1 (25.0) | 17 (36.9) | 0.24 | 6 (42.8) | 8 (17.4) | 0.10 |
| Total | 4 | 46 | 14 | 46 | ||
pdg, Per diploid genome.
We then examined CCL3L1–transmission relationships stratifying by maternal viral load, ignoring maternal drug regimens. The association was attenuated if mothers had low viral loads and was strongest if mothers had high viral loads. Combining 165 mothers with viral loads greater than 10 000 copies/ml, there was a significant association between infant CCL3L1 copies and transmission: 32.0 versus 12.2% of infected compared with uninfected infants had CCL3L1 copies of three pdg or less, and 16.0 versus 28.7% of infected compared with uninfected infants had CCL3L1 copies greater than five pdg (P=0.006). There was no trend in this direction among 118 mothers with viral loads less than 10 000 copies/ml (P=0.78).
Stratifying by both maternal viral load and maternal drug exposure, the association between infant CCL3L1 gene copies and transmission was strongest if maternal viral loads were high and there was no exposure to maternal nevirapine (Table 4).
The ratio of intrapartum to intrauterine transmission was higher in the absence of maternal antiretroviral drugs. Among infected infants whose mothers did not receive antiretroviral drugs, 73.9% (17/23) had infections attributable to the intrapartum period (excluding those with unknown timing) compared with 46.2% (12/26) whose mothers received nevirapine (P=0.049). When the association between infant CCL3L1 copies was examined for intrauterine and intrapartum transmission separately, the association remained significant for intrapartum transmission with high maternal viral loads and in the absence of antiretroviral drugs. Breastfeeding was rare or of short duration and no infection could be clearly attributable to the postnatal period.
CCL3, CCL4 and CCL5 production in cord blood mononuclear cells
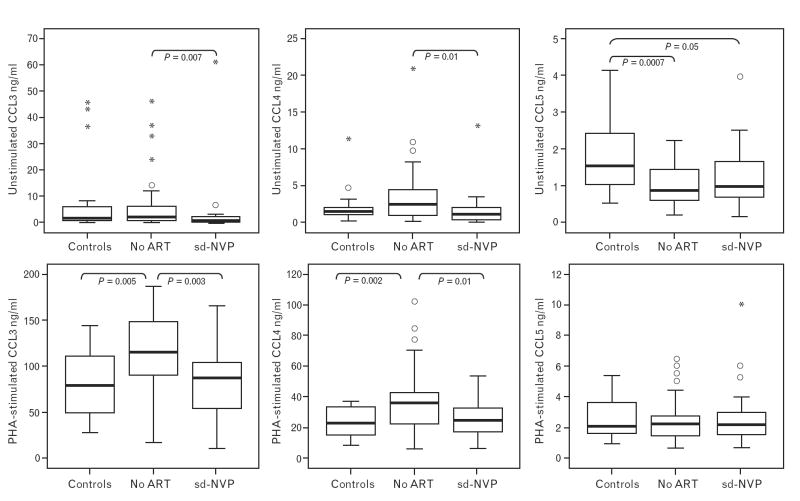
Within cohort 1, CCL3, CCL4 and CCL5 production was measured in infant CBMC from 20 uninfected infants born to HIV-infected mothers who received single-dose nevirapine, 45 uninfected infants born to HIV-infected mothers who received no antiretroviral drugs before delivery, and 20 control uninfected infants born to uninfected mothers. Spontaneously released CCL3 (P=0.007) and phytohemagglutinin-stimulated CCL3 (P=0.005) was reduced for HIV-exposed, uninfected infants whose mothers took nevirapine compared with those whose mothers received no antiretroviral drugs. Maternal nevirapine use appeared to ‘normalize’ CCL3 levels because levels among nevirapine-exposed infants born to HIV-infected mothers were similar to levels among infants of HIV-negative mothers (controls); whereas levels among drug-unexposed infants born to HIV-positive mothers were higher than levels among controls (Fig. 1). Spontaneously released CCL4 (P=0.01) and phytohemagglutinin-stimulated CCL4 (P=0.01) followed the same pattern, being lower with nevirapine exposure, and higher with HIVexposure in the absence of nevirapine compared with controls.
