Summary
The signals that determine whether axons are ensheathed or myelinated by Schwann cells have long been elusive. We now report that threshold levels of neuregulin-1 (NRG1) type III on axons determine their ensheathment fate. Ensheathed axons express low levels whereas myelinated fibers express high levels of NRG1 type III. Sensory neurons from NRG1 type III deficient mice are poorly ensheathed and fail to myelinate; lentiviral-mediated expression of NRG1 type III rescues these defects. Expression also converts the normally unmyelinated axons of sympathetic neurons to myelination. Nerve fibers of mice haploinsufficient for NRG1 type III are disproportionately unmyelinated, aberrantly ensheathed, and hypomyelinated, with reduced conduction velocities. Type III is the sole NRG1 isoform retained at the axon surface and activates PI 3-kinase, which is required for Schwann cell myelination. These results indicate that levels of NRG1 type III, independent of axon diameter, provide a key instructive signal that determines the ensheathment fate of axons.
Introduction
Axons profoundly regulate Schwann cells during development of the peripheral nervous system (PNS) (Jessen and Mirsky, 2003). Mitogenic and trophic axonal signals drive the generation of Schwann cells. Subsequently, Schwann cells adopt one of two alternative phenotypes distinguishable by their anatomic relationship to the axon (Peters et al., 1991) and the repertoire of proteins they express. Individual Schwann cells either ensheath multiple small, unmyelinated axons–forming a Remak bundle–or sort larger axons into a 1:1 relationship, upregulate specific transcription factors and structural proteins, and form a multilammelar myelin sheath around the axon. This binary choice of axon ensheathment fates is reflected in correspondingly distinct patterns of impulse propagation, i.e., cable or saltatory conduction, respectively.
Classic studies first performed more than a century ago (Langley and Anderson, 1903), in which myelinated and nonmyelinated nerves were cross-anastomosed, demonstrated that axons determine the mode of Schwann cell ensheathment and their subsequent phenotype (Aguayo et al., 1976; Hillarp and Olivecrona, 1946; Simpson and Young, 1945; Weinberg and Spencer, 1976). Myelination typically commences around axons with a diameter of 1 μm or greater, and above that size, myelin sheath thickness is correlated to axon diameter (Peters et al., 1991). These findings led to the hypothesis that a critical axonal diameter triggers Schwann cell myelination, reflecting either the size of the axon (Friede, 1972; Murray, 1968; Voyvodic, 1989) or the quantity of signaling molecules on its surface (Spencer and Weinberg, 1978). An alternative hypothesis is that distinct cell surface components are present on axons destined to be myelinated that regulate Schwann cell differentiation. Such molecules have yet to be identified.
Proteins encoded by the neuregulin-1 (NRG1) gene (Adlkofer and Lai, 2000; Garratt et al., 2000a) are candidate axonal signals for regulating Schwann cell differentiation. At least 15 different secreted or transmembrane NRG1 isoforms resulting from alternative promoter usage and RNA splicing are expressed by neurons and glia, including Schwann cells (Falls, 2003a). These can be classified into three major types (I, II, and III) that differ in their amino terminal sequences (Garratt et al., 2000a; Buonanno and Fischbach, 2001). Each isoform contains an EGF domain that is sufficient to activate their cognate receptors, members of the erbB family (Yarden and Sliwkowski, 2001). NRG1 type III is the major iso-form expressed by neurons that project into the PNS (Ho et al., 1995; Meyer et al., 1997; Yang et al., 1998).
NRG1 has a key role at many stages of the Schwann cell lineage (Garratt et al., 2000a). These include promoting commitment of neural crest cells to the glial lineage (Leimeroth et al., 2002; Shah et al., 1994) and the proliferation, survival, and maturation of Schwann cells and their precursors (reviewed in Garratt et al., 2000a). Striking confirmation of NRG1's importance in the Schwann cell lineage is evidenced by the severe deficiency of Schwann cells in mice with targeted disruptions of the NRG1 gene or its receptors (Garratt et al., 2000a). Isoform-specific NRG1 knockouts, and the pattern of expression, provide compelling evidence that type III is the key isoform required for Schwann cell generation (Meyer et al., 1997; Wolpowitz et al., 2000).
The role of NRG1 in regulating axon ensheathment and myelination by Schwann cells has remained elusive. Analysis has been complicated by the failure of Schwann cells to develop in mice with targeted mutations of NRG1 and its receptors, together with the embryonic lethality of these mice. Recent studies using transgenic and conditional knockout strategies have established that levels of axonal NRG1, signaling through the erbB2 coreceptor, determine the number of lamellae that myelinating Schwann cells form around axons (Garratt et al., 2000b; Michailov et al., 2004). A fundamental unanswered question is whether NRG1 also dictates the binary choice of axon ensheathment fates by regulating the alternative phenotypes of Schwann cells. We now report that threshold levels of NRG1 type III, independent of axon diameter, provide the long-sought instructive signal that determines whether axons become ensheathed or myelinated.
Results
NRG1 Type III Is Required for Ensheathment and Myelination of Axons by Schwann Cells
We first examined the role of NRG1 type III in myelination by explanting dorsal root ganglia (DRG) from wild-type (wt) and knockout (NRG1 type III−/−) mice (Wolpowitz et al., 2000) into culture. Neurites from wt mice were quickly repopulated by endogenous Schwann cells migrating out from the explant and associating with individual nerve fibers. In contrast, there was a marked deficiency of Schwann cells in explants from the NRG1 type III−/− mice (see Figure S1 in the Supplemental Data online); over time (weeks), such cultures partially repopulated with endogenous Schwann cells. Interestingly, many of the Schwann cells in the NRG1 type III−/− cultures preferentially grew out along the substrate rather than attaching to the neurites, which instead remained fasciculated and poorly ensheathed (Figure S1). This phenotype was never observed in the wt cocultures. These explant studies are consistent with the known role of NRG1 type III in the generation of Schwann cells and also suggest that NRG1 type III is required for proper ensheathment.
To directly examine the role of NRG1 type III in ensheathment and myelination, mature Schwann cells, isolated from postnatal rats, were plated onto established cultures of either wt or NRG1 type III−/− DRG neurons, thereby circumventing the requirement for NRG1 during the early Schwann cell lineage. Ascorbate was added to the media to initiate myelination, and cocultures were maintained for up to 60 days. Under these conditions, Schwann cells attached to both wt and NRG1 type III−/− neurites and persisted throughout the period of coculture. Schwann cells were more closely aligned with and draped around wt compared to NRG1 type III−/− neurites based on S100 staining (data not shown). Of particular note, Schwann cells extensively myelinated wt and NRG1 type III+/− neurons but consistently failed to myelinate NRG1 type III−/− neurons (Figure 1Ad). The lack of myelin protein expression was confirmed by Western blotting culture lysates (Figure 1B). Similarly, Oct-6/SCIP/Tst-1, a key transcription factor of promyelinating Schwann cells (Scherer et al., 1994), is readily detected in wt but not in NRG1 type III−/− cocultures (Figures 1B and 1C). These results indicate that NRG1 type III is required for Oct-6 activation and myelin induction.
Figure 1. NRG1 Type III Is Essential for Schwann Cell Ensheathment and Myelination.
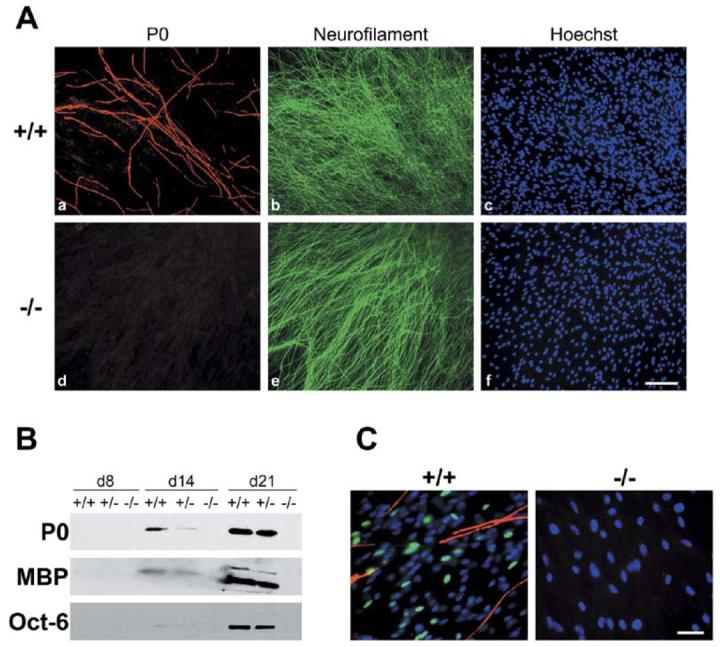
(A) Rat Schwann cells were maintained in vitro for 3 weeks with sensory neurons obtained from wt (Aa–Ac) or NRG1 type III−/− (Ad–Af) mice, fixed, and stained for the myelin protein P0 (Aa and Ad), neurofilament (Ab and Ae), and cell nuclei (Ac and Af). Numerous myelin segments are evident in wt cultures; none form in NRG1 type III−/− cultures. Scale bar, 100 μm.
(B) Cocultures of wt, NRG1 type III+/−, and NRG1 type III−/− neurons and rat Schwann cells were maintained in myelinating conditions for 8, 14, or 21 days; extracts prepared from these cocultures were probed for P0, MBP, and Oct-6. All proteins are detected in extracts of wt cocultures at both day 14 and day 21, are present at reduced levels in extracts of NRG1 type III+/− cocultures, and are absent in extracts from NRG1 type III−/− cocultures. Oct-6 is detectable at day 14 in wt and NRG1 type III+/− with longer exposures (data not shown).
(C) Schwann cells were maintained with wt and NRG1 type III−/− neurons for 15 days in myelinating conditions and stained for Oct-6 (fluorescein), MBP (rhodamine), and with the Hoechst nuclear dye (blue). Oct-6 is expressed in Schwann cell nuclei in the wt cocultures just prior to the onset of myelination; no expression was observed in Schwann cells cocultured with NRG1 type III−/− neurons. Scale bar, 50 μm.
Typically, after several weeks, there were more Schwann cells in wt compared to the NRG1 type III−/− cocultures (compare Figures 1Ac and 1Af), consistent with the known mitogenic and trophic effects of NRGs. To exclude a failure of myelination in NRG1 type III−/− cocultures due to limiting numbers of Schwann cells, we added a 5-fold excess of Schwann cells (106/cover-slip) to some NRG1 type III−/− neuron cultures. Despite a considerable excess of Schwann cells, these co-cultures also failed to myelinate (data not shown). Schwann cells also failed to myelinate NRG1 type III−/− neurons that had been grown continuously with BDNF and NT3 to promote survival of larger neurons that are normally more heavily myelinated (data not shown). Taken together these data demonstrate that NRG1 type III is an essential neuronal signal for Schwann cell myelination of all size classes of DRG neurons, independent of its mitogenic and trophic activity.
Abnormalities of ensheathment and myelination of NRG1 type III−/− axons were confirmed by electron microscopy (Figure 2). Several striking differences between these sets of cocultures were observed. The wt cocultures were robustly myelinated, whereas no myelin was present in the NRG1 type III−/− cocultures. In addition, unmyelinated axons in the wt cocultures were fully ensheathed and separated by Schwann cell processes, whereas many of the NRG1 type III−/− axons lacked any investment by Schwann cell processes, remaining directly in contact with other neurites. Other NRG1 type III−/− axons were only partially ensheathed or were incompletely sorted into separate “pockets” of the Schwann cell (Figures 2B and 2D, asterisks). Even large axons (>1 μm) remained unsorted despite close association with Schwann cells (Figure 2F, asterisks). These studies confirm that Schwann cells do not myelinate NRG1 type III−/− axons and indicate that NRG1 type III is also required for proper segregation and ensheathment of axons by Schwann cells.
Figure 2. Ultrastructure of Schwann Cell Association with Wt and NRG1 Type III−/− Neurites.
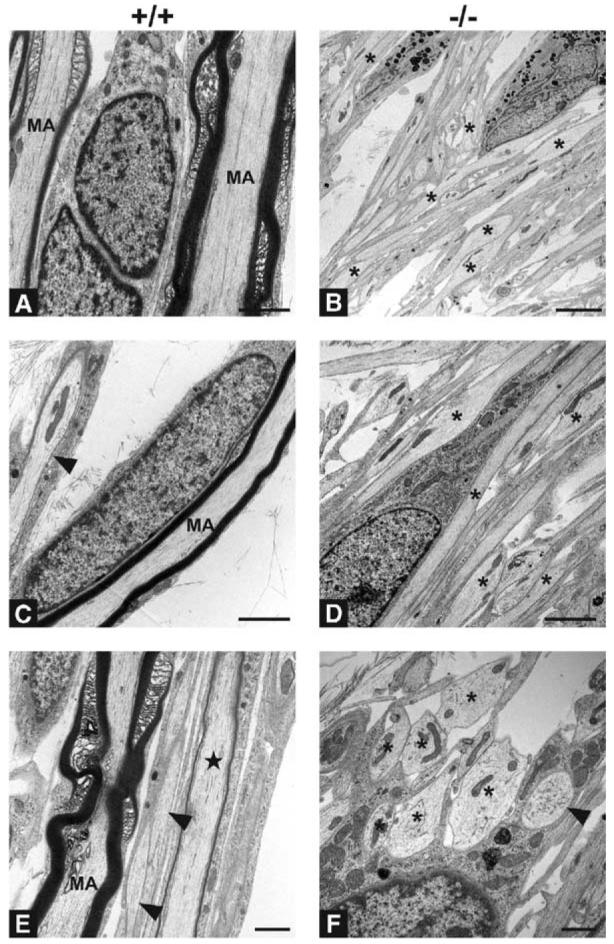
Electron micrographs of cocultures of Schwann cells and wt (A, C, and E) and NRG1 type III−/− (B, D, and F) neurons maintained for 60 days. Myelinated axons (MA) are common in wt cocultures. Interruptions in the surrounding myelin sheaths represent typical non-compacted specializations of the myelin sheath; a thinly myelinated axon is also indicated (star) in panel (E). Smaller axons are not myelinated, but are consistently ensheathed (arrowheads, [C and E]). Despite apposition of Schwann cell processes, most axons in NRG1 type III−/− cocultures lack any ensheathment or are incompletely ensheathed (asterisks). No myelin is detectable in any of the NRG1 type III−/− cocultures, and many large diameter axons (asterisks, [F]) remain unsorted; one fully ensheathed fiber is apparent ([F], arrowhead). Scale bars, 1 μm (E and F), 2 μm (A, B, D, and E). Schwann cell nuclei are visible in many of the micrographs.
NRG1 Type III Expression Levels Determine the Ensheathment Fate of Axons
We next asked whether NRG1 type III is not only necessary but may provide the long-sought instructive signal for Schwann cell myelination. We first confirmed that NRG1 levels were deficient in NRG1 type III−/− neurons (Figure 3A). Lysates from these neurons were probed with an antibody against an NRG1 epitope common to the “a” type cytoplasmic tail, the most abundant variant expressed in the nervous system (Falls, 2003b). This antibody recognizes the uncleaved type III (135 kDa) and Ig (types I/II) NRG1 (105 kDa) pro-proteins as well as an ∼65 kDa cleaved fragment of these isoforms (Frenzel and Falls, 2001). The NRG1 type III pro-protein (indicated with an arrow) was present in wt extracts, significantly reduced in the NRG1 type III+/−, and undetectable in the NRG1 type III−/− extracts (Figure 3A). Although the 105 kDa type I NRG1 pro-protein is not visible in any of the extracts, an ∼65 kDa NRG1 band, which likely corresponds to a cleaved NRG1 intracellular fragment, was prominently expressed in all of the extracts, including the NRG1 type III−/− lane. Preincubation of the pan-neuregulin antibody with the peptide to the cytoplasmic tail abolished staining of the 135 and 65 kDa bands, underscoring the specificity of the results (data not shown). These results indicate that NRG1 type III+/− and NRG1 type III−/− neurons have reduced and deficient type III expression, respectively.
Figure 3. Forced Expression of NRG1 Type III Rescues the Myelination Defect of NRG1 Type III−/− Neurons and Results in Hypermyelination.
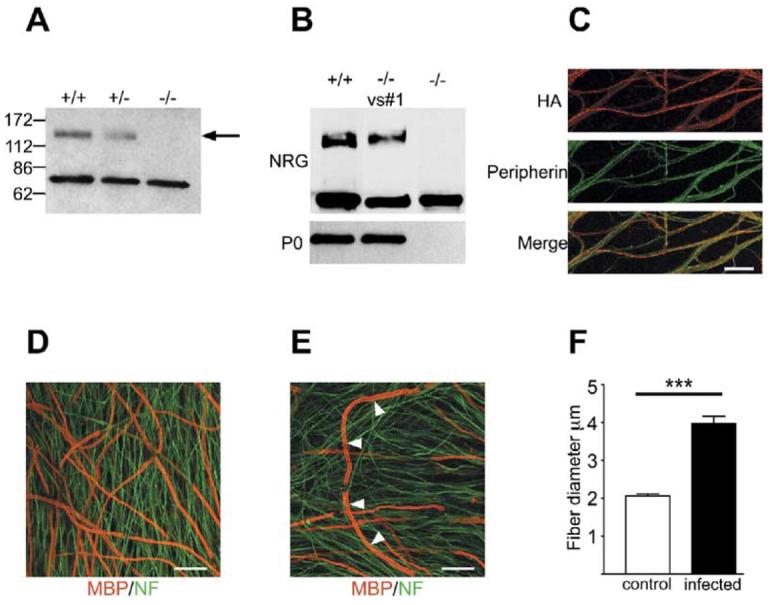
(A) Detergent lysates of wt, NRG1 type III+/−, and NRG1 type III−/− DRG neurons were fractionated by SDS PAGE and blotted with an antibody to the “a-tail” C-terminal epitope. The 135 kDa band corresponding to the full-length NRG1 type III pro-protein (indicated with an arrow) is present in wt, reduced in NRG1 type III+/−, and completely missing in NRG1 type III−/− lysates; a cleaved NRG1 band of ∼65 kDa is present in all extracts.
(B) Detergent lysates of cocultures of wt or NRG1 type III−/− neurons infected (Vs#1) or not with a lentiviral construct driving expression of an HA-tagged NRG1 type III construct were probed with antibodies to NRG1 and P0.
(C) NRG1 type III expression by infected NRG1 type III−/− neurons was detected by live staining for the HA epitope (rhodamine); neurons were double stained for the intermediate filament protein peripherin (fluorescein). Scale bar, 40 μm.
(D) Rescue of myelination of NRG1 type III−/− neurons by expression of NRG1 type III is shown in cocultures double stained for MBP (rhodamine) and neurofilament (fluorescein). Scale bar, 40 μm.
(E) A fiber with hypermyelinated segments (indicated with white arrows) is illustrated. Scale bar, 40 μm.
(F) Quantitation of hypermyelination showing the mean ± SEM. The thickest myelin segments in the infected NRG1 type III−/− cocultures were approximately twice the thickness of those in uninfected wt cocultures (p < 0.0001).
We next infected NRG1 type III−/− neurons with a lentivirus to drive expression of HA-tagged NRG1 type III. This resulted in efficient and persistent expression of NRG1 type III. Expression was comparable to that of wt neurons based on Western blotting (Figure 3B). Live staining for the HA tag indicated that NRG1 type III was robustly expressed at the surface of many axons (Figure 3C). Addition of Schwann cells to these infected NRG1 type III−/− neurons demonstrated a striking rescue of myelination to levels similar to wt neuron cocultures based on immunofluorescence (Figure 3D) and Western blotting for the myelin protein, P0 (Figure 3B). We also observed numerous examples of unusually thick myelin sheaths after just 2 weeks of coculture in myelinating conditions (Figure 3E); the most heavily myelinated segments were on average twice as thick in infected NRG1 type III−/− as compared to wt cultures (Figure 3F). While the HA epitope was consistently masked in cocultures, the sequential arrangement of these segments along individual axons (Figure 3E) suggests that those fibers may express unusually large amounts of NRG1. These results indicate that deficient myelination of NRG1 type III−/− neurons is specifically due to the lack of NRG1 type III expression and demonstrate that driving type III expression can result in a substantial increase in the thickness of the myelin sheath.
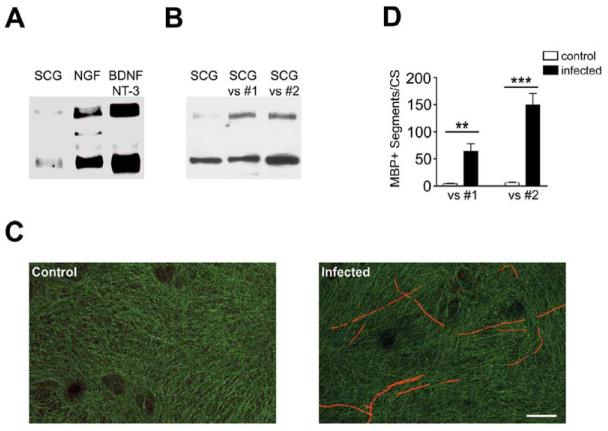
To address further a potential instructive role of NRG1 type III in myelination, we compared the levels of NRG1 on three sets of peripheral neurons: NGF-dependent sympathetic neurons of the superior cervical ganglia (SCG), which are ensheathed but unmyelinated; NGF-dependent neurons from DRGs, which are thinly myelinated; and the larger BDNF- and NT3-dependent neurons from DRGs, which are more consistently and heavily myelinated (Snider and Wright, 1996). Western blotting demonstrates that each set of neurons express both NRG1 bands (Figure 4A). However, SCG neurons express minimal levels of NRG1 type III, whereas DRG neurons, particularly the BDNF- and NT3-dependent neurons, express much higher levels. These results provide an explanation for the relatively limited mitogenic effects of SCG compared to DRG neurons for Schwann cells (R. Devon and P. Wood, cited in Obremski et al., 1993). Importantly, they indicate that levels of NRG1 vary widely on different axon types in a manner correlated to their ensheathment fate. As each lane was loaded with equal amounts of protein, NRG1 expression levels do not strictly correlate with axon size.
Figure 4. NRG1 Type III Expression Induces Myelination.
(A) Detergent lysates of SCG, NGF-dependent, and BDNF/NT3-dependent DRG neurons were fractionated by SDS PAGE and blotted with an antibody to the “a-tail” C-terminal epitope. The 135 kDa and ∼65 kDa are present in all extracts although levels vary considerably between these populations of neurons
(B) SCG neurons were infected with two separate lentiviral stocks to drive expression of NRG1type III. Detergent extracts were prepared and blotted for NRG1 as shown; the blot is slightly overexposed by comparison to panel (A) to better show the increase in NRG1 levels.
(C) Cocultures of Schwann cells and control (left) or SCG neurons infected with NRG1 type III lentivirus were stained for MBP (rhodamine) and neurofilament (fluorescein). Scale bar, 100 μm.
(D) Quantitation of the number of myelin segments in control and infected SCG cocultures from two separate experiments (four coverslips/condition/experiment) showing the mean ± SEM. The increase in the number of myelin segments is statistically significant (**p = 0.006, ***p = 0.0005).
A key question is whether increasing the expression of NRG1 type III in SCG neurons can promote myelination of these normally unmyelinated nerve fibers. To address this possibility, SCG neurons cultured from P2 rats were infected with the NRG1 type III-lentiviral construct. This resulted in detectable NRG1 type III expression, although the level of expression achieved after lentiviral infection was consistently lower in SCG than in NRG1 type III−/− DRG neurons, based on staining for the HA epitope (data not shown) and blotting for NRG1 (Figure 4B). Rat Schwann cells were then added to control or infected SCG neurons, and cocultures were maintained under myelinating conditions, fixed, and stained for MBP expression. As expected, myelin segments were rarely observed in the cocultures of uninfected SCG neurons. In contrast, significant numbers of myelin segments formed when Schwann cells were cocultured with infected neurons (Figure 4C) and continued to accumulate over time (data not shown). These results are quantitated in Figure 4D. This increase was quite remarkable, considering the limited expression of NRG1 type III by these neurons. These results demonstrate that expression of NRG1 type III by itself is sufficient to switch axons that are normally unmyelinated to a myelinating fate and provide compelling evidence for its instructive role.
NRG1 Type III Is the Sole NRG1 Isoform on the Axon Surface and Determines PI 3-Kinase Activity
Results of Western blotting suggested that sensory neurons express several NRG isoforms: type III which is expressed as an uncleaved pro-protein at the axon surface and other, cleaved forms (Figure 3A). To determine if these other isoforms are expressed at the axon surface, we incubated neurons with erbB2/3-Fc (Figure 5); this chimeric protein contains the extracellular domains of erbB2 and erbB3 fused to the Fc portion of human IgG and binds to NRG1 with high affinity (Fitzpatrick et al., 1998). ErbB2/3-Fc bound strongly to wt (Figure 5Aa), modestly to NRG1 type III+/− (Figure 5Ab), and not at all to the NRG1 type III−/− neurons (Figure 5Ac). The failure of erbB2/3-Fc to bind to NRG1 type III−/− neurons indicates that type III is the sole NRG1 isoform on the axon surface. Binding studies with erbB2/3-Fc also corroborated that SCG neurites express negligible amounts of NRG1 on their surface, that these levels increase after lentiviral infection, and are higher still on DRG neurites (data not shown).
Figure 5. Type III Is the Sole Isoform on the Axon Surface and Regulates PI 3-Kinase Activity.
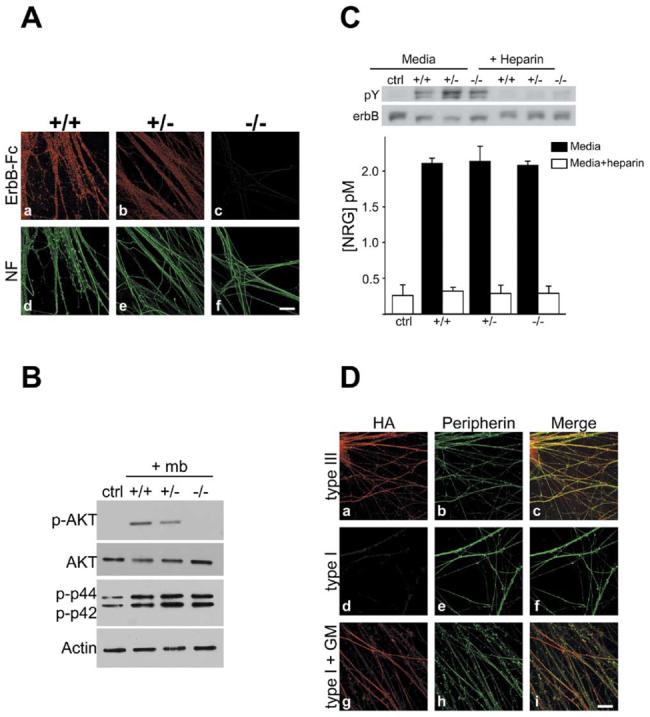
(A) Cultures of wt, NRG1 type III+/−, and NRG1 type III−/− neurons were incubated with erbB2/3-Fc and then fixed and visualized with rhodamine-conjugated anti-human Fc antibodies (Aa–Ac); neurofilament staining from the corresponding fields is shown (Ad–Af). ErbB2/3-Fc binds strongly to wt (Aa), modestly to NRG1 type III+/− (Ab), and not at all to NRG1 type III−/− neurons (Ac). Scale bar, 40 μm.
(B) Membranes prepared from wt, NRG1 type III+/−, and NRG1 type III−/− neurons were centrifuged onto Schwann cell cultures, and after 20 min, lysates were prepared, blotted, and probed for phospho-Akt, total Akt, and phospho-erk as shown.
(C) NRG levels were assayed in culture media from wt, NRG1 type III+/−, and NRG1 type III−/− neurons by measuring erbB receptor phosphorylation in L6 myotubes. A representative Western blot revealing erbB receptor phosphorylation is shown. Following the phosphotyrosine (pY) Western blot, the membrane was stripped and reprobed for total erbB. The ratio of pY to total erbB is a quantitative measure of the amount of NRG in the media. Results from two separate experiments are shown below (mean ± SD).
(D) Rat DRG neurons were nucleofected with HA-tagged NRG1 type III (Da–Dc) or type I (Dd–Di) constructs were stained for HA (Da, Dd, and Dg) and peripherin (Db, De, and Dh); merged images (Dc, Df, and Di) are also shown. NRG1 type III is expressed at the axon surface (Da), whereas type I NRG1 is detectable at the surface of neurons treated for 48 hr with the MMP inhibitor GM 6001 (Dg) but not in untreated cultures (Dd). Scale bar, 40 μm.
As type III is the only isoform expressed on the axon surface, the inability of NRG1 type III−/− neurons to myelinate suggests that contact-dependent signaling by axons to Schwann cells might be impaired. To investigate this possibility, we determined whether different levels of NRG1 type III affected activation of two key signaling pathways in Schwann cells: PI 3-kinase, which is required for myelination; and MAP kinase activity, which is not required and has been reported to have an opposing effect (Maurel and Salzer, 2000; Ogata et al., 2004). We plated membranes prepared from wt, NRG1 type III+/−, and NRG1 type III−/− neuron cultures onto Schwann cells for 20 min and then measured the levels of phospho-Akt, a key effector of PI 3-kinase, and phospho-Erk (Figure 5B). Both signaling pathways were robustly activated when wt neurite membranes were plated onto Schwann cells. Activation of PI 3-kinase was consistently reduced with the NRG1 type III+/− membranes and absent with the NRG1 type III−/− membranes. In contrast, MAP kinase activation by NRG1 type III−/− neurite membranes was equivalent to wt membranes or only slightly reduced. These results indicate that NRG1 type III on the axon membrane is the key activator of PI 3-kinase and that activation is graded to the levels of NRG1type III. They also strongly suggest that other non-NRG1 signals on the axon activate MAP kinase.
Ig-NRG1 Isoforms Are Shed by Metalloproteinase Cleavage
To determine whether other NRG isoforms expressed by NRG1 type III−/− neurons are cleaved and shed, we measured NRG activity in conditioned media from wt, NRG1 type III+/−, and NRG1 type III−/− neuron cultures using a sensitive bioassay (Esper and Loeb, 2004). Significant and comparable amounts of NRG1 were released from wt, NRG1 type III+/−, and NRG1 type III−/− neurons (Figure 5C). In each case, the NRG activity was largely blocked by incubation with soluble heparin, indicating that it reflects Ig (type I or II) isoforms. To examine the mechanism of shedding, cDNA constructs encoding HA-tagged NRG1 isoforms were nucleofected into rat DRG neurons. Neurons were grown for an additional 3 weeks, live stained for HA, fixed, permeabilized, and stained for peripherin to visualize nerve fibers. In agreement with its expression in NRG1 type III−/− neurons (Figure 3C), the epitope tag of NRG1 type III was present on the extracellular surface of rat sensory neurons (Figure 5Da). In contrast, type I NRG1 was not expressed at the surface of these axons (Figure 5Dd) unless neurons were also treated for 48 hr with the metalloproteinase (MP) inhibitors TAPI-1 (data not shown) or GM 6001 (Figure 5Dg). Thus, MPs differentially regulate the expression of NRG1 isoforms at the surface of neurons; type III is retained, whereas Ig forms are shed.
NRG1 Type III Is an Instructive Juxtacrine Signal that Requires Other Axonal Signals
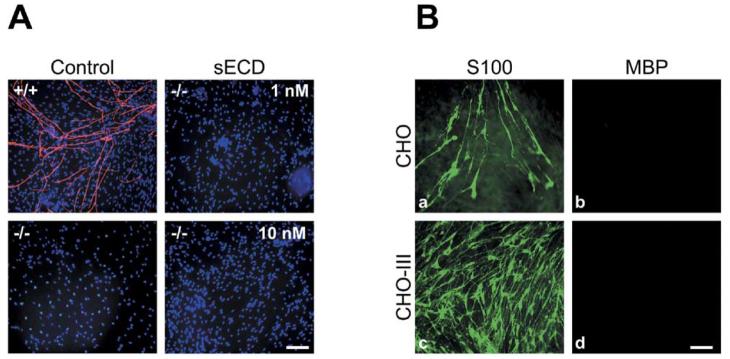
As NRG1 type III−/− neurons are poorly ensheathed and unmyelinated, the released Ig isoforms are not functionally redundant with the type III isoform, suggesting that type III may promote ensheathment and myelination only as a contact-dependent signal. We thus examined the efficacy of a paracrine source of NRG1 type III in rescuing myelination in the NRG1 type III−/− cocultures. We supplemented the media of the NRG1 type III−/− cultures with a recombinant, soluble form of the type III ectodomain (sECD), which retains its mitogenic effect and ability to activate downstream signaling pathways in rat Schwann cells (Figure S2). Cocultures were maintained in myelin-promoting media, supplemented with sECD at 1 or 10 nM for up to 30 days. Despite addition of sECD, NRG1 type III−/− cocultures failed to myelinate (Figure 6A). Interestingly, the sECD was effective in promoting repopulation of NRG1 type III−/− neurites when only small numbers of Schwann cells were added to these neurons (data not shown). Together, these findings indicate that type III is effective in promoting proliferation as a paracrine or a juxtacrine signal, but is effective in promoting myelination only as a juxtacrine signal.
Figure 6. NRG1 Type III Induces Myelination as a Juxtacrine Signal from Axons.
(A) Schwann cells were grown with wt and NRG1 type III−/− neurons in myelinating conditions for 14 days without (control) or with the addition of 1 nM or 10 nM of recombinant, soluble extracellular domain of type III (sECD). Cultures were fixed and stained for MBP (rhodamine) and cell nuclei (Hoechst, blue). Numerous myelin segments are present in wt cocultures but none in NRG1 type III−/− cocultures, even when supplemented with sECD.
(B) Schwann cells were plated onto control CHO cells (Ba and Bb) or CHO cells that stably express NRG1 type III (Bc and Bd). After 14 days, Schwann cells were stained for S100 (fluorescein, [Ba and Bc]) and MBP (rhodamine, [Bb and Bd]). There was a marked increase in the number of Schwann cells grown on the CHO-III cell monolayer but no MBP expression was detected. Scale bars, 100 μm.
We also investigated whether a heterologous, juxtacrine source of NRG1 type III can induce myelin protein expression in Schwann cells. We transfected CHO cells with NRG1 cDNA constructs and selected for cells expressing high levels of the HA-tagged NRG1 type III (CHO-III). Rat Schwann cells were then plated onto confluent monolayers of either CHO-III or control (nontransfected) CHO cells (Figure 6B). Cultures were maintained for 14 days in myelinating conditions and fixed. Schwann cells were stained with S100 antibodies and MBP expression was assessed. There was a significant accumulation of Schwann cells grown on the CHO-III monolayers over time compared to control CHO cells, confirming that NRG1 type III expressed on CHO cells retains its activity as a mitogen. However, no MBP expression was observed even after 1 month of coculture. These results indicate that NRG1type III, while necessary and instructive, requires other signals present on the axon to induce Schwann cell myelination.
NRG1 Type III+/− Mice Are Hypomyelinated and Exhibit Ensheathment Defects
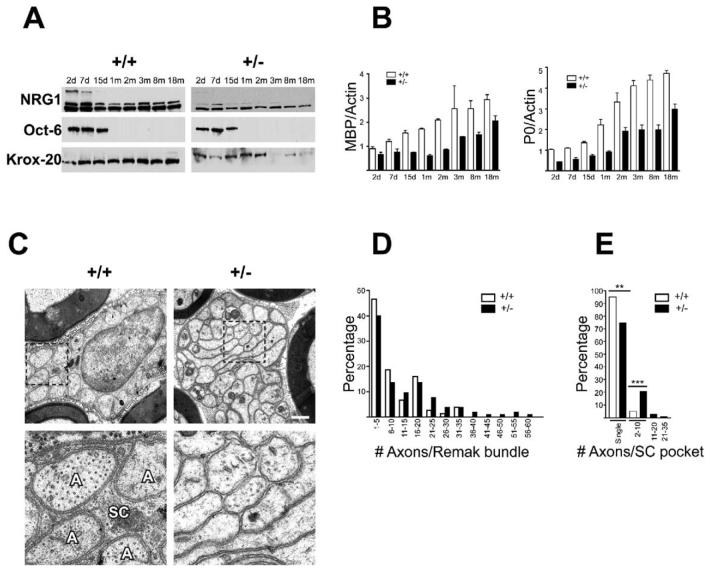
To extend these findings, we characterized PNS myelination and ensheathment in NRG1 type III+/− mice, which are viable and appear phenotypically normal in contrast to NRG1 type III−/− mice which die at birth (Wolpowitz et al., 2000). We first confirmed that NRG1 levels were reduced at all developmental stages in the PNS of NRG1 type III+/− compared to wt mice, in agreement with in vitro results (Figure 7A). We next compared the expression of myelin transcription factors (Figure 7A) and the myelin proteins P0 and MBP (Figure 7B) in wt and NRG1 type III+/− sciatic nerve. Each showed significant reductions in the heterozygotes. Thus, myelin protein levels normalized to actin were decreased by ∼30%–50%; similar results were observed for MAG (data not shown). Reductions were apparent at all ages, indicating that it reflects true hypomyelination and not a delay in the onset or progression of myelination. Changes were myelin specific, as neurofilament and actin expression were comparable to or slightly increased in the NRG1 type III+/− sciatic nerve lysates (Figure S3). These results corroborate a key role of NRG1 type III in myelination.
Figure 7. NRG1 Type III+/− Mice Are Hypomyelinated and Aberrantly Ensheathed.
(A) Extracts were prepared from sciatic nerve at the postnatal time points shown and fractionated by SDS-PAGE, blotted, and probed with antibodies NRG1 and the transcription factors Oct-6 and Krox-20.
(B) Semiquantitative analysis of PNS myelin protein levels was performed by probing Western blots with 125I protein A and quantitating by PhosphorImager analysis. Myelin protein levels are normalized to actin as indicated; the means (±SEM) from two different experiments are shown.
(C) Electron micrographs of Remak bundles in sciatic nerves from wt (left panels) and NRG1 type III+/− (right panels) adult mice show altered axonal segregation; insets are shown at higher magnification in the lower panels. In the wt mice, axons (A) are fully ensheathed by Schwann cell processes (Sc), whereas NRG1 type III+/− axons frequently directly appose each other without intervening Schwann cells processes. All of the profiles seen in the inset from the NRG1 type III+/− mice are axons, a number of which are flattened or elongated in cross-section. Scale bar, 1 μm.
(D) The number of axons per Ramak bundle for wt and NRG1 type III+/− sciatic nerves were binned into separate groups and are shown as a percentage of the total axons. Over 120 bundles for each genotype were counted (n = 2 mice of each genotype). The maximum number of axons/bundle in the wt mice was 34; in NRG1 type III+/− mice, bundles containing up to 60 unmyelinated fibers were observed (p < 0.0001).
(E) The percentages of axons in Schwann cell pockets in Remak bundles were binned into four categories: a single axon/pocket; 2–10 axons/pocket; 11–20 axons/pocket, and 21–35 axons/pocket. Over 800 unmyelinated axons for each genotype (n = 2) were counted. While almost all axons are present in individual Schwann cell pockets in wt nerves, the number in NRG1 type III+/− nerves is significantly reduced (**p = 0.006). The maximum number of axons per pocket in the wt nerves was 7 versus 35 in the NRG1 type III+/− nerves (***p < 0.0001).
Reduction in myelin protein levels could result from a lower percentage of myelinated axons, thinner myelin sheaths, or both. Quantitation of axon ensheathment on electron micrographs demonstrated that the proportion of myelinated axons is reduced in the PNS of NRG1 type III+/− mice (Table 1); thus, 41% of fibers are myelinated in wt mice, whereas only 28% were myelinated in NRG1 type III+/− littermates. These results strongly suggest that fibers that would normally be myelinated in wt mice are unmyelinated in NRG1 type III+/− mice and are consistent with culture studies indicating that the level of NRG1 type III controls the binary choice between ensheathment and myelination. In agreement with a recent report (Michailov et al., 2004), myelin sheaths in NRG1 type III+/− sciatic nerves are thinner than comparably sized fibers in wt mice (Figure S4). Morphometry confirmed a significant increase in the g ratio (axon diameter/total fiber diameter) in NRG1 type III+/− versus wt mice (Table 1 and Figure S4) but comparable axon diameters in adults (wt 3.31 μm ± 0.12 versus NRG1 type III+/− 3.30 μm ± 0.13; mean ± SEM). The ultrastructural appearance and periodicity of myelin lamellae were also unaltered (Figure S4), indicating that differences in myelin sheath thickness are due to differences in the number of lamellae.
Table 1.
Morphometric and Physiological Analyses of Wt and NRG1 type III+/− Nerves
| +/+ | +/− | ||
|---|---|---|---|
| % myelinated | 40.5% | 27.8% | |
| % unmyelinated | 59.5% | 72.2% | p < 0.0001 |
| g ratios | 0.68 ± 0.004 | 0.73 ± 0.004 | p < 0.0001 |
| Unsegregated axons/Remak bundle | 5% | 25% | p < 0.0001 |
| # axons/Remak bundle | 8.7 | 13.1 | p < 0.0001 |
| NCV Myelinated (37°C) | 39.89 ± 3.75 | 31.93 ± 2.29 | p = 0.01 |
| NCV C fibers (37°C) | 0.61 ± 0.04 | 0.46 ± 0.02 | p = 0.006 |
Summary of the morphometric analyses performed in adult wt and NRG1 type III+/− mice showing the g ratio values for sciatic nerve, the percentage of unmyelinated and myelinated fibers in sciatic nerves, and the percentage of unsegregated axons in Remak bundles. Morphologic analyses were performed using NIH Image J and Prism software. G ratios were calculated from the data set in Figure S4.
The organization of the Remak bundles was also quite different in the heterozygotes (Figure 7 and Table 1). In NRG1 type III+/− mice, nonmyelinating Schwann cells associate with more axons, and these axons are frequently incompletely segregated into Schwann cell pockets (Figure 7C). Remak bundles in NRG1 type III+/− nerves had ∼50% more axons per Schwann cell than in the wt nerves. There were several examples of more than 50 axons/bundle in NRG1 type III+/− nerves; wt nerves rarely had more than 30 axons/Remak bundle (Figure 7D), consistent with a recent report (Murinson and Griffin, 2004). In addition, many of the axons in Remak bundles of NRG1 type III+/− mice were not appropriately segregated into separate pockets of the Schwann cell but rather were bundled together as axon fascicles and lacked intervening Schwann cell processes. Wild-type nerves rarely contained more than seven axons/pocket, whereas NRG1 type III+/− nerves had as many as 35 axons/pocket (Figure 7E). Interestingly, many of the bundled axons in the NRG1 type III+/− nerves were tightly packed together and exhibited flattened, elongated profiles. These findings suggest that Schwann cell ensheathment is required for appropriate axon morphology in the adult. Corresponding abnormalities of nerve conduction velocity (NCV) were observed, with both Remak/C fibers and myelinated fibers in the heterozygotes conducting significantly more slowly than their wild-type counterparts (Table 1 and Figure S5). In addition, C fiber traces were typically more heterogeneous and exhibited reduced amplitudes in NRG1 type III+/− mice compared to their wt littermates (Figure S5).
Taken together, these findings indicate that limiting amounts of NRG1 type III increased the proportion of unmyelinated axons, resulted in aberrant sorting and ensheathment of axons in Remak bundles, thinner sheaths, and corresponding abnormalities of nerve conduction. A summary of morphologic abnormalities observed in the heterozygotes and cultures are illustrated in Figure 8.
Figure 8. Schematic Summary of Abnormalities in Nerves with Altered NRG1 Type III Levels.
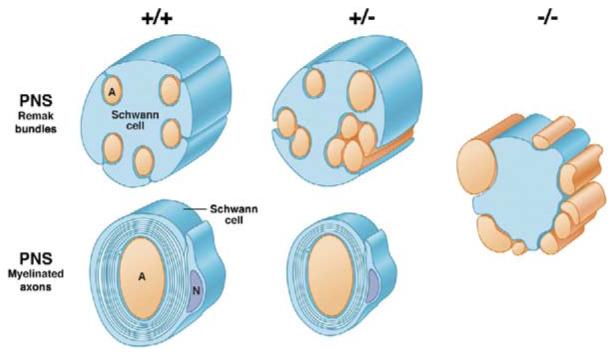
Alterations in axon ensheathment and myelination as a function of NRG1 type III levels are illustrated based on in vivo and in vitro analysis. In wt mice, unmyelinated axons in the PNS are segregated into separate pockets of Remak bundles and myelinated axons are fully wrapped. In NRG1 type III+/− mice, a greater proportion of axons remain unmyelinated and are frequently fasciculated together in Remak bundles, sometimes with distorted cross-sectional profiles; myelinated axons have thinner sheaths. In the absence of NRG1type III, Schwann cells fail to ensheath axons and even large axons remain unsegregated and unmyelinated.
Discussion
The nature of the signal(s) that determines whether axons are ensheathed or myelinated has long been elusive. We demonstrate here that NRG1 type III regulates the binary choice between ensheathment and myelination in the PNS. Low levels are required for ensheathment of PNS axons, while high levels trigger myelination. At levels above this threshold, the amount of myelin formed is graded to the levels of NRG1 type III.
NRG1 Type III Is an Essential Signal for Ensheathment and Myelination in the PNS
Peripheral nerves contain a mixture of small, unmyelinated axons organized as Remak bundles and larger, myelinated axons. The axonal signals that determine whether axons are ensheathed or myelinated have been thought to be distinct. Findings in this study indicate that NRG1 type III plays an essential role in both processes. Its crucial role in ensheathment is most strikingly demonstrated by the failure of Schwann cells in cocultures to wrap NRG1 type III−/− neurites or to physically sort even larger axons into a 1:1 relationship, despite attachment to these fibers (Figure 2). Remak bundles in the NRG1 type III+/− mice exhibit intermediate defects of ensheathment characterized by persistence of unsegregated axon fascicles together with a marked increase in the number of unmyelinated axons per Schwann cell (Figure 7). These results indicate that sorting and ensheathment of axons by Schwann cells require NRG1 type III signaling and that its levels determine the number of axons per Remak bundle.
Our studies also indicate that full ensheathment of axons is necessary for normal morphology of unmyelinated axons and conduction velocity in C fibers (Figure 7 and Figure S5). Abnormalities of C fiber conduction in the heterozygotes likely reflect an altered microenvironment, as many of these axons were incompletely ensheathed by Schwann cell processes and, consequently, not electrically isolated. These findings differ from those of a recent study (Chen et al., 2003) in which overexpression of a dominant-negative (truncated) erbB4 in adult, nonmyelinating Schwann cells resulted in increased numbers of Schwann cells and fewer axons per Remak bundle. These differences probably reflect distinctions in the animal models used. The studies of Chen et al. focused on the role of erbB signaling in adult Remak bundles, and hence their maintenance, rather than their formation. In addition, overexpression of this mutant erbB4 receptor may have gain-of-function effects or, alternatively, block erbB signaling provided by other EGF-related ligands in peripheral nerves (Ling et al., 2005).
A striking finding of the current study was the consistent failure of Schwann cells to myelinate NRG1 type III−/− axons (Figures 1 and 2). This failure may result, in part, from the inability of Schwann cells to segregate NRG1 type III−/− axons into a 1:1 relationship, a likely prerequisite for myelination (Peters et al., 1991). However, NRG1 type III also directly regulates myelination itself apart from its role in axon segregation. Myelination is strictly dependent on the activation of a series of transcription factors, including Oct-6 and Krox-20 (Jessen and Mirsky, 2003). Expression of both of these transcription factors requires axonal signals (Murphy et al., 1996; Scherer et al., 1994). We have shown that expression of these transcription factors is sensitive to the levels of NRG1 on the axon (Figures 1 and 7). These results are consistent with studies showing that membrane-associated, but not soluble, NRG1 type III induces Oct-6 expression in multipotential crest-derived progenitors (Leimeroth et al., 2002). Reduced expression of transcription factors in the PNS of heterozygotes, in turn, likely accounts for the reduction of myelin proteins and the thinner myelin sheaths of appropriately segregated axons. Taken together, these findings indicate that NRG1 type III is essential for Schwann cell myelination by promoting the physical sorting of large axons and regulating the expression of key transcription factors.
While our data demonstrate that NRG1 type III is necessary for PNS myelination, they also show that it is not sufficient (Figure 6B). Candidate molecules that may act in concert with NRG1 type III include cell adhesion molecules on the axon surface as well as signals from the extracellular matrix (Bunge et al., 1986) and growth factors such as the neurotrophins (Chan et al., 2004; Cosgaya et al., 2002) and GDNF (Chen et al., 2003; Hoke et al., 2003).
Threshold and Graded Effects of NRG1 Type III on Myelination
This study establishes NRG1 type III as a key axonal signal that determines the binary choice between ensheathment and myelination. Thus, Schwann cells fail to myelinate NRG1 type III−/− neurites, and NRG1 type III+/− sciatic nerves are substantially hypomyelinated due, in part, to a reduction in the proportion of myelinated fibers (Table 1). The latter result suggests that fibers that would normally be myelinated are instead present in Remak bundles. In potential agreement, a number of unmyelinated axons in the type III heterozygotes had very large axon circumferences; some were nearly twice that of the smallest myelinated fibers in wt nerves (data not shown).
We have also shown that NRG1 levels on different fiber types correlate with their ensheathment fate. SCG neurites, which are poorly ensheathed by Schwann cells in cocultures (Obremski et al., 1993), express negligible levels of NRG1 (Figure 4A). In contrast, DRG neurites, which express much higher levels of NRG1, are fully ensheathed and many fibers become myelinated. The highest expression of NRG1 is by the heavily myelinated, BDNF/NT3-dependent neurons (Figure 4A). These findings also indicate that expression of NRG1 is independent of axon diameter. Thus, SCG and NGF-dependent DRG neurons have fibers of comparable diameter when cultured in the absence of Schwann cells (Estridge and Bunge, 1978; Windebank et al., 1985) yet express widely different levels of NRG1 even with correction for protein loading (Figure 4). Differences in NRG1 levels on these neurons appear to result from both transcriptional (Marchionni et al., 1993) and posttranscriptional mechanisms; the latter is consistent with limited expression even after lentiviral infection.
Remarkably, expression of NRG1 type III not only rescues myelination of the NRG1 type III−/− neurites and leads to hypermyelinated segments (Figure 3) but also promotes myelination of SCG neurites (Figure 4). SCG fibers are nearly uniformly unmyelinated and fail to induce myelin protein expression by Schwann cells in vivo and in vitro (Brunden et al., 1992; Roufa et al., 1986). However, even with modest expression of NRG1 type III in SCG neurons, there is a significant increase in myelination (Figures 4C and 4D). These results indicate that expression of this single molecule is sufficient to alter the ensheathment fate of the axon.
Together, these results provide compelling evidence that a threshold level of NRG1 type III is critical in determining which axons become myelinated. Neurites that are normally myelinated express NRG1 type III at substantial levels but are unmyelinated in its absence; neurites that are normally unmyelinated express limited amounts and become myelinated with its expression. Haploinsufficiency results in a shift from myelinated to unmyelinated fibers in peripheral nerves. Above threshold levels, myelin sheath thickness is graded to the amount of NRG1 type III (Figures 3E, 3F, and 7), in agreement with Michailov et al. (2004). It has long been known that axon diameter (Friede and Samorajski, 1967) and surface area (Smith et al., 1982) are strongly correlated to the number of myelin lamellae generated in the PNS. Our data indicate that large axons not only have increased surface area but also express greater amounts of NRG1 per unit membrane area (Figure 4A).
Implications for the Mechanisms of Ensheathment and Myelination
NRG1 isoforms are synthesized as secreted proteins or as membrane-spanning precursors (pro-NRGs). We have demonstrated that pro-NRGs undergo MP cleavage in neurons: heparin binding (Ig) forms are shed as soluble NRGs, whereas only type III is retained at the membrane (Figures 5A and 5D), presumably via its hydrophobic N terminus, in agreement with studies in heterologous cells (Cabedo et al., 2002; Schroering and Carey, 1998; Wang et al., 2001). These findings indicate that type III functions as a juxtacrine signal and type I as a paracrine signal as previously proposed (Falls, 2003b). They also suggest that these distinct modes of signaling have important functional consequences.
Indeed, NRG1 type III promotes ensheathment and myelination via a juxtacrine mechanism since the addition of soluble NRG1 type III to the culture media does not rescue the myelination defect in the NRG1 type III−/− cocultures (Figure 6). The reason why NRG1 type III is much more effective as a juxtacrine signal in promoting myelination is not known, although several possibilities may be considered. Potentially, membrane tethering would enhance receptor activation by concentrating the ligand at the axon surface and provide for sustained activation. In contrast, a soluble ligand is likely to have more transient effects; indeed, we have previously found that a soluble NRG downregulates erbBs on Schwann cells within a few hours, which would limit the duration of its effects (Zanazzi et al., 2001). Short-term and long-term signaling by growth factors, or signaling components such as PI 3-kinase, are known to have very distinct effects on differentiation (Auger et al., 2000; Marshall, 1995). Another possibility is that NRG1 on the axon surface would activate only those receptors localized on the adaxonal glial membrane, whereas soluble NRG1 may activate receptors on the abaxonal membrane. Determining the functional consequences of modifying the duration of NRG1 type III signaling and activating receptors with different subcellular localizations on the Schwann cell will be of considerable interest for future study.
Although Schwann cells fail to ensheath NRG1 type III−/− axons in the cocultures, they do attach to and appose these neurites (Figures 2D and 2F). Axon-Schwann cell apposition is mediated by cell adhesion molecules expressed by Schwann cells and axons, such as N-cadherin and L1 (Haney et al., 1999; Wanner and Wood, 2002). This study indicates that, while these adhesion molecules are necessary for attachment, they are not sufficient for ensheathment; rather, they require activation of intracellular signaling pathways by NRG1 type III. The PI 3-kinase pathway is likely to mediate many of the downstream effects of NRG1 type III. We show here that NRG1 type III is the key signal on the axon that regulates the activity of PI 3-kinase in Schwann cells and this activation is graded to NRG1 levels (Figure 5B). PI 3-kinase activity is known to be required for appropriate axon segregation and is essential for and promotes subsequent Schwann cell myelination (Maurel and Salzer, 2000; Ogata et al., 2004). Indeed, the defects of the NRG1 type III−/− cocultures phenocopy the failure of axon sorting and myelin initiation in cocultures in which PI 3-kinase activity is blocked (Maurel and Salzer, 2000).
Together, these studies suggest a model for myelination in which cell adhesion molecules promote glial apposition and NRG1 type III on the axon surface, via activation of PI 3-kinase signaling at the inner glial membrane, promotes ensheathment and myelination. Our data also suggest that different levels of activation regulate these different outcomes. Both NRG and PI 3-kinase have been implicated in the reorganization of the actin cytoskeleton required for cell motility and lamellipodia formation in other cell types (for example, see Nagata-Ohashi et al., 2004). Future studies to elucidate how graded signaling of NRG1 regulates effectors of PI-3 kinase and the glial cytoskeleton, as well as identification of NRG1-independent axonal signals, including those that activate MAP kinase (Figure 5B), will provide important insights into the mechanisms by which NRG1 type III dictates distinct axon ensheathment fates.
NRG1 Type III Coordinates Schwann Cell Numbers to Cell Fate
NRG1 has now been implicated as an axonal signal that regulates a remarkable range of stages in the lineage of Schwann cells. NRG1 promotes the generation of Schwann cells via commitment of neural crest cells to the glial lineage and the proliferation, survival, and migration of Schwann cells and their precursors (Garratt et al., 2000a; Mahanthappa et al., 1996; Meintanis et al., 2001; Morrissey et al., 1995), thereby regulating Schwann cell numbers along the axon. As we have shown here, NRG1 also regulates the Schwann cell phenotype. An important question is why a single molecule has such a prominent role in regulating Schwann cells at so many different points of their lineage.
