Abstract
Little has been done to investigate the effects of opioid exposure during adolescence. Therefore, our first objective was to determine behavioral differences in response to acutely administered morphine (e.g., antinociception and locomotion) between periadolescent and adult male and female rats. Our second objective was to determine the impact of age of morphine exposure on sensitivity to morphine-induced locomotion later in life. For the acute morphine studies, antinociceptive responses (using tail-flick and hot plate latencies) were assessed using cumulative morphine dosing (0.5–12 mg/kg) followed by a time course after the last morphine injection (up to 4 hr), and dose-response curves for motor activity (2 h test) were determined following saline and morphine (0.1–3.0 mg/kg) administration. For the long-term study, periadolescent and adult rats were given one of four treatment regimens (saline or one, three, or five days of morphine; 5.0 mg/kg, 2X/day). Changes in locomotor activity in response to saline or morphine (0.1–3.0 mg/kg) were determined one month later. A number of age- and sex-related behavioral differences were observed: basal differences in behavior were assay-dependent; however, male periadolescent rats were generally more sensitive to acute morphine-induced motor stimulation, while both male and female periadolescent rats tended to be less sensitive to morphine-induced antinociception. Lastly, following morphine exposure, activity was dependent on age of treatment and treatment regimen, with the greatest effects in five-day periadolescent-treated animals. These findings demonstrate that the sensitivity of periadolescent rats to the acute and protracted effects of morphine is different from that of adult rats.
Keywords: Adolescence, drug abuse, opioid, behavior, elevated plus-maze, hot plate, tail-flick, sex difference, motor activity
1. Introduction
Periadolescence represents a critical ontogenic period during which maturational changes and neuronal development in the brain occur (Spear, 2000). Compared to other developmental periods, periadolescence is characterized by hormonal, metabolic, and neurochemical changes (Spear, 2000), which result in specific behavioral features. For example, compared with adult (and younger) rats adolescent rats exhibit greater exploration in novel situations (Spear and Brake, 1983; Spear, 2000). These ontogenic differences also result in differential sensitivity to the locomotor stimulatory effect of drugs of abuse, including cocaine (Laviola et al., 1995; Collins and Izenwasser, 2002), methylphenidate (Brandon et al., 2001), and nicotine (Faraday et al., 2001; Schochet et al., 2004). These age-related differences extend to other drug-induced behavioral effects as well. For example, repeated daily exposure to nicotine conditions significant place preference in adolescent rats but not in adult rats (Vastola et al., 2002), and adolescent rats exhibit greater ethanol-induced impairment of spatial memory in the Morris Water Maze than adult rats do (Markwiese et al., 1998).
Drug exposure during adolescence can also have long-lasting consequences. For example, in Golden hamsters, voluntary ethanol consumption during adolescence significantly enhanced aggression towards smaller intruders in adulthood compared to control subjects (Ferris et al., 1998). Additionally, nicotine exposure in periadolescent, but not postadolescent, rats led to increases in nicotine self-administration as adults (Adriani et al., 2003). The latter study also suggests that drug exposure during adolescence can alter sensitivity to later drug exposure. Taken together, these studies suggest that the long-term consequences of drug exposure during adolescence may include increased "susceptibility" to the effects of drugs of abuse in adulthood.
Given the evidence suggesting a link between age and sensitivity to drugs of abuse, there have been surprisingly few preclinical studies exploring the behavioral impact of opioids on adolescents (versus adults). In one study, acutely administered morphine (1.0–10 mg/kg) induced greater locomotor responses in male and female periadolescent rats compared with adult rats (Spear et al., 1982). Additionally, previous work from our laboratory demonstrated that periadolescent rats treated with morphine (10 mg/kg/day) for three days were more responsive to morphine-induced locomotion compared to saline-treated cohorts when tested as young adults- an effect not shared by their adult-treated counterparts (White and Holtzman, 2005). In light of these findings, the purpose of the current study was extend our previous observations (White and Holtzman, 2005) as well as those of others (Spear et al., 1982), using well-validated assays of effects of morphine. First, we determined the effects of age on acutely administered morphine-induced antinociception. Second, we assessed the effects of gender on age-related differences in acute morphine-induced motor activity and antinociception. Lastly, we determined whether or not limited morphine exposure during periadolescence (versus adulthood) could give rise to an altered functional state of endogenous opioid systems in female rats later consistent with our earlier study using males (White and Holtzman, 2005).
Recently it was shown that periadolescent male rats are more sensitive to morphine-induced antinociception and show greater tolerance using the hot plate test as compared to adult male rats (Ingram et al., 2007). Additionally, a number of gender differences have been reported in several aspects of opioid pharmacology, including antinociceptive (Cicero et al., 1996; Bartok and Craft, 1997) effects as well as drug-induced locomotion (Spear et al., 1982) and discriminative-stimulus (Craft et al., 1996) effects. Based on these studies, and our previous findings suggesting that periadolescent rats are more sensitive to the long-term effects of morphine, we hypothesized that periadolescent male and female rats would show greater morphine-induced antinociception and locomotor activity compared to their adult counterparts. We also predicted that, like periadolescent males, morphine-treated periadolescent females would more sensitive to subsequent morphine exposure later in life as compared their saline-treated counterparts and adult females.
2. Methods
2.1 Subjects
A summary of the subjects used for this study is provided in Table 1. Male and female Sprague-Dawley rats were approximately 22 or 57 days old upon arrival (Charles River, Wilmington, NC). All animals were pair-housed in standard polycarbonate cages in a centralized climate-controlled facility and were maintained on a 12-h light:dark cycle with food provided ad libitum. Subjects were maintained according to the “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, 1996), and all procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
Table 1.
Summary of experimental details
| Test Order | Experiment | Gender | Age of morphine treatment/exposure | Morphine treatment | Age of testing | n/group | total N |
|---|---|---|---|---|---|---|---|
| 1 | Antinociception Experiment: tail-flick and hot plate | Males | Periadolescence | Periadolescence | 8 | ||
| Adulthood | Acute | Adulthood | 8 | 32 | |||
| Females | Periadolescence | Periadolescence | 8 | ||||
| Adulthood | Adulthood | 8 | |||||
| 2 | Locomotor Experiment: Acute Effects of Morphine | Males | Periadolescence | Periadolescence | 8 | 40 | |
| Adulthood | Acute | Adulthood | 8 | ||||
| Females | Periadolescence | Periadolescence | 12 | ||||
| Adulthood | Adulthood | 12 | |||||
| 3 | Locomotor Experiment: 1 month after drug exposure | Males | Periadolescence | Periadolescence | 8 | 112 | |
| Adulthood | 0, 1, 3, or 5 days | Adulthood | 8 | ||||
| Females | Periadolescence | Periadolescence | 6 | ||||
| Adulthood | Adulthood | 6 | |||||
See section 2.1 Subjects for more detail.
All experiments were conducted between 0800–1800 h, during the light phase of the light:dark cycle. With the exception of the initial locomotor experiments, experiments assessing the acute effects of morphine or making direct age comparisons between periadolescents and adults contained groups of both male and female cohorts representing both ages (See below for further detail.).
2.1.1 Acute Morphine- Antinociception Experiment
The total number of animals used for the antinociception experiment was 32 (16 males, 16 females; n=8/ group).
2.1.2 Acute Morphine- Locomotor Experiment
The total number of animals for the acute morphine-induced locomotor activity experiment was 40 (16 males, 24 females; n=8 and 12 per group, respectively), which were tested as two sets of cohorts. The first cohort consisted of periadolescent and adult males, while the second cohort comprised periadolescent and adult females.