Fig. 1. Spontaneously released (unstimulated) and phytohemagglutinin-stimulated CCL3, CCL4, and CCL5 in cord blood mononuclear cells among 20 infants born to uninfected mothers (controls), and among 45 infants born to HIV-infected mothers who either received no antiretroviral drugs before delivery or 20 who received single-dose nevirapine before delivery.
Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), 10th and 90th percentiles (bars), outliers (dots) and extremes (stars). Significant differences between groups are indicated. ART, Antiretroviral therapy; PHA, phytohemagglutinin; sd-NVP, single-dose nevirapine.
In contrast, CCL5 followed a different pattern: no association with drug exposure and, for spontaneously released CCL5, levels were lower among HIV-exposed uninfected infants than among controls. It should be noted that levels of CCL5 were more than 10-fold lower than levels of CCL3. Maternal viral loads did not differ between the nevirapine-exposed (median 9240 HIV-1-RNA copies/ml) and unexposed (median 15 050 HIV-1-RNA copies/ml) HIV-infected mothers (P=0.66), and maternal viral load was not correlated with infant chemokine production in CBMC.
Discussion
Our results support the original observation [3] of a dose-dependent relationship between duplication of the CCL3L1 gene and perinatal HIV transmission. Within this southern African black population, the average number of CCL3L1 gene copies was four to five copies/pdg. Infants with fewer copies were more likely to acquire HIV infection from their mothers; infants with more copies were less susceptible to infection. Infant genotype was pertinent; maternal genotype did not explain the association.
Most vertical transmission is thought to occur with virus that requires the CCR5 co-receptor for cell entry [13,14]. It is well established that CCL3L1 gene duplications increase CCL3 protein production [2,3], which is a major ligand for CCR5. We have previously reported that CCL3 production at birth is increased among infants born to HIV-infected mothers compared with unexposed infants born to uninfected mothers [5]. Furthermore, those infants who acquire HIV infection intrapartum fail to produce as much CCL3 at birth (median phytohemagglutinin-stimulated CCL3 production in CBMC 79 432 ng/ml) than those infants of HIV-infected mothers who escape infection (median 114 185 ng/ml, P=0.001) [5]. Blocking of CCR5 may not, however, be the sole mechanism responsible for reduced transmission because in our previous data the increased production of CCL4 (another ligand of CCR5) did not compensate for deficits in CCL3 [5]. Other biological functions specific to CCL3 may be pertinent, including its support for the development of effective adaptive immunity [12]. We also observed that ‘not all CCL3L1 copies are created equal’ with respect to protein production, as the copy number alone did not entirely explain differences in CCL3 production between infants who became infected and those who did not [5]. These findings demonstrate greater complexity in the expression and function of host CCL3 gene products and that additional genetic or other determinants need to be identified that identify individuals at greater risk of acquiring HIV infection [15].
Intriguingly, we observed that the association between CCL3L1 gene duplication and perinatal HIV transmission was attenuated if mothers received nevirapine. The attenuation of this otherwise strong association occurred in the same population in which we observed that nevirapine consumed by the mother at the onset of labor reduced the spontaneous and phytohemagglutinin-stimulated cellular production of CCL3 and CCL4 in newborn mononuclear cells.
One explanation for these related findings might be that, through a reduction in viral replication in the mother, nevirapine reduced both the stimulus for elevated CCL3 production and the viral exposure needed for infection. Consistent with this explanation, we observed that the association between CCL3L1 duplication and transmission was strongest with high maternal viral loads. In other words, a genotype that predicts a more effective response to viral exposure is no longer necessary. This explanation is not entirely satisfactory, however, because viral suppressive effects of single-dose nevirapine are only partial [16]. Although the correlation between maternal viral load and transmission is strong, there is no clear threshold at which transmission always or never occurs [17].