We suggest that the dual regulation of Schwann cell generation and differentiation by NRG1 type III coordinates Schwann cell numbers to their alternative phenotypes. In Remak bundles, multiple axons are associated with a single Schwann cell, requiring relatively few Schwann cells per axon; in contrast, Schwann cells have a unitary relationship with an axon in myelinated fibers requiring a higher density of Schwann cells per axon. The lower levels of NRG1 type III characteristic of neurons with unmyelinated axons would be expected to generate fewer Schwann cells and promote an ensheathing, nonmyelinating phenotype. In contrast, axons that express higher levels of NRG1 type III would generate the additional Schwann cells required by myelinated fibers and, subsequently, drive axon segregation and myelination as shown here.
Experimental Procedures
Mice and Genotyping
Generation of NRG1 type III knockout mice has been described previously (Wolpowitz et al., 2000). Mice were genotyped by PCR using the following primers: 5′-ACTTTCTTCTTCCCATTCTGT-3′, 5′-TTTACTCTTCCTTACGGTCTA-3′, and 5′-TTTCTCTTGATTCCCACTTTG-3′. PCR was carried out at 94°C for 30 s, 52°C for 60 s, and 72°C for 60 s, followed by 10 min extension at 72°C for 30 cycles. The expected 700 nt product for wild-type allele and 734 nt product for the mutant allele were separated on a 2% agarose gel.
Cell Cultures
Mouse and rat DRG were isolated from E14.5 and E16.5 embryos, respectively, and established on collagen-coated glass coverslips as described (Zanazzi et al., 2001). Explants were cycled with FUDR to eliminate all nonneuronal cells. Neuronal media was supplemented with 50 ng/ml NGF (Harlan, Bioproducts for Science) and, in some cases, 25 ng/ml BDNF (PeproTech) and 10 ng/ml NT3 (Austral Biologicals). Primary rat Schwann cells were prepared as described (Einheber et al., 1997) and maintained in DMEM (BioWhittaker), 10% FBS (Hyclone Laboratories), 2 mM L-glutamine (Invitrogen), until used. Rat Schwann cells (200,000 cells/coverslip) were added to established explant cultures of DRG neurons, and myelination was initiated by supplementing media with 50 μg/ml ascorbic acid (Sigma-Aldrich). Phase contrast images of wt and NRG1 type III−/− Schwann cell neuronal cocultures were acquired using an Axiovert 100 microscope (Zeiss).
SCG neurons were isolated from P2 Sprague-Dawley rats, stripped of their cellular capsule, dissociated in 0.1% trypsin (Sigma-Aldrich) and 1 μg/ml DNase (Sigma-Aldrich), and triturated. Approximately 0.8 ganglia were placed on collagen-coated coverslips, and cultures were maintained in Neurobasal Media (Invitrogen) supplemented with 0.4 g/L glucose (Sigma-Aldrich), B27 supplements (Invitrogen), and 50 ng/ml NGF. Cultures were cycled with FUDR to eliminate nonneuronal cells and were repopulated with primary rat Schwann cells as for DRG cultures. Culture media was supplemented with 50 μg/ml of ascorbic acid in myelination studies. To quantitate the extent of myelination, the total number of myelin segments, stained with MBP, was counted in each coverslip (total of eight coverslips per condition).
Rat DRG neurons were transfected with pcDNA3.1 vector containing either NRGβ1a type I or NRGβ1a type III tagged with an HA epitope (Wang et al., 2001), immediately after dissection using the Rat Neuron Nucleofector kit (Amaxa Biosystems). Neurons were cycled with antimitotics and maintained for 21 days prior to analysis. In some cases, nucleofected neurons were treated with metalloprotease inhibitors (10 μM or 100 μM GM 6001 or 20 μM or 200 μM TAPI-1; both from Calbiochem) for 48 hr prior to analysis. CHO cells were transfected with NRGβ1a type III HA using Lipofectamine 2000 (Invitrogen). Transfectants were selected with G418 (Invitrogen), pooled, live stained for the HA epitope, and FACS sorted for high-level expression using the MoFlo DakoCytomation FACScan. Expression was confirmed by Western blotting and binding of erbB2/3-Fc.
Antibodies and Immunofluorescence
Mouse monoclonal antibodies included anti-MBP (SMI-94, SMI-99), neurofilament (SMI-31 and SMI-32) (Sternberger Monoclonals), and HA 1.1 (Covance). Rabbit polyclonal antibodies included anti-NRG sc348 (Santa Cruz), MBP (DakoCytomation), MBP (Chemicon), MAG, P0 (M. Filbin, Hunter College, New York, NY), actin (Sigma-Aldrich), Oct-6 (D. Meijer, Erasmus University, Rotterdam, Netherlands), Krox-20 sc190 (Santa Cruz), peripherin (Chemicon International), S100 (DakoCytomation), phospho-AKT, total AKT, and phospho-MAP kinase (Cell Signaling Technologies). Secondary antibodies conjugated to rhodamine, fluorescein, or HRP were obtained from Jackson Immunoresearch. Cocultures were permeabilized in methanol and stained as described previously (Zanazzi et al., 2001) and examined by epifluorescence on a Nikon E800 microscope and by confocal microscopy on a Zeiss LSM 510.
Preparation of Detergent Lysates and Immunoblotting
Tissues and cell cultures were Dounce homogenized in a lysis buffer containing 2% SDS, 95 mM NaCl, 10 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 20 μM leupeptin, 1 mM orthovanadate, and 2.5 mM sodium pyrophosphate in 25 mM Tris buffer (pH 7.4). Protein concentrations were determined by the BCA method (Pierce); samples (20–40 μg of protein) were fractionated by SDS-PAGE and blotted onto nitrocellulose (Protran Biosciences). Appropriate regions were excised, incubated with specific primary and secondary antibodies, and developed with the SuperSignal chemiluminescent substrate (Pierce). To compare levels of MBP, P0, and MAG, nitrocellulose membranes were incubated with the appropriate primary antibody followed by 125I-labeled protein A (Valeant ICN Pharmaceutical). Quantitation was performed using a Molecular Dynamics PhosphorImager and the ImageQuant Software.
Measurement of NRG Release from Cultured Neurons
Conditioned media from ∼30 cultures each of wt, NRG1 type III+/−, and NRG1 type III−/− DRG were collected, concentrated 200-fold, and halved. One aliquot was treated with 100 μg/ml soluble heparin to bind heparin binding forms of NRG (types I and II). Media samples were then assayed for NRG as previously described (Esper and Loeb, 2004). L6 myoblasts were plated at 50,000 cells per well and allowed to fuse and differentiate for 7 days. The cells were exposed to the DRG media samples for 45 min, lysed, and the erbB2 and erbB3 proteins were immunoprecipitated and analyzed by Western blotting. Following a probe with anti-phosphotyrosine (4G10), the membrane was stripped and reprobed for total erbB protein. The intensity of each protein band was quantitatively measured with MetaMorph software.
Neurite Membrane Preparation
Neurite membranes were prepared from wt, NRG1 type III+/−, and NRG1 type III−/− DRG neurons grown in 35 mm dishes as previously described (Maurel and Salzer, 2000). Membranes were centrifuged onto serum starved, rat primary Schwann cells. Cells were then incubated at 37°C for an additional 20 min, lysed, and analyzed by Western blotting.
Lentivirus Production and Infection
A lentivirus expressing NRGβ1a type III HA was generated using the ViraPower Lentiviral Expression System (Invitrogen) according to the manufacturer's instructions. The cDNA encoding NRGβ1a type III HA was cloned in a pLenti6/V5 plasmid using the Directional TOPOR Cloning Kit (Invitrogen) and confirmed by sequencing. 293FT cells were transfected together with pLP1, pLP2, and pLP/VSVG plasmids (Invitrogen) using Lipofectamine 2000 (Invitrogen). Supernatants were collected after 48 hr and stored at −80°C until used. Primary neurons, DRGs and SCGs, were infected the day after dissection with 100 μl of the produced virus. Cells were kept in the presence of the virus for 48 hr in MEM, 10% FBS, 2 mM L-glutamine, and antimitotic agents. Viral expression was confirmed by HA staining 11 days after infection. To quantitate the fiber diameter in infected NRG1 type III−/− and control cultures, high-power images were acquired by confocal microscope and analyzed using Volocity software.
Fc Fusion Proteins and Binding Experiments
293FT cells were cotransfected with plasmids encoding erbB2-Fc and erbB3-Fc fusion proteins in media containing DMEM, 1% Ultra-Low IgG FBS (BioWhittaker), and 2 mM L-glutamine. After 72 hr, the supernatant was collected, filtered, and erbB-Fc expression was confirmed by Western blotting. Established explants of wt, NRG1 type III+/−, and NRG1 type III−/− DRG neurons were incubated with supernatant containing erbB2/erbB3 Fc for 2 hr at 37°C and an anti-human Fc (Jackson ImmunoResearch) for 1 hr at 25°C. Neurons were then fixed, permeabilized, and stained for neurofilament.
Electron Microscopy and Morphometry
Cocultures were processed for EM as previously described (Melendez-Vasquez et al., 2004). Analysis of sciatic nerves from wt and NRG1 type III+/− littermates was performed as described (Bhat et al., 2001). Mice were anesthetized with pentobarbital and fixed via transcardial perfusion with 2% paraformaldehyde and 3.75% acrolein in 0.1 M phosphate buffer or with 2% paraformaldehyde and 3% glutaraldehyde in cacodylate buffer (pH 7.3). One micron sections of whole sciatic nerves were stained with toluidine blue for survey by light microscopy. Ultrathin sections (70–100 nm thick) of sciatic nerves were cut and counterstained with uranyl acetate and Reynold's lead citrate or alcoholic uranyl acetate and permanganate. Micrographs were taken with a Philips EM300, JEOL JEM 1200EXII, or a Philips CM10 electron microscope (Eindhoven, The Netherlands) (Einheber et al., 1997; Rosenbluth et al., 2003).
Nonoverlapping digitized images of fiber cross-sections were obtained and analyzed from sciatic nerve using Image J software (National Institute of Health). G ratios were determined by dividing the diameter of the axon by that of the total fiber diameter from ∼600 fibers in the sciatic nerve (four animals per genotype). The total number of unmyelinated fibers, the number of axons/Remak bundle, and the number of axons in Schwann cell pockets were determined from the same digitized images used for g ratio analysis.
Electrophysiology
Compound action potentials were measured by drawing each end of a nerve into a suction electrode; the n varied between 5 and 10 for all measurements. At the proximal end, the entire trunk was held in the pipette, and stimuli were applied. At the distal end, tibial and peroneal branches were placed sequentially in a pipette for recording. Nerves were bathed in oxygenated Locke's solution containing 154 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM HEPES (pH 7.4). Bath temperature was controlled with a proportional heater. Sweeps were collected and analyzed in a laboratory computer. The stimulus amplitude was first set for the myelinated axons and was then raised until the signal from the unmyelinated axons was maximal. To increase the signal-to-noise ratio for C-fibers, 8–32 sweeps were averaged. Further details have been published (Vabnick et al., 1999).
Statistical Analysis
Statistical analyses (Fischer' s exact test, χ2, and t test) were performed using the Prism Software package (GraphPad).
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health Grants NS26001 (J.L.S.), NS037475 (J.R.), NS17965 (P.S.), NS051282 (M.V.C.), and NS29071 (L.R.), by National Multiple Sclerosis Society Grants RG2311-C-6 (J.L.S.), RG-3410-A-2 (J.L.), and RG2539 (J.R.), by NYS CO19772-3784 and CRPF XBC-0302-1 (P.S.), and by an award from the Emory University Research Committee (D.L.F.). C.T. is a recipient of a postdoctoral fellowship from the National Multiple Sclerosis Society (FG 1541A1/1). We thank Marie Filbin and Dies Meijer for antibodies; Teresa Milner and Lee Cohen-Gould for assistance with electron microscopy; Wenbiao Gan and David Talmage for comments on the manuscript; and Yosi Yarden for the erbB-Fc plasmids.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J. Neurocytol. 1976;5:565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- Auger KR, Wang J, Narsimhan RP, Holcombe T, Roberts TM. Constitutive cellular expression of PI 3-kinase is distinct from transient expression. Biochem. Biophys. Res. Commun. 2000;272:822–829. doi: 10.1006/bbrc.2000.2806. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Ding Y, Hennington BS. Myelin protein expression in dissociated superior cervical ganglia and dorsal root ganglia cultures. J. Neurosci. Res. 1992;32:507–515. doi: 10.1002/jnr.490320406. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu. Rev. Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr. Opin. Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Cabedo H, Luna C, Fernandez AM, Gallar J, Ferrer-Montiel A. Molecular determinants of the sensory and motor neuron-derived factor insertion into plasma membrane. J. Biol. Chem. 2002;277:19905–19912. doi: 10.1074/jbc.M201587200. [DOI] [PubMed] [Google Scholar]
- Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann Cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like para-nodal junctions that assemble during myelination. J. Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper RM, Loeb JA. Rapid axoglial signaling mediated by neuregulin and neurotrophic factors. J. Neurosci. 2004;24:6218–6227. doi: 10.1523/JNEUROSCI.1692-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estridge M, Bunge RP. Compositional analysis of growing axons from rat sympathetic neurons. J. Cell Biol. 1978;79:138–155. doi: 10.1083/jcb.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J. Neurocytol. 2003a;32:619–647. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 2003b;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick VD, Pisacane PI, Vandlen RL, Sliwkowski MX. Formation of a high affinity heregulin binding site using the soluble extracellular domains of ErbB2 with ErbB3 or ErbB4. FEBS Lett. 1998;431:102–106. doi: 10.1016/s0014-5793(98)00737-6. [DOI] [PubMed] [Google Scholar]
- Frenzel KE, Falls DL. Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts. J. Neurochem. 2001;77:1–12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. [DOI] [PubMed] [Google Scholar]
- Friede RL. Control of myelin formation by axon caliber (with a model of the control mechanism) J. Comp. Neurol. 1972;144:233–252. doi: 10.1002/cne.901440207. [DOI] [PubMed] [Google Scholar]
- Friede RL, Samorajski T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J. Comp. Neurol. 1967;130:223–231. doi: 10.1002/cne.901300304. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays. 2000a;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J. Cell Biol. 2000b;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J. Cell Biol. 1999;146:1173–1184. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillarp NÅ, Olivecrona H. The role played by the axon and the Schwann cells in the degree of myelination of the peripheral nerve fibre. Acta Anat. (Basel) 1946;2:17–32. doi: 10.1159/000140193. [DOI] [PubMed] [Google Scholar]
- Ho WH, Armanini MP, Nuijens A, Phillips HS, Osheroff PL. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J. Biol. Chem. 1995;270:14523–14532. doi: 10.1074/jbc.270.24.14523. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. J. Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Schwann cell development. In: Lazzarini RA, editor. Myelin Biology and Disorders. Academic Press; New York: 2003. pp. 329–370. [Google Scholar]
- Langley JN, Anderson HK. On the union of the fifth cervical nerve with the superior cervical ganglion. J. Physiol. 1903;30:439–442. doi: 10.1113/jphysiol.1904.sp001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent progenitor cells. Dev. Biol. 2002;246:245–258. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- Ling B, Wu J, Miller SJ, Monk KR, Rizvi TA, Shamekh R, Hadley J, Vogel KS, DeClue JE, Ratner N. Role of the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanthappa NK, Anton ES, Matthew WD. Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J. Neurosci. 1996;16:4673–4683. doi: 10.1523/JNEUROSCI.16-15-04673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J. Neurosci. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintanis S, Thomaidou D, Jessen KR, Mirsky R, Matsas R. The neuron-glia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia. 2001;34:39–51. [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates Schwann cell myelination and formation of associated axons. J. Neurosci. 2004;24:3953–3963. doi: 10.1523/JNEUROSCI.4920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murinson BB, Griffin JW. C-fiber structure varies with location in peripheral nerve. J. Neuropathol. Exp. Neurol. 2004;63:246–254. doi: 10.1093/jnen/63.3.246. [DOI] [PubMed] [Google Scholar]
- Murphy P, Topilko P, Schneider-Maunoury S, Seitanidou T, Baron-Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Murray MA. An electron microscopic study of the relationship between axon diameter and the initiation of myelin production in the peripheral nervous system. Anat. Rec. 1968;161:337–352. doi: 10.1002/ar.1091610306. [DOI] [PubMed] [Google Scholar]
- Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R, et al. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J. Cell Biol. 2004;165:465–471. doi: 10.1083/jcb.200401136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obremski VJ, Johnson MI, Bunge MB. Fibroblasts are required for Schwann cell basal lamina deposition and ensheathment of unmyelinated sympathetic neurites in culture. J. Neurocytol. 1993;22:102–117. doi: 10.1007/BF01181574. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, d Webster H. The Fine Structure of the Nervous System. Third Edition Oxford University Press; New York: 1991. [Google Scholar]
- Rosenbluth J, Dupree JL, Popko B. Nodal sodium channel domain integrity depends on the conformation of the para-nodal junction, not on the presence of transverse bands. Glia. 2003;41:318–325. doi: 10.1002/glia.10179. [DOI] [PubMed] [Google Scholar]
- Roufa D, Bunge MB, Johnson MI, Cornbrooks CJ. Variation in content and function of non-neuronal cells in the outgrowth of sympathetic ganglia from embryos of differing age. J. Neurosci. 1986;6:790–802. doi: 10.1523/JNEUROSCI.06-03-00790.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. J. Neurosci. 1994;14:1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroering A, Carey DJ. Sensory and motor neuron-derived factor is a transmembrane heregulin that is expressed on the plasma membrane with the active domain exposed to the extracellular environment. J. Biol. Chem. 1998;273:30643–30650. doi: 10.1074/jbc.273.46.30643. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Simpson SA, Young JZ. Regeneration of fibre diameter after cross-unions of visceral and somatic nerves. J. Anat. 1945;79:48–65. [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, Murray JA, Patterson RC. Internodal myelin volume and axon surface area. A relationship determining myelin thickness? J. Neurol. Sci. 1982;55:231–246. doi: 10.1016/0022-510x(82)90103-4. [DOI] [PubMed] [Google Scholar]
- Snider WD, Wright DE. Neurotrophins cause a new sensation. Neuron. 1996;16:229–232. doi: 10.1016/s0896-6273(00)80039-2. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Weinberg HJ. Axonal specification of Schwann cell expression and myelination. In: Waxman SG, editor. Physiology and Pathobiology of Axons. Raven Press; New York: 1978. pp. 389–405. [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J. Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J. Biol. Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J. Neurosci. 2002;22:4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg HJ, Spencer PS. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976;113:363–378. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Wood P, Bunge RP, Dyck PJ. Myelination determines the caliber of dorsal root ganglion neurons in culture. J. Neurosci. 1985;5:1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J. Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.