2.1.3 Protracted Effects One Month Following Morphine Exposure- Locomotor Experiment
In the studies assessing the protracted effects of morphine one month after treatment, several morphine treatment regimens (i.e. 0, 1, 3, or 5 days of drug treatment) were implemented. Consistent with the acute morphine study, groups were composed of animals of both ages but were of a single sex. For these experiments a total of 112 animals (i.e. 32 periadolescent males, 32 adult males, 24 periadolescent females, and 24 adult females; n=6–8 per dosing regimen) was used.
2.2 Apparatuses and Measurement of Behavior
2.2.1 Antinociception Testing Apparatuses
Tail-flick test: response latencies were determined using a modified version (Gellert and Holtzman, 1978) of the radiant heat tail-flick procedure (D'Amour and Smith, 1941) and a Model 33 Tail Flick Analgesia Meter (IITC, Inc., Life Science Instruments, Woodland Hills, CA). At the beginning of the test, radiant heat from a 24-V, 150-W bulb was focused on the lower third of the rat’s tail. An automatic timer was also activated. Tail movement activated a photocell, which subsequently turned off the light source and the timer. The light intensity was adjusted to 80% of maximum. Lowering the intensity increases sensitivity of the assay (White et al., 2004) and the chance to reveal age- or sex-dependent differences. Baseline latencies ranged from 2.0 s to 4.0 s, and a 10 s cutoff time was employed to minimize tissue damage. Tail-flick latency was recorded to the nearest tenth of a second.
Hot-plate test: the surface temperature of the plate (Model 39D, Hot Plate Analgesia Meter; IITC, Inc., Woodland Hills, CA) was set at 50.0 ±0.2°C. The surface of the hot plate measured 26.5×29×3 cm and was surrounded by 28.5-cm-high Plexiglas walls and removable cover. The test was stopped when an animal licked its hind paws or jumped off the surface or if a response was not made within 38 s (i.e., 38 s-cutoff). The surface temperature was set so that the baseline latencies were between 7.0 s and 13 s.
2.2.2 Locomotor Apparatus
Locomotor activity was measured using eight Accuscan Digiscan Activity Monitors (AccuScan Instruments, Inc., Columbus, OH), with the aid of VersaMax® software (Version 1.30, AccuScan Instruments, Inc.). For locomotor testing, rats were placed individually in one of eight clear acrylic chambers (40 × 40 × 30 cm). Each chamber was inside a ventilated, sound attenuating cabinet illuminated by incandescent light (approximately 45 lux). During testing, a number of behaviors were measured, including total movement in the horizontal plane (horizontal activity), with an array of infrared beams surrounding the chambers. Movements were determined by breaks in photobeams and were converted into locomotor activity counts with the aid of the software VersaDat® (Version 1.3; AccuScan Instruments Inc.). Out of the several measures of motor activity we analyzed two key, mutually exclusive, behaviors: horizontal activity counts (ambulation) and vertical activity counts (rearing).
2.3 Drugs
Morphine sulfate (Penick, Newark, NJ) was dissolved in saline and administered s.c. in a volume of 1.0 ml/kg body weight. All doses are expressed as the free base.
2.4 Procedures
2.4.1 Acute Morphine- Antinociception Experiment
For each animal, the two antinociception tests were conducted in succession, tail-flick followed by hot-plate, as described elsewhere (Kalinichev et al., 2000). A cumulative-dosing procedure was used to generate dose–effect curves for each animal. Two predrug trials were conducted approximately 30 min apart. The mean of these trials served as the baseline measure for that subject. Immediately after the second baseline test, rats received an injection of saline followed 20 min later by the sequence of antinociception tests (Kalinichev et al., 2000). Next, they received increasing doses of morphine (0.5, 0.5, 1.0, 2.0, 4.0, and 4.0 mg/kg) at 20-min intervals for cumulative doses of 0.5, 1.0, 2.0, 4.0, 8.0, and 12 mg/kg. As with saline, morphine injections were given immediately after each series of antinociceptive tests (i.e. tail-flick test followed by hot plate test). After the last injection, response latencies were measured at 20-, 30-, or 60-min intervals (40, 60, 90, 120, 180, and 240 min) in order to determine the time course of the drug effect.
2.4.2 Acute Morphine- Locomotor Experiment
The first experiment was designed to determine age- and sex-dependent differences following acutely administered morphine. One week after arrival, on postnatal days 29 (periadolescents) and 64 (adults), basal horizontal activity was measured for 2 h. Beginning the next day (postnatal days 30 and 65 for periadolescent and adults, respectively), dose-response curves for horizontal activity were determined following saline and morphine (0.1–3.0 mg/kg) administration. Testing occurred over 5 days, and drug doses were given in ascending order. Dosing was based on pilot experiments conducted in our laboratory.
2.4.3 Protracted Effects One Month Following Morphine Exposure- Locomotor Experiment
A subsequent set of experiments was performed to determine the impact of age of morphine exposure on subsequent sensitivity to the drug in both males and females. The experimental design was based on one from a previous study in our laboratory (White and Holtzman, 2005). In short, eight days after arrival, beginning on postnatal days 30 (periadolescents) and 65 (adults), subjects were treated with one of four injection regimens: five days of saline (S-S-S-S-S), one day of morphine (10 mg/kg) followed by four days of saline (M-S-S-S-S), three days morphine followed by two days of saline (M-M-M-S-S), or five days of morphine (M-M-M-M-M). In pilot experiments, several subjects died in response to a single injection of 10 mg/kg, therefore the total morphine dose was given in two 5.0 mg/kg injections (s.c.) administered 10–14 h apart. The first injection each day was given in the laboratory (0900–1530 h). The second daily injection was given in the vivarium during the dark phase 10–14 h later. Animals receiving morphine for the first time were individually housed in polycarbonate cages lacking bedding for 2 h of observation. Once it was determined that subjects could tolerate the dose they were returned to their home cage following subsequent injections. As a control, saline-treated animals were also injected twice daily and individually housed after their first injection. Five weeks later, basal horizontal activity was measured for 2 h. Beginning the next day, dose-response curves for horizontal activity were determined following saline and morphine (0.1–5.6 mg/kg). Testing occurred over 6 days with subjects tested once per day. Each animal received saline and all doses of drug. Doses were given in ascending order.
2.5 Statistics
2.5.1 Acute Morphine- Antinociception Experiment
For the antinociception testing, response latencies following morphine treatment (dose-response and time-course curves) are expressed as percentage of maximum possible effect (%MPE; Dewey and Harris, 1975).
The transformed data were analyzed by a mixed three-factor ANOVA (sex-repeated X age-repeated X dose or time). Newman-Keuls post hoc comparison was used, when appropriate, to determine significant differences among means. The % MPE was also used to calculate the dose at which 50% antinociception occurred (i.e. ED50) and the time at which morphine-induced antinociception reached one-half its maximal value following the last injection (i.e. t½). The latter served as an estimate of the offset rate of morphine. The ED50 and t½ estimates were based on linear regressions of the ascending portion of the dose-response curve and the descending portion of the time-course curve, respectively, for each individual. From these, means and 95% confidence intervals (95% C.I.) were derived for each group. Comparisons between groups were made using a two-factor ANOVA (sex X age). The alpha level chosen for all statistical measures was P≤0.05.
2.5.2 Acute Morphine- Locomotor Experiment
For experiments involving the acute locomotor effects of morphine, male and female cohorts were tested at different times. Therefore, the untransformed data for each group (males and females) were analyzed separately. Basal activity prior to treatment between periadolescents and adults was analyzed using Student's t-test. To compensate for the potential impact of differences in basal activity and to facilitate comparisons between males and females, horizontal and vertical activity data were then normalized as a percentage of within-subject basal activity using either a mixed, two-factor (age-repeated X dose) or three-factor ANOVA (sex-repeated X age-repeated X dose). The alpha level chosen for all statistical measures was P≤0.05.
2.5.3 Protracted Effects One Month Following Morphine Exposure- Locomotor Experiment
For the studies assessing the protracted effects of morphine exposure during periadolescence versus adulthood, both sexes (for the one month experiment) and age groups were equally represented throughout testing as appropriate. As with the data from the acute morphine study, data were normalized as a percentage of within-subject basal activity to compensate for differences in basal activity. Determinations of significant effects of age of treatment or sex on morphine-induced motor activity were made using mixed, three-factor ANOVAs (age- or sex-repeated X morphine-treatment regimen-repeated X morphine dose). Newman-Keuls post hoc comparison was used to determine significant differences among means. The alpha level chosen for all statistical measures was P≤0.05.
3. Results
3.1 Acute Morphine- Antinociception Experiment
3.1.1 Acute Morphine- Antinociception Experiment: Tail-flick Test
Baseline latencies (periadolescent males: 2.8 ± 0.1 s; adult males: 3.6 ± 0.2 s; periadolescent females: 2.9 ± 0.1 s, adult females: 3.2 ± 0.2 s) were compared using a two-factor ANOVA (sex X age). There was a significant effect of age (F(1,28)=10.63, P≤0.01). The average latency for adult males was significantly greater than that of periadolescent males. There was no effect of sex (F(1,28)=0.63, P=0.43) nor a significant interaction between the two factors (F(1,31)=1.83, P=0.19).
A mixed, three-factor ANOVA (sex-repeated X age-repeated X dose) revealed a significant effects of sex (F(1,31)=4.77, P≤0.05), age (F(1,31)=30.85, P≤0.001), and drug (F(5,160)=212.25, P≤0.001). Further inspection of the data also revealed significant interactions between gender and age (F(1,31)= 3.90, P≤0.05), gender and drug dose (F(5,191)= 2.62, P≤0.05) and between age and drug dose (F(5,191)= 8.01, P≤0.001). Analysis of morphine ED50 data demonstrated an overall significant effect of age of treatment (Table 2; F(1,25)= 17.53, P≤0.001) and a significant interaction between gender and age of treatment (F(5,191)= 8.01, P≤0.001). As shown in Fig. 1, morphine dose-dependently increased tail-flick latencies in both males (Fig. 1A) and females (Fig. 1C). Periadolescents were less sensitive to morphine-induced analgesia compared to adults and required more drug to reach 100% MPE (Figs. 1A and 1C).
Table 2.
Treatment age and sex significantly affect morphine-induced antinociception- periadolescent- versus adult-treated rats
| Sex | Age group | N | Tail-Flick ED50 and 95% C.I. (mg/kg) | N | Hot Plate ED50 and 95% C.I. (mg/kg) | N | Tail-Flick t1/2 and 95% C.I. (min) | N | Hot Plate t1/2 and 95% C.I. (min) |
|---|---|---|---|---|---|---|---|---|---|
| Males | Periadolescents | 8 | 2.12 (1.75, 2.50)@ | 7 | 2.30 (1.74, 2.85)# | 8 | 120 (101, 139)# | 8 | 105 (81, 128) |
| Adults | 5 | 1.82 (1.67, 1.97) | 8 | 11.6 (2.46, 20.7) | 7 | 183 (172, 194) | 6 | 111 (69, 152) | |
| Females | Periadolescents | 8 | 3.20 (2.64, 3.76)# | 8 | 4.25 (3.01, 5.50) | 7 | 113 (92, 133)# | 7 | 68 (55, 82)#@ |
| Adults | 8 | 1.67 (1.12, 2.22) | 7 | 5.70 (4.25, 7.15) | 6 | 206 (161, 252) | 7 | 122 (105, 140) |
ED50 indicates the dose at which 50% antinociception occurred. T½ is the time at which morphine-induced antinociception reached one-half its maximal value following the last drug injection. 95% C.I. is the confidence interval of the mean at 95%.
Indicates a significant difference from adult group of same gender, P<0.05;
Indicates significant gender effect, P<0.05.
Fig. 1. Periadolescent rats are less sensitive than adults to morphine-induced antinociception as measured using the tail-flick test.
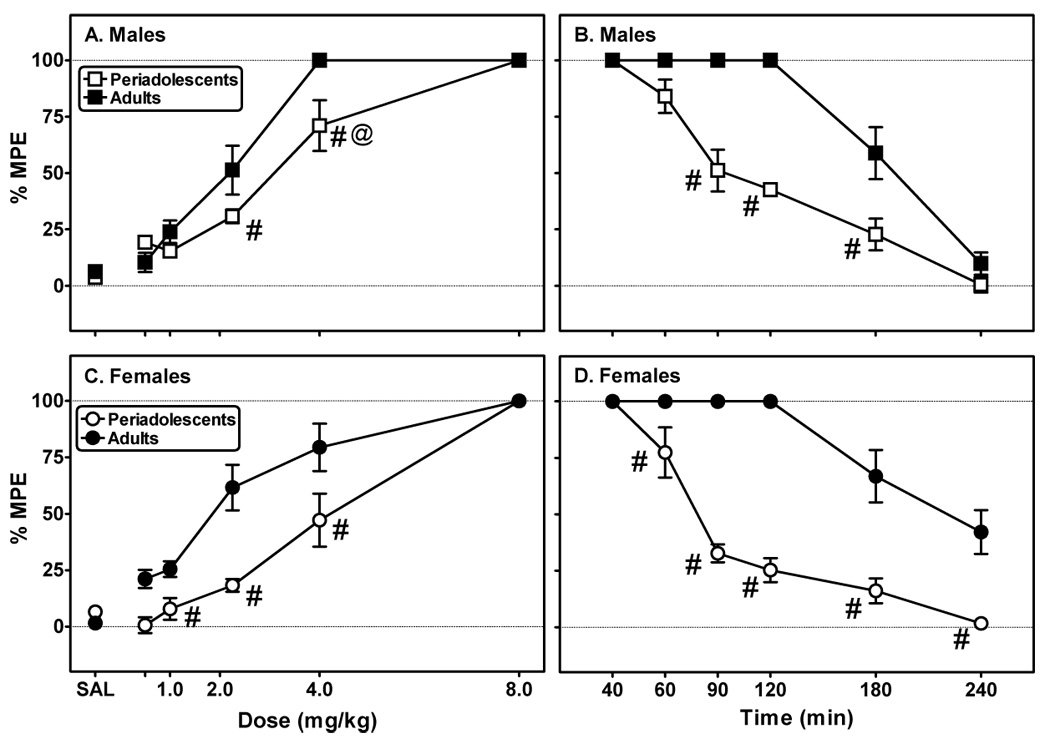
Tail-flick latencies were measured in 20 min intervals after administration of either saline (SAL) or cumulative doses of morphine (A males; C: females). Tail-flick latencies continued to be recorded (20–60 min intervals) following the last injection of morphine (B: males; D: females). Data are presented as a percentage of the maximum possible effect (% MPE), as defined in the text. Each point represents the mean ± S.E.M. (n=8/group). #Indicates a significant difference between periadolescents and adults, P<0.05. @Indicates significant differences between genders of same age group, P<0.05.
For the time course portion of the experiment (Figs. 3B and 3D), there were significant effects of time (F(5, 160)=132.80) and age of treatment (F(1,31)= 174.11, P≤0.001). There were also significant interactions between gender and age (F(1,31)= 7.59, P≤0.01), gender and time (F(5,191)= 2.91, P≤0.05), and age and time (F(5,191)= 20.38, P≤0.001). The effects of morphine were shorter-lived in the periadolescent animals, resulting in significantly quicker offset rates (Table 2; F(1,24)= 60.57, P≤0.001).
Fig. 3. Periadolescent males, but not females, are less active in a novel environment but are more sensitive to morphine-induced locomotion compared to adults.
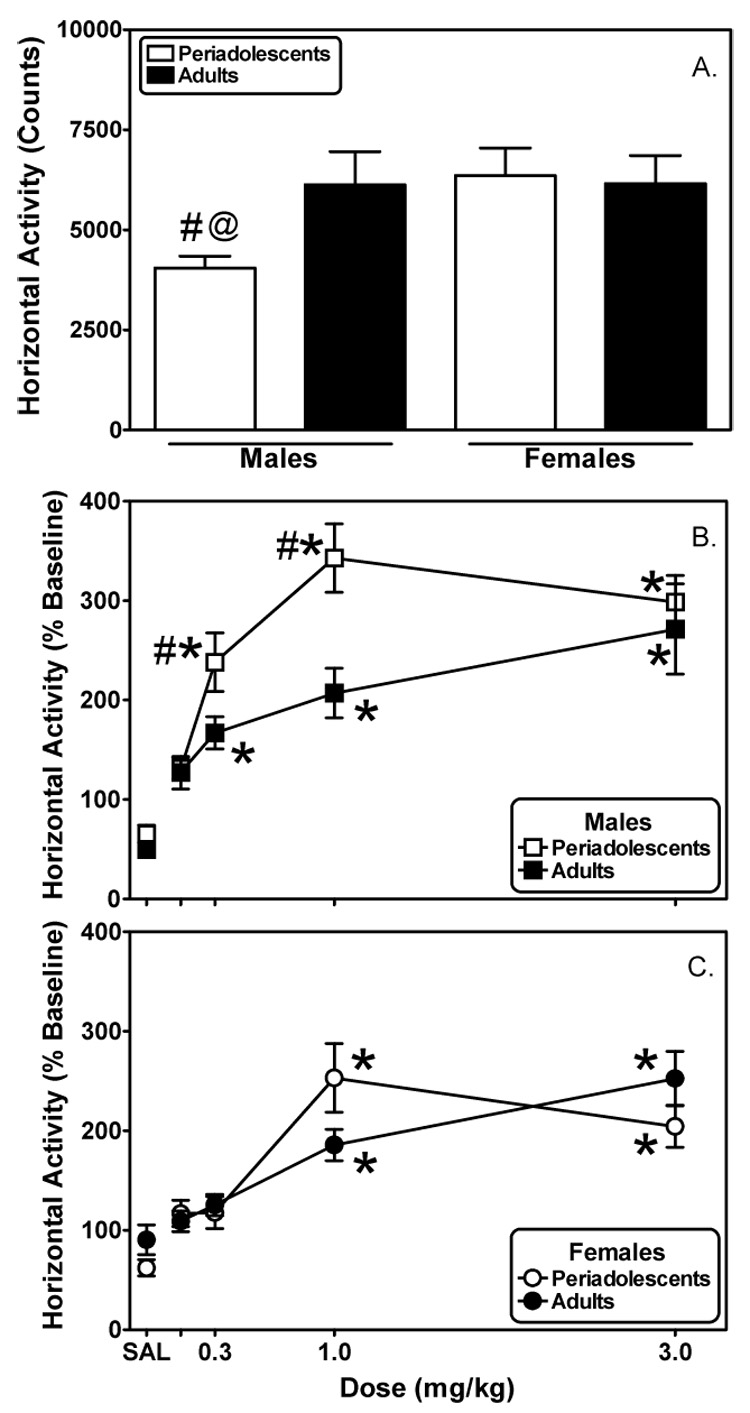
Basal horizontal activity (A.) was determined in periadolescent and adult male and female rats for 2 h during initial exposure to the locomotor activity chambers. Periadolescent and adult rats were 29 and 64 days old, respectively. Beginning the next day, horizontal activity was assessed (2 h) in males (B.) and females (C.) immediately following the administration of saline or increasing doses of morphine. Dose-response data are normalized as a percentage of within-subject baseline activity. Each data point represents the mean ± S.E.M. (males n=7–8; females n=12). #Indicates significantly different from adults, p<0.05. *Indicates a significant difference from saline, p<0.05. @Indicates a significant gender effect, P<0.05.
3.1.2 Acute Morphine- Antinociception Experiment: Hot Plate Test
All four groups of rats had similar baseline latencies on the hot plate prior to morphine administration (periadolescent males: 10.2 ± 0.7 s; adult males: 10.0 ± 0.8 s; periadolescent females: 9.7 ± 0.6 s; and adult females: 10.3 ± 0.7 s, respectively). As a result, there were no effects of gender (F(1,28)= 0.026, P=0.87) or age (F(1,28)= 0.077, P=0.78) on latency.
As with the tail-flick test, there were significant effects of sex (F(1,31)=9.64, P≤0.01), age (F(1,31)=9.21, P≤0.01), and drug (F(5,160)=111.60, P≤0.001) on hot plate latencies for both males (Fig. 2A) and females (Fig. 2C). Analysis further revealed significant interactions between sex and morphine dose (F(5,160)=2.75, P≤0.05) and between age and morphine dose (F(5,160)=4.65, P≤0.001). A comparison of morphine ED50s (Table 2) demonstrated a significant effect of age of treatment (F(1,26)=11.25, P≤0.01). There was no effect of sex (F(1,26)=0.50, P=0.48) or interaction between sex and age (F(1,29)=3.86, P=0.06) on morphine ED50s.
Fig. 2. Periadolescent males, but not females, are more sensitive than their adult counterparts to morphine-induced antinociception as measured using the hot plate test.
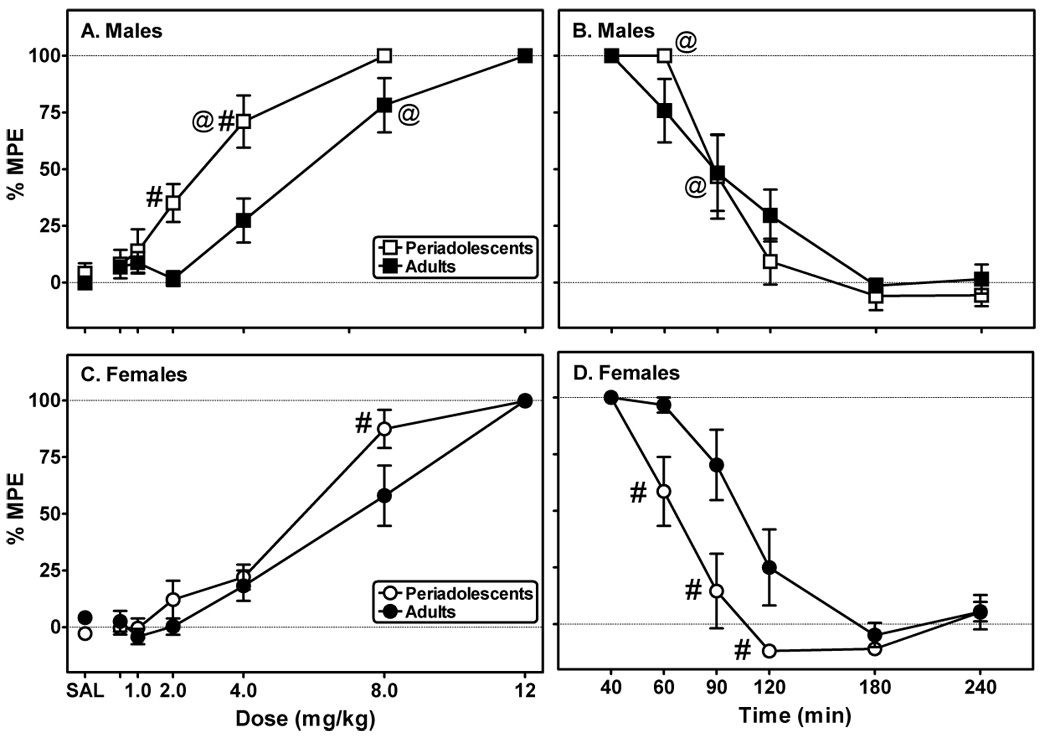
Hot plate latencies were measured in 20 min intervals after administration of either saline (SAL) or cumulative doses of morphine (A: males; C: females). Hot plate latencies continued to be recorded (20–60 min intervals) following the last injection of morphine (B: males; D: females). Data are presented as a percentage of the maximum possible effect (% MPE), as defined in the text. Each point represents the mean ± S.E.M. (n=8/group). #Indicates a significant difference between periadolescents and adults, P<0.05. @Indicates significant differences between genders of same age group, P<0.05.
Analysis of the time course data (Figs. 2B and 2D) revealed a significant main effects of time (F(5,160)=104.86, P≤0.001) and age (F(1,31)=4.42, P≤0.05). There were also significant interactions between sex and time (F(5,191)=2.24, P≤0.05), age and time (F(5,191)=2.30, P≤0.01), and between sex, age, and time (F(5,191)=2.30, P≤0.01). A two-factor ANOVA of the t½ data (Table 2) demonstrated an effect of age (F(1,24)=5.67, P≤0.01) and an interaction between sex and age (F(1,27)=5.67, P≤0.05). The offset rate for periadolescent females was significantly less than that of adult females (Table 2). Post hoc analysis also revealed morphine’s effects waned significantly quicker in periadolescent females than in periadolescent males.
3.2 Acute Morphine- Locomotor Experiment
Periadolescent males exhibited significantly less basal horizontal active compared to their adult counterparts upon their initial exposure to the locomotor activity chambers (Fig. 3A; t(13)=−2.57, P≤0.05). Periadolescent and adult female rats exhibited comparable levels of basal activity (Fig. 1A).
Horizontal activity data following morphine treatment were expressed as a percentage of within-subject basal horizontal activity for males (Fig. 3B) and females (Fig. 3C) and were analyzed using mixed, two-factor ANOVAs (age-repeated X morphine dose). Analysis revealed significant effects of drug in both males (F(4,60)=11.74, P≤0.001) and females (F(4,96)=22.34, P≤0.001) and significant effects of age in males (F(1,14)=5.54, P≤0.05) but not females (F(1,23)=0.063, P=0.80). To determine the effects of gender, a subsequent analysis (mixed, three-factor ANOVA: sex-repeated X age-repeated X morphine dose) was performed. There was a significant effect of sex (F(1,19)=8.38, P≤0.01) as well as a significant interaction between sex and morphine dose (F(4,199)=8.38, P≤0.001).
Periadolescent male (t(14)=−3.69, P≤0.05) and female (t(22)=−2.80, P≤0.05) rats displayed significantly less vertical activity compared to adults during their initial exposure to the locomotor activity chambers (Fig. 4A). In males, there were significant effects of age (F(1,14)=9.91, P≤0.01) and morphine dose (F(4,60)=3.45, P≤0.05) (Fig. 4B). Consistent with the males, there were significant effects of age (F(1,23)=4.28, P≤0.05) and morphine dose (F(4,96)=2.91, P≤0.05) in females (Fig. 4C). There was no overall effect of gender (F(1,19)=0.65, P=0.63) or significant interactions for any of the analyses performed on vertical activity.
Fig. 4. Periadolescent rats exhibit different sensitivity to morphine-induced vertical activity than adult rats.
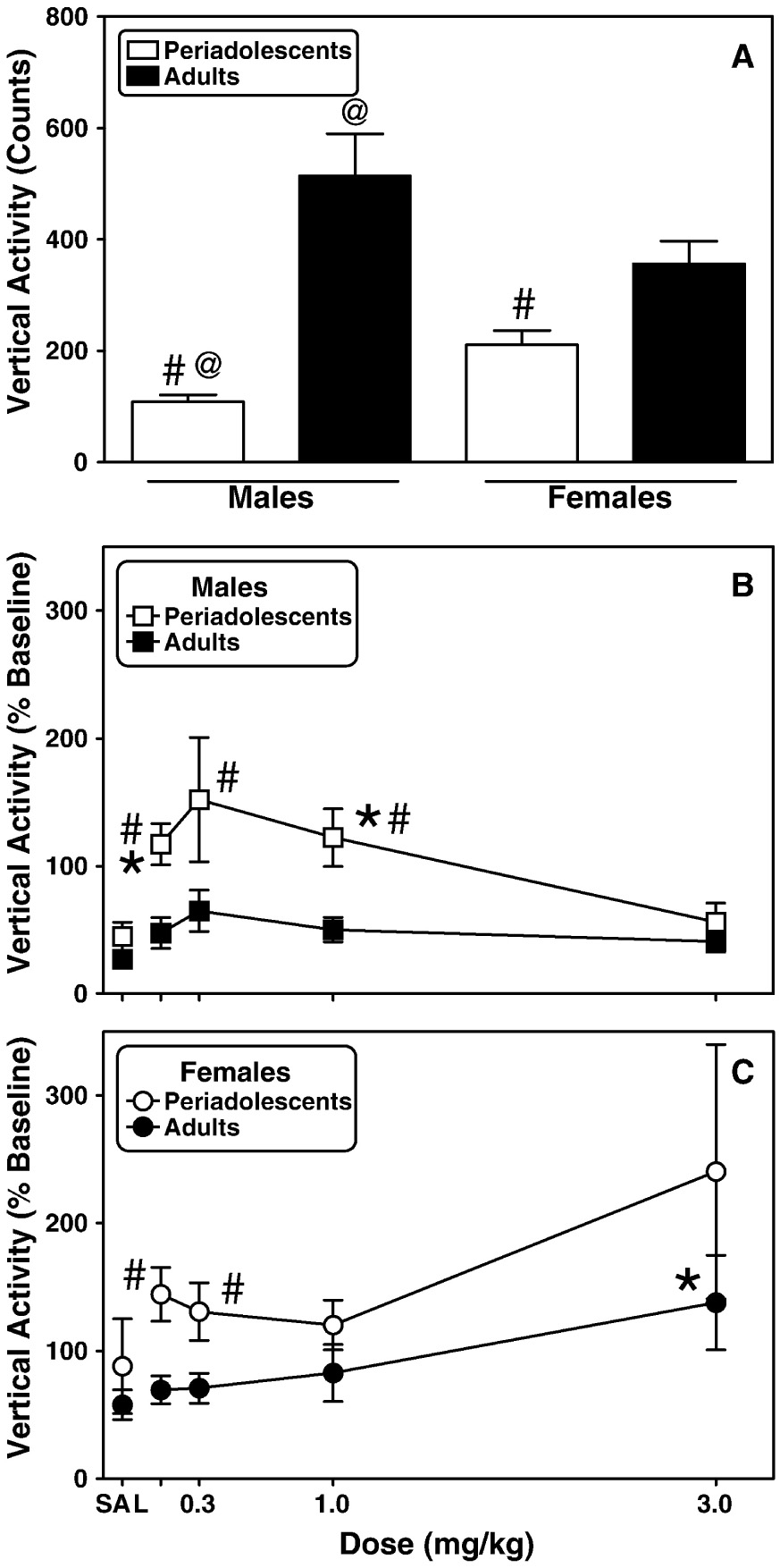
Basal vertical activity (A.) was determined in periadolescent and adult male and female rats for 2 h during initial exposure to the locomotor activity chambers. Beginning the next day, activity was assessed (2 h) in males (B.) and females (C.) immediately following the administration of saline or increasing doses of morphine. Each data point represents the mean ± S.E.M. (males n=7–8; females n=12). Other details are as in Fig. 1. #Indicates significantly different from adults, p<0.05. *Indicates a significant difference from saline, p<0.05. @Indicates a significant gender effect, P<0.05.
3.3 Protracted Effects One Month Following Morphine Exposure- Locomotor Experiment
Five weeks after treatment, when periadolescent rats had reached adulthood, motor activity in response to morphine was assessed (Fig. 5 and Fig. 6). A mixed, three-factor ANOVA (age-repeated X treatment-repeated X dose) revealed morphine dose-dependently and significantly increased horizontal activity in males (Figs. 5A, 5B; F(5,320)=45.61, P≤0.001) and females (Figs. 5C, 5D; F(5,240)=30.35, P≤0.001). For the males, there were also significant effects of age (F(1,63)=5.57, P≤0.05) and treatment regimen (F(3,63)=4.00, P≤0.01) as well as significant interactions between age of treatment and morphine dose and between treatment regimen and morphine dose (F(5,383)=4.27, P≤0.001 and F(15,383)=2.79, P≤0.001, respectively). Consistent with the males, there was an effect of treatment regimen (F(3,47)=5.13, P≤0.01) for females. There were also interactions between age of treatment and morphine dose (F(5,287)=9.15, P≤0.001), between treatment regimen and morphine dose (F(15,287)=2.69, P≤0.001) and between all three factors (F(15,287)=2.49, P≤0.01).
Fig. 5. Morphine exposure differentially impacts subsequent morphine-induced locomotor responses in rats treated as periadolescents or adults.
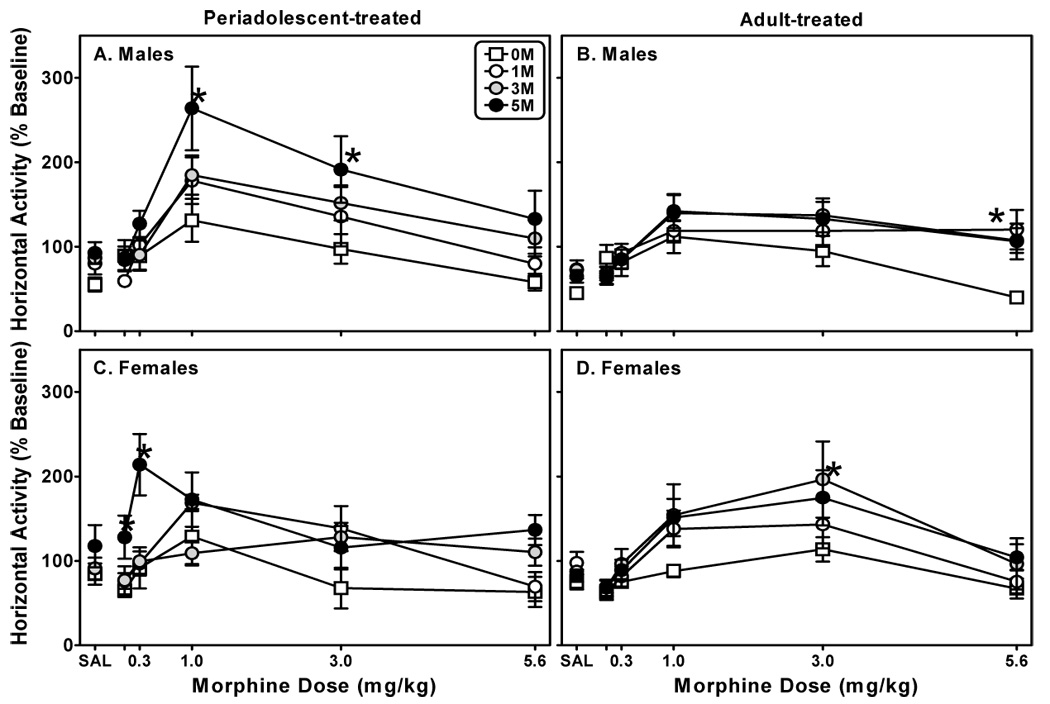
Periadolescent male (A) and female (C) and adult male (B) and female (D) rats were treated with one of four injection regimens: five days of saline (S-S-S-S-S), one day of morphine (10 mg/kg/day) followed by four days of saline (M-S-S-S-S), three days of 10 mg/kg morphine (M-M-M-S-S), or five days of 10 mg/kg morphine (M-M-M-M-M). Five weeks later, horizontal activity was assessed (2 h) immediately following the administration of saline or increasing doses of morphine. Each data point represents the mean ± S.E.M. (n=6–8). *Indicates a significant difference from S-S-S-S-S treated cohorts; mixed three-factor ANOVA, Newman-Keuls post hoc, P<0.05.
Fig. 6. Morphine exposure differentially impacts subsequent morphine-induced vertical activity in male rats but not female rats.
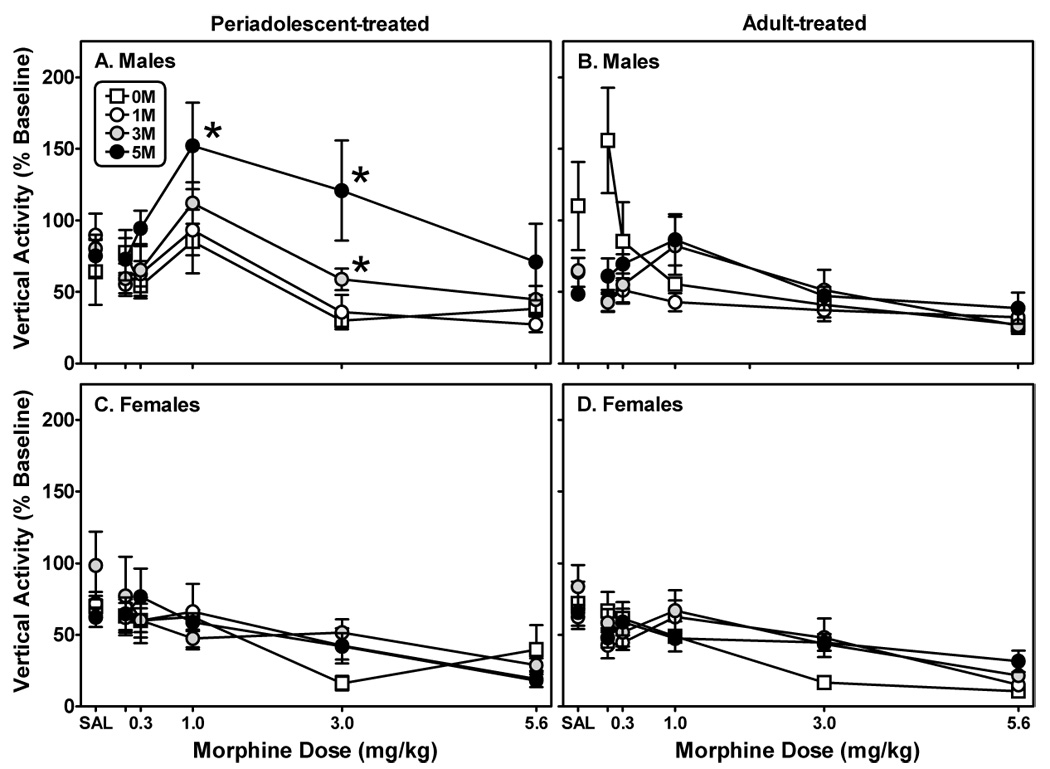
Male (A, B) and female (C, D) rats were treated with one of four injection regimens as detailed in Fig. 6. Five weeks later, vertical activity was assessed (2 h) immediately following the administration of saline or increasing doses of morphine. Each data point represents the mean ± S.E.M. (n=6–8). *Indicates a significant difference from S-S-S-S-S treated cohorts; mixed three-factor ANOVA, Newman-Keuls post hoc, P<0.05.
Subsequent analyses (mixed, three-factor ANOVA; sex-repeated X treatment-repeated X morphine dose) were used to determine the impact of gender on periadolescent- and adult-treated animals’ responses to morphine treatment regimen and drug-induced motor activity. For periadolescent-treated animals, there were significant interactions between gender and morphine dose (F(5,335)=6.38, P≤0.001) and between gender, treatment, and morphine dose (F(15,335)=2.42, P≤0.01). As with the periadolescent-treated animals, there was a significant interaction between gender and morphine dose for adult-treated animals (F(5,335)=3.91, P≤0.01).
There was a dose-dependent effect of morphine on vertical activity for males (Figs. 6A and 6B; F(5,315)=18.10, P≤0.001) and females (Figs. 6C and 6D; F(5,240)=40.39, P≤0.001). For males, there was a significant interaction between age of treatment and treatment regimen (F(3,62)=2.73, P≤0.05). There were also interactions between both factors and morphine dose (F(5,377)=4.72, P≤0.001, and F(15,377)=5.14, P≤0.001, respectively). For females, the only interaction that occurred was between morphine dose and treatment regimen (F(15,287)=2.38, P≤0.01).
In periadolescent-treated animals a mixed three-factor ANOVA (gender-repeated X treatment-repeated X morphine dose) revealed a significant effect of gender on response to morphine (F(1,54)=6.14, P≤0.01) and a significant interaction between gender and morphine dose (F(5,329)=6.29, P≤0.001). The only impact that gender had on adult-treated animal behavior was revealed in a significant interaction between gender, treatment regimen, and morphine dose (F(15,335)=2.52, P≤0.01).
4. Discussion
Our data confirm the findings of previous preclinical studies demonstrating both age- and sex-related behavioral differences in motor activity between periadolescent and adult rats under basal conditions and in response to morphine (Spear et al., 1982; White and Holtzman, 2005). The principal new contributions of this study were to extend those observations to include a) age-related differences in response to the acute antinociceptive effects of morphine, and b) the impact of varying levels of morphine exposure during periadolescence (versus adulthood) on sensitivity to morphine-induced motor activity later in life in both male and female rats. Taken together, these results suggest that periadolescent males, but not necessarily females, are more sensitive to the motor-stimulatory effects of morphine compared to their adult counterparts. However, this age-related difference in sensitivity is not global (e.g., morphine-induced antinociception varied in an assay-dependent manner). Our data also suggest that limited morphine exposure during periadolescence results in animals that are sex-dependently more sensitive to opioids later in life. Lastly, regardless of age of initial exposure, morphine exposure can have quite profound and long lasting effects.
In the tail-flick test, periadolescent rats were less sensitive than adult rats were to the antinociceptive effects of morphine, requiring more drug to attain 100% MPE; moreover, the effects of the drug abated more quickly. Response profiles were markedly different using the hot-plate test as periadolescent males were more sensitive than adults and both groups of females responded comparably. During the time course, the antinociceptive response to morphine wore off more quickly in periadolescent females as compared to adults, consistent with the tail-flick assay, but the effects of morphine waned at comparable rates in males. The influences of age and gender on analgesic processes have been reviewed elsewhere (Hamm and Knisely, 1988; Cook et al., 2000; Craft, 2003). We are unaware of any studies that have made comparisons of the antinociceptive potency of morphine between adolescent and adult rats while considering the impact of gender. However, two studies have directly examined the impact of age (adolescence versus adulthood) on antinociceptive potency of morphine (Nozaki et al., 1975; Ingram et al., 2007). In one study in which an electric foot-shock technique was used, the antinociceptive effects of morphine were greater in older (12 weeks old) versus younger (4 and 7 weeks old) rats (Nozaki et al., 1975). Younger rats also developed tolerance to morphine-induced antinociception more quickly. These findings are generally in keeping with ours from the tail-flick test in terms of the relationship between age and antinociceptive potency. However, a more appropriate comparison would be between the 4- and 7-week old animals. (Nozaki and colleagues 1975) failed to find differences between the two groups in response to morphine-induced analgesia, but 4-week old animals still showed a more rapid onset of tolerance with repeated dosing.
In a more recent article (Ingram et al., 2007), adolescent male rats (28–35 days old) were more sensitive to morphine-induced antinociception and showed greater tolerance as compared to adult male rats (73–75 days old) using the hot plate test. We found a similar effect using the hot plate test. However, this was exclusive to males. The greater antinociceptive effect of morphine in periadolescent males versus females is consistent with reviewed findings, where males tended to be more sensitive to morphine-induced analgesia than females using the hot plate test (Craft, 2003). Given the age of the subjects in the current study, the differences in morphine-induced analgesia between periadolescent males and females are likely not due to circulating gonadal hormones. However, these results may stem from sex-related differences in opioid pharmacokinetics (e.g., disposition, metabolism) or pharmacodynamics (e.g., receptor density, affinity, and signaling transduction) as has been suggested elsewhere (Craft, 2003). The consistency between periadolescent males and females in the tail-flick test would argue against such a notion. However, there is evidence that there are greater brain levels of morphine in males versus females following systemic administration (Candido et al., 1992). Although both the tail-flick and hot-plate antinociceptive assays measure the response to thermal stimuli, the tail-flick response is mediated predominantly by spinal mechanisms, whereas the hot-plate response is mediated predominantly by supraspinal mechanisms (Yaksh, 1981; Grossman et al., 1982).
Despite consistency with the literature regarding vertical activity (Faraday et al., 2001; Schochet et al., 2004), we found that periadolescent males were less active upon their initial exposure to the locomotor testing apparatus compared to adult males. This is in contrast to the notion that adolescent rats are typically hyperactive in novel situations compared with adult rats (Spear and Brake, 1983; Spear, 2000). However, this finding is not uncommon. Sometimes adolescent males have less activity (versus adult males) upon their initial exposure to a testing environment under basal conditions (Faraday et al., 2001;Faraday et al., 2003) or following vehicle injection (Collins and Izenwasser, 2004), and other times there are no differences between the two age groups (Spear et al., 1982; Schochet et al., 2004; White and Holtzman, 2005).
In the current study, periadolescent male rats were more sensitive to the acute motor stimulatory effects of morphine than were adult male rats, while periadolescent female rats tended to show greater morphine-induced vertical activity (e.g., 0.1 and 0.3 mg/kg) as compared to adult female rats. These results are generally consistent with those of (Spear and colleagues 1982), where adolescent rats, particularly females, exhibited greater horizontal (matrix crossings) and vertical (rearing) activity at higher doses of morphine (e.g. 5.0 and 10 mg/kg) compared to adults. Our findings differed somewhat in that the greatest effects were observed at lower doses of morphine and in periadolescent males as opposed to periadolescent females. Different results between the two studies are likely attributable to differences in route of administration (s.c. versus i.p.) and experimental design (e.g., dosing regimen). We only tested up to a dose of only 3.0 mg/kg of morphine because in pilot studies higher doses suppressed equally the motor activity of all experimental groups. On the other hand, in the study by (Spear and colleagues 1982), drug doses were expressed as the salt form, whereas drug doses in the current study were expressed in terms of the base, potentially reducing differences between the two studies. Regardless, the overall findings of this study and those of (Spear and colleagues 1982) are in line with those of other studies of age-related (Spear and Brake, 1983; Collins and Izenwasser, 2002; Vastola et al., 2002; Schochet et al., 2004) and age- and sex-related (Collins and Izenwasser, 2004) differences in motor response to drugs of abuse.
Subsequent sensitivity to the locomotor stimulatory effects of morphine was exposure- and age-dependent. This was true for both measures but particularly evident with horizontal activity, where the effects of morphine exposure were marked in periadolescent-treated male and female rats, persisting into young adulthood (and even longer for males). The findings with males are consistent with a previous study from our laboratory, where three days of morphine treatment (10 mg/kg) during periadolescence resulted in a marked increase in sensitivity to morphine in adulthood (White and Holtzman, 2005). Periadolescent-treated females receiving morphine for five days demonstrated a qualitatively different response to the drug as compared to the males. Greater stimulation occurred at the lowest two doses of morphine (0.1 and 0.3 mg/kg) compared to saline, one-day, and three-day morphine-treated cohorts and at half log unit lower dose than their male morphine-treated counterparts. These findings are clearly suggestive of a leftward shift of the dose-response curve for this particular group, which raises the possibility that we might have observed greater activity relative to the cohorts’ activity at even lower doses of morphine. This apparent increased sensitivity of periadolescent females versus their male counterparts clearly requires further investigation. The impact of morphine exposure on subsequent sensitivity to the drug was less marked in adult-treated rats, with no clear relationship between days of morphine exposure and subsequent sensitivity; however, adult morphine-exposed females did show greater horizontal activity in response to 1.0 and 3.0 mg/kg of morphine than saline-treated cohorts. This is in contrast with the males, where there were no differences in horizontal activity between morphine- and saline-treated rats until the highest dose of morphine. At that dose, morphine-treated rats were resistant to the activity-suppressing effects of morphine compared to their saline-treated counterparts. Given the purported links between sensitivity to drug-induced locomotion and increased vulnerability to drug taking (Vezina, 2004), our findings are suggestive of a possible relationship between gender and abuse liability of opiates. In support of this, (Cicero and colleagues 2000) demonstrated that male and female rats differentially responded to the positive reinforcing properties of morphine as measured in the place conditioning procedure. Although a preference for a chamber paired with morphine was conditioned in both groups, female rats exhibited a stronger preference for the morphine-paired chamber over a broader range of doses. Moreover, in a separate study (Cicero et al., 2003), female rats self-administered greater amounts of heroin and morphine under a fixed-ratio schedule of responding and showed higher “breakpoints” under a progressive ratio of responding compared to male rats.
In comparison with our previous study (White and Holtzman, 2005), we observed an increased motor response to morphine following periadolescent exposure to one and three days of morphine in males; however, activity was not significantly different from that of saline-treated cohorts for either group until the animals received five days of morphine treatment. The need for two more days of morphine exposure to produce comparable effects to those reported in our previous study is probably the result of one key difference in the treatment protocols between the two studies. In our previous study, we immediately placed the subjects in the locomotor chambers after the first injection each day and measured their activity. Exposure to the locomotor chambers probably resulted in some degree of conditioning (i.e. context-dependent sensitization) of the subjects. Such profound effects are well documented (for review see Ohmori et al., 2000) and could confound our results despite corrective measures (e.g. data normalization). Subjects also received the second injection in their home cage, which probably muted, but did not eliminate, the effect. In the current study, we housed animals individually in clear Plexiglas cages for observation during the treatment period to eliminate any possibility of conditioning. Regardless, these data support our earlier contention (White and Holtzman, 2005) that, unlike the situation with adult treatment, there is a minimum number of exposures to morphine during adolescence required to alter subsequent sensitivity to morphine. However, once the threshold requirement is achieved, the effects are profound and long lasting.
Epidemiological evidence suggests that there is an increased propensity for excessive use of and development of addiction to various drugs of abuse (e.g. alcohol, nicotine, cocaine) when initial exposure to and use of those drugs occur early in adolescence (Estroff et al., 1989; Spear, 2000; Kandel and Chen, 2000; Spear, 2004). According to the 2006 Monitoring the Future Report (Johnston et al., 2007), general use of opioids other than heroin has leveled off over the past few years after substantial and sustained increases in use since the early 1990s, but still remains high (approximately 9%). However, two prescription narcotics, Vicodin® and OxyContin®, continue to show evidence of increasing use. In fact, after marijuana, Vicodin was the most frequently reported illicitly-used drug among high school seniors (Johnston et al., 2007). Thus far, opioids have received little attention in the adolescent preclinical and clinical literature. The considerable addictive potential of these drugs and frequent use among adolescents make preclinical studies to assess their acute and longer-term effects timely and important. We have shown that age-dependent differences in response to morphine are not global and vary depending on the assay. Our data also suggest that limited morphine exposure during periadolescence results in animals that are sex-dependently more sensitive to opioids later in life. Lastly, morphine exposure can have profound and long lasting effects regardless of age of initial exposure.
Acknowledgements
We thank Dr. Mi Zhou and Ms. Phuong N. Nguyen for their technical assistance in conducting these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnotes This research was supported in part by N.I.H. grants DA00541, DA14122, and K05 DA00008 awarded to SGH and by a URC Award from Emory University presented to DAW.
Reference List
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Inturrisi CE, Yoburn BC. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav. 1992;42:685–692. doi: 10.1016/0091-3057(92)90015-8. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav. 2000;65:91–96. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kalivas PW, Stratmann JA. Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharmacol. 1996;7:764–778. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method of determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Dewey WL, Harris LS. The tail flick test. In: Ehrenpreis S, Neidle A, editors. Methods in Narcotic Research. New York: Dekker; 1975. pp. 101–109. [Google Scholar]
- Estroff TW, Schwartz RH, Hoffmann NG. Adolescent cocaine abuse. Addictive potential, behavioral and psychiatric effects. Clin Pediatr (Phila) 1989;28:550–555. doi: 10.1177/000992288902801201. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Shtiegman K, King JA. Voluntary ethanol consumption in male adolescent hamsters increases testosterone and aggression. Physiol Behav. 1998;63:739–744. doi: 10.1016/s0031-9384(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Grossman ML, Basbaum AI, Fields HL. Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms) J Comp Neurol. 1982;206:9–16. doi: 10.1002/cne.902060103. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Knisely JS. Developmental aspects of nociception. Brain Res Bull. 1988;21:933–946. doi: 10.1016/0361-9230(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32:600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Bethesda, MD: National Institute on Drug Abuse; Monitoring the Future national survey results on adolescent drug use: Overview of key findings. 2006 NIH Publication No. 07-6202. 2007.
- Kalinichev M, Easterling KW, Holtzman SG. Periodic postpartum separation from the offspring results in long- lasting changes in anxiety-related behaviors and sensitivity to morphine in Long- Evans mother rats. Psychopharmacology (Berl) 2000;152:431–439. doi: 10.1007/s002130000556. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991 – 1993. Nicotine Tob Res. 2000;2:263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Nozaki M, Akera T, Lee CY, Brody TM. The effects of age on the development of tolerance to and physical dependence on morphine in rats. J Pharmacol Exp Ther. 1975;192:506–512. [PubMed] [Google Scholar]
- Ohmori T, Abekawa T, Ito K, Koyama T. Context determines the type of sensitized behaviour: a brief review and a hypothesis on the role of environment in behavioural sensitization. Behav Pharmacol. 2000;11:211–221. doi: 10.1097/00008877-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology (Berl) 2004;175:265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescence and the trajectory of alcohol use: introduction to part VI. Ann N Y Acad Sci. 2004;1021:202–205. doi: 10.1196/annals.1308.025. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periado lescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. II. Agonist-induced antinociception and antagonist-induced suppression of fluid consumption. Psychopharmacology (Berl) 2004;177:68–78. doi: 10.1007/s00213-004-1921-8. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981;11:293–346. doi: 10.1016/0304-3959(81)90633-3. [DOI] [PubMed] [Google Scholar]