Nevirapine may also have altered the timing of vertical transmission. It is generally thought that nevirapine preferentially reduces transmission occurring during delivery (intrapartum) with fewer, if any, effects on earlier transmission occurring during pregnancy (intrauterine) [6]. Our data supported a change in the ratio of intrapartum to intrauterine infection with maternal nevirapine. If the CCL3L1 genotype only affects intrapartum transmission, then the change in ratio might explain our findings. We were not able to test all infants at birth to determine the timing of transmission, and are thus unable to confirm or refute this. Nevirapine may also cross the placenta and influence intrauterine transmission if, for example, its efficacy is partly a result of preventing the establishment of infection.
Alternatively, nevirapine may have immunomodulatory effects that operate independently or in combination with a reduction in viral load and changes in the timing of transmission. Immunomodulatory effects of nevirapine, and of other antiretroviral drugs used for the prevention of perinatal transmission, have been described [18]. These include increases in markers of activation (neopterin and soluble l-selectin) [9], reductions in HIV-specific cellular immune responses [11], and lower levels of neutrophils, lymphocytes, and hemoglobin concentrations [19-21]. A limitation of our study is that we only investigated nevirapine, but whether similar attenuations of genotype–transmission relationships are observed with other antiretroviral drugs will be important to investigate. No modifying effect of zidovudine prophylaxis was mentioned in the original study among Argentinean children [3] but detailed analysis is not presented.
Maternal nevirapine reduced CCL3 and CCL4 production among exposed-uninfected infants. The capacity to produce CCL3 and CCL4 is greater in infants than in adults (whether HIV infected or not) consistent with the expected skewing of immune responsiveness in early life towards stronger innate compared with adaptive immunity [5,22]. HIV-exposed infants, however, have higher levels than unexposed infants, thus the reductions seen with nevirapine, in effect ‘normalize’ these levels. Although greater production of CCL3 was associated with less transmission in our data, because maternal nevirapine is known to reduce HIV transmission [7], we do not hypothesize that the reductions in neonatal CCL3 levels increase vulnerability to HIV. Rather, nevirapine renders redundant a protective genotype possibly by providing protection against transmission through different mechanisms. HIV-infected women who did not receive nevirapine were women who had not accessed antenatal care early enough or at facilities with well functioning prevention programmes. These women did not differ from women who received single-dose nevirapine in terms of viral load (the most powerful predictor of transmission risk), and maternal viral loads did not explain the differences in infant CC chemokine production at birth. All infants received postexposure prophylaxis (mostly with nevirapine). We thus observed a strong association between more infant CCL3L1 copies and lower transmission in the presence of only infant drug exposure, but not when there is both maternal and infant exposure.
In contrast to CCL3 and CCL4, defective production of CCL5 by cord blood cells, compared with adult peripheral blood mononuclear cells, has previously been reported [23]. In our study, CCL5 was not affected by nevirapine exposure. Nor was it elevated in HIV-exposed uninfected infants, and production levels were at the low end of detectable concentrations. Although CCL5 may be relevant to other routes of HIV transmission, it may not be important for HIV transmission in the context of the maturing neonatal immune system.
Our results have important implications for future studies of mother-to-child HIV transmission. First, for ethical reasons, it is unlikely in the future to find contexts in which vertical transmission can be studied in the absence of antiretroviral drugs. It appears, however, that some antiretroviral drugs currently used for prevention may obscure genotype–transmission relationships. Second, a vaccine to prevent breastfeeding HIV transmission would be extremely beneficial for settings in which avoiding all breastfeeding is neither safe nor acceptable. It is likely that studies of infant vaccines will include antiretroviral drugs given at least over the perinatal period. If these drugs influence CCL3 production, they may affect the immunogenicity of vaccines that rely on appropriate support from this component of innate immunity. For these reasons, it is important that the indirect consequences of antiretroviral drugs used for the prevention of perinatal HIV transmission be investigated further.
Acknowledgments
Sponsorship: This study was supported in part by grants from NICHD (HD 42402, HD 36177), the South African AIDS Vaccine Initiative (SAAVI) and Wellcome Trust. CTT is the recipient of a Wellcome Trust International Senior Research Fellowship (076352/Z/05/Z).
Footnotes
Conflicts of interest: None.
References
- 1.Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, et al. Recent segmental duplications in the human genome. Science. 2002;297:1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 2.Townson JR, Barcellos LF, Nibbs RJ. Gene copy number regulates the production of the human chemokine CCL3-L1. Eur J Immunol. 2002;32:3016–3026. doi: 10.1002/1521-4141(2002010)32:10<3016::AID-IMMU3016>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 4.Arenzana-Seisdedos F, Parmentier M. Genetics of resistance to HIV infection: role of co-receptors and co-receptor ligands. Semin Immunol. 2006;18:387–403. doi: 10.1016/j.smim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Meddows-Taylor S, Donninger SL, Paximadis M, Schramm DB, Anthony FS, Gray GE, Kuhn L. Reduced ability of newborns to produce CCL3 is associated with increased susceptibility to perinatal human immunodeficiency virus 1 transmission. J Gen Virol. 2006;87:2055–2065. doi: 10.1099/vir.0.81709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis. 2006;6:726–732. doi: 10.1016/S1473-3099(06)70629-6. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 8.Gray GE, Urban M, Chersich MF, Bolton C, van Niekerk R, Violari A, et al. for the PEP Study Group. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS. 2005;19:1289–1297. doi: 10.1097/01.aids.0000180100.42770.a7. [DOI] [PubMed] [Google Scholar]
- 9.Schramm DB, Kuhn L, Gray GE, Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr. 2006;42:545–553. doi: 10.1097/01.qai.0000225009.30698.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn L, Meddows-Taylor S, Gray G, Trabattoni D, Clerici M, Shearer GM, Tiemessen C. Reduced HIV-stimulated T-helper cell reactivity in cord blood with short-course antiretroviral treatment for prevention of maternal-infant transmission. Clin Exp Immunol. 2001;123:443–450. doi: 10.1046/j.1365-2249.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasik TJ, Bratosiewicz J, Wierzbicki A, Whiteman VE, Rutstein RR, Starr SE, et al. Protective role of B-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J Immunol. 1999;162:4355–4364. [PubMed] [Google Scholar]
- 13.Ometto L, Zanchetta M, Mainardi M, De Salvo GL, Garcia-Rodriguez MC, Gray L, et al. Co-receptor usage of HIV-1 primary isolates, viral burden, and CCR5 genotype in mother-to-child HIV-1 transmission. AIDS. 2000;14:1721–1729. doi: 10.1097/00002030-200008180-00006. [DOI] [PubMed] [Google Scholar]
- 14.Salvatori F, Scarlatti G. HIV type 1 chemokine receptor usage in mother-to-child transmission. AIDS Res Hum Retroviruses. 2001;17:925–935. doi: 10.1089/088922201750290041. [DOI] [PubMed] [Google Scholar]
- 15.Tiemessen CT, Kuhn L. CC chemokines and protective immunity: insights gained from mother-to-child transmission of HIV. Nat Immunol. 2007;8:219–222. doi: 10.1038/ni0307-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, John-Stewart GC. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–1422. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thea DM, Steketee RW, Pliner V, Bornschlegel K, Brown T, Orloff S, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. AIDS. 1997;11:437–444. doi: 10.1097/00002030-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Heagy W, Crumpacker C, Lopez P, Finberg R. Inhibition of immune functions by antiviral drugs. J Clin Invest. 1991:1916–1924. doi: 10.1172/JCI115217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, Blanche S Enquete Perinatale Francaise Study Group. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 20.European Collaborative Study. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS. 2004;18:2009–2017. doi: 10.1097/00002030-200410210-00005. [DOI] [PubMed] [Google Scholar]
- 21.Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Women and Infants Transmission Study. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the Women and Infants Transmission Study. J Infect Dis. 2006;194:1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 22.Tiemessen CT, Kuhn L. Immune pathogenesis of pediatric HIV infection. Curr HIV/AIDS Rep. 2006;3:13–19. doi: 10.1007/s11904-006-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariharan D, Ho W, Cutilli J, Campbell DE, Douglas SD. C-C chemokine profile of cord blood mononuclear cells: selective defect in RANTES production. Blood. 2000;95:715–718. [PubMed] [Google Scholar]