Stem cells are undifferentiated, self-renewing, and multipotent (able to differentiate into multiple cell types). Unlike traditional treatment modalities, these unique characteristics may enable stem cells to undo irreversible cellular damage and rebuild injured or diseased tissue. Recent evidence suggests that stem cells may influence positively the recovery from injury via paracrine factors that promote tissue repair. Insights into paracrine mechanisms of stem cells may help the surgeon scientist devise more effective therapies and earlier widespread application. This review: (1) unravels the pathways for stem cell mediated paracrine protection; (2) highlights the growth factors and cytokines expressed; (3) and explores the potential of using stem cells clinically.
THE STEM CELL PARACRINE THEORY
Stem cells hold great promise for potential clinical therapeutic strategies. More than a decade of research in experimental models of animal injury suggests that stem cell treatment or transplantation of stem cells remodels and regenerates injured tissue, improves function, and protects tissue from further insult. Indeed, these encouraging results have led to phase I and II clinical trials regarding stem cell treatment for a variety of surgical diseases (Table 1). Despite these encouraging advances, the mechanism of this protection is still not well-characterized. It was initially hypothesized that immature stem cells differentiated into the phenotype of injured tissue, repopulated the diseased organ with healthy cells, and subsequently improved function, but, recent research indicates that this stem cell-mediated protection probably did not result from differentiation into the target tissue type.1 Instead, several lines of evidence suggest that stem cells may mediate their beneficial effects, at least in part, by paracrine mechanisms. First, studies demonstrate that donor stem cell engraftment and survival after transplantation is only 1–5% which is too few to be relevant therapeutically and influence directly organ function. Second, stem cells have been shown to confer acute improvement in end organ function less than 72 h after injury, precluding differentiation as a cause due to time required for meaningful differentiation and regeneration of these donor cells. Third, and perhaps most importantly, in vitro and in vivo animal studies have revealed that much of the functional improvement and attenuation of injury afforded by stem cells can be replicated by cell free, conditioned media derived from stem cells. Taken together, these indirect and direct data suggest that stem cells may improve injured organ performance and limit injury not via differentiation but rather via complex paracrine actions.
Table 1.
Surgical Diseases and Stem Cell Clinical Trials
| Disease | Stem Cell Type | Clinical Phase |
|---|---|---|
| Myocardial Ischemia | BMSCs, HSCs, EPCs, MSCs | Phase 1 and 2 |
| Liver insufficiency | HSCs, EPCs | Phase 1 |
| Crohn’s Disease | MSCs | Phase 1 |
| Peripheral Vascular Ischemia | HSCs, EPCs, MSCs | Phase 1 |
| Skin Flaps | MSCs | Phase 1 |
| CVA | MSCs | Phase 1 |
| Spinal Cord Injury | BMSCs, MSCs | Phase 1 |
| Brain Trauma | BMSCs, neural stem | Phase 1 |
| Chronic Renal Failure | Renal stem + device | Phase 1 |
A variety of stem and progenitor cell populations have been used to repair injured tissue, and each cell type has its own profile of advantages, limitations, and practicality issues (Table 2). Notably, several different stem cell types exhibit paracrine effects and mesenchymal stem cells (MSC) appear to have the fewest disadvantages. However, the ideal stem cell type remains unknown as further study is necessary to elucidate which stem cell type provides the greatest potency and paracrine protection with the least adverse effects.
Table 2.
Stem Cell Based Therapy: Common cell types, advantages, disadvantages, and evidence for paracrine effects
| Cell Type | Source | Markers | Advantages | Disadvantages | Paracrine Effects |
|---|---|---|---|---|---|
| Unfractionated Bone marrow cells | Bone marrow | c-kit, Sca-1 | Expansion | Yes | |
| Hematopoetic Stem Cells (HSCs) | Bone marrow | CD133, CD34 | Well characterized | Harvest Expansion | Yes |
| Mesenchymal Stem Cells (MSCs) | Bone marrow, Adipose | CD29, CD44, CD105 | Isolation Immune Tolerance | Yes | |
| Embryonic Stem Cells (ESCs) | Allogeneic cell lines | CD30 | Pluripotency Expansion | Teratoma Allogeneic | Unknown |
| Endothelial Progenitor Cells | Peripheral Blood, Bone Marrow | CD34, VEGFR2 | Limited Potency | Yes | |
| Adipose Progenitor Cells | Adipose Tissue | Harvest | Limited Potency | Yes |
STEM CELL PARACRINE PATHWAYS
Stem cells transplanted into injured tissue express paracrine signaling factors including cytokines, chemokines, and growth factors, which are involved in orchestrating the stem cell-driven repair process. While our understanding of the mechanistic basis for stem cell-mediated paracrine enhancement of end organ function remains incomplete, several mechanisms have been proposed.
Increased angiogenesis
First, stem cells produce local signaling molecules that may improve perfusion and enhance angiogenesis to chronically ischemic tissue. Although the particular growth factors contributing to this neovascular effect remain to be defined, the list includes vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and basic fibroblast growth factor (FGF2).2, 3 VEGF is a strong promoter of angiogenesis. Although originally associated with liver regeneration, HGF also exerts beneficial effects on neovascularization and tissue remodeling. FGF2, a specific member of the FGF signaling family is involved intimately with endothelial cell proliferation and may be a more potent angiogenic factor than VEGF. When exposed to either insult or stress, mesenchymal stem cells (MSC) in cell culture and in vivo significantly increase release of VEGF, HGF, and FGF2, which may improve regional blood flow as well as promote autocrine self survival. Increased perfusion due to the production of stem cell angiogenic growth factor has also been associated with improved end organ function. Further, VEGF overexpressing bone marrow stem cells demonstrate greater protection of injured tissue than controls. Thus, VEGF, HGF, and FGF2 may be important paracrine signaling molecules in stem cell-mediated angiogenesis, protection, and survival.
Decreased inflammation
Stem cells appear to attenuate infarct size and injury by modulating local inflammation. When transplanted into injured tissue, the stem cell faces a hostile, nutrient-deficient, inflammatory environment and may release substances which limit local inflammation in order to enhance its survival. Recent studies implicate the release of the anti-inflammatory cytokine IL-10 as playing an integral role in modulating the activity of innate and adaptive immune cells, such as dendritic cells, T cells, and B cells. Transforming growth factor beta (TGF- β) appears to be involved in suppression of inflammation by stem cells. TGF-β1 plays a role in T cell suppression, and its anti-inflammatory effect may be further potentiated by concomitant HGF. Modulation of local tissue levels of pro-inflammatory cytokines by anti-inflammatory paracrine factors released by stem cells, thus, are important in conferring improved outcome after stem cell therapy.4
Anti-apoptotic and chemotactic signaling
Stem cells in a third pathway promote salvage of tenuous or malfunctioning cell types at the infarct border zone. Injection of MSC into a cryo-induced infarct reduces myocardial scar width 10 weeks later. MSCs appear to activate an anti-apoptosis signaling system at the infarct border zone which effectively protects ischemia-threatened cell types from apoptosis. Furthermore, expression profiling of adult progenitor cells reveals characteristic expression of genes associated with enhanced DNA repair, upregulated anti-oxidant enzymes, and increased detoxifier systems. HGF has been observed to improve cell growth and to reduce cell apoptosis.
Evidence also exists that both endogenous and exogenous stem cells are able to “home” or migrate into the area of injury from the site of injection or infusion. MSC in the bone marrow can be mobilized, target the areas of infarction, and differentiate into target tissue type. Granulocyte colony-stimulating factor (G-CSF) has been studied widely and promotes the mobilization of bone marrow–derived stem cells in the setting of acute injury. This homing mechanism may also depend on expression of stromal cell-derived factor 1 (SDF-1), monocyte chemoattractant protein-3 (MCP-3), stem cell factor (SCF), and / or IL-8.
Beneficial remodeling of the extracellular matrix
Fourth, stem cell transplantation alters the extracellular matrix, resulting in more favorable post-infarct remodeling, strengthening of the infarct scar, and prevention of deterioration in organ function. Acute human 5 and murine MSC infusion prior to ischemia improve myocardial developed pressure, contractility, and compliance after ischemia/reperfusion (I/R) injury and decrease end diastolic pressure. Similarly, direct injection of human MSC into ischemic hearts decreased fibrosis, left ventricular dilation, apoptosis, and increased myocardial thickness with preservation of systolic and diastolic cardiac function without evidence of myocardial regeneration. MSCs appear to achieve this improved function by increasing acutely the cellularity and decreasing production of extracellular matrix proteins such as collagen type I, collagen type III, and TIMP-1 which result in positive remodeling and function.
Activation of neighboring resident stem cells
Finally, exogenous stem cell transplantation may activate neighboring resident tissue stem cells. Recent work demonstrated the existence of endogenous, stem cell-like populations in adult hearts, liver, brain, and kidney. These resident stem cells may possess growth factor receptors that can be activated to induce their migration and proliferation and promote both the restoration of dead tissue and the improved function in damaged tissue. Mesenchymal stem cells have also released HGF and IGF-1 in response to injury 6 which when transplanted into ischemic myocardial tissue may activate subsequently the resident cardiac stem cells.
Although the definitive mechanisms for protection via stem cells remains unclear, stem cells mediate enhanced angiogenesis, suppression of inflammation, and improved function via paracrine actions on injured cells, neighboring resident stem cells, the extracellular matrix, and the infarct zone. Improved understanding of these paracrine mechanisms may allow earlier and more effective clinical therapies.
FUTURE THERAPEUTIC APPLICATIONS
The therapeutic capacity of stem cells to modulate tissue repair after injury may be enhanced by ex vivo priming of the stem cells to be transplanted to maximize secreted paracrine factors and minimize deleterious consequences for surgical patients. For instance, transduction of VEGF-overexpressing genes into MSCs and subsequent delivery into injured tissue decreases infarct size, increases capillary density, and improves function compared to non modified MSCs. Similarly, hypoxic preconditioning of stem cells prior to transplantation has also resulted in improved protection, possibly via increased synthesis of VEGF and increased activation of endothelial nitric oxide synthase.7 Furthermore, recent studies have shown that female mesenchymal stem cells, compared to male mesenchymal stem cells, may confer improved protection after ischemia, and this protection may be associated with greater production of paracrine factors by female stem cells. Thus, identifying the ideal stem cell type for paracrine actions and modifying the stem cell ex vivo with gene therapy or preconditioning may increase stem cell paracrine-mediated protection.8
Recent studies also suggest that enhanced survival of transplanted stem cells may also increase their cytoprotective efficacy and paracrine ability to rescue ischemic tissue. Modification of transplanted stem cells via Akt overexpression, Bcl-2 overexpression, or preconditioning decreased inflammation, apoptosis, and increased functional recovery. Moreover, conditioned media derived from Akt-modified MSC but without the actual stem cells also conferred protection on cardiomyocytes as well as an experimentally induced myocardial infarction. Similarly, Bcl-2 overexpression and preconditioning increases VEGF production. Therefore, pro-survival modification of stem cells prior to transplantation may enhance both stem cell survival and protective effects mediated via stem cell derived paracrine pathways.9
Clinically, stem cells for bone marrow transplants have been used in the treatment of patients with hematopoietic malignancies and diseases for more than 40 years., Only recently, however, have human trials of stem cell therapy for surgical disease been conducted. In 2002, the first of these trials, the TOPCARE-AMI trial for patients with acute myocardial infarction and the TACT trial for chronic limb ischemia demonstrated that stem cell therapy was safe. Since that time, several studies have followed, demonstrating both promising efficacy as well as minimal beneficial effects over placebo. This discrepancy between promising preclinical animal research and the uncertain efficacy of human clinical trials highlights the need to further our understanding of the mechanisms of stem cell protection, delineate their paracrine effects, and develop therapies which maximize their effects.
CONCLUSION
Will surgeons be able to grow new tissue to replace that which has been removed surgically? At this moment, no; however, stem cells have great promise for potential therapeutic strategies via beneficial paracrine mechanisms and effects. Paracrine mediated actions derived from stem cells have proven to potentiate angiogenesis and beneficial extracellular remodeling as well as reduce apoptosis, inflammation, and infarct size. Interestingly, this paracrine protection may be enhanced by ex vivo modification. Nevertheless, several crucial questions remain regarding mechanism and optimal treatment methodology. Further investigations are needed to address the potency of intrinsic paracrine factors released by stem cells as well as optimal localization, timing, delivery, and stem cell type to be transplanted. Clinical trials indicate that stem cell treatments are safe, although future trials designed to assess efficacy and protection need to be carried out. Continued enthusiasm, coupled with rigorous scrutiny, may enable earlier widespread therapeutic application in surgical disease.
Figure 1. Proposed stem cell paracrine mechanisms.
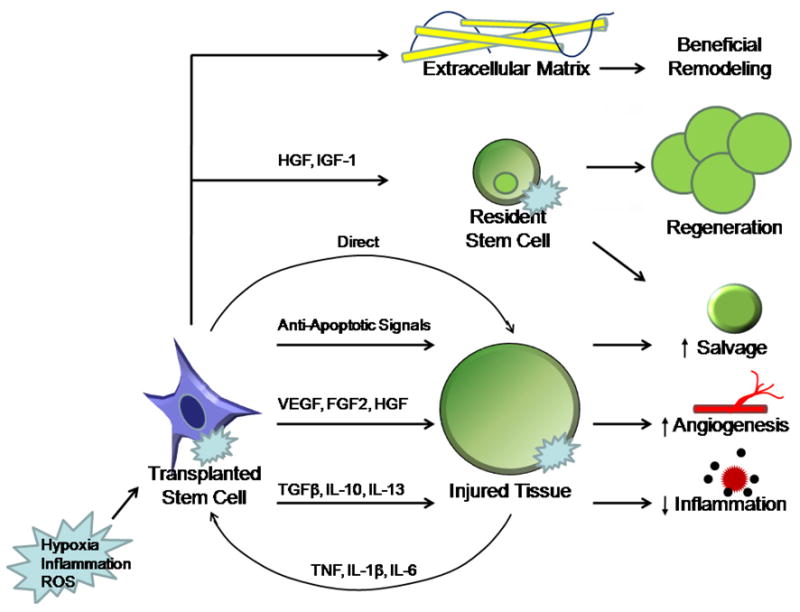
Stem cell mediated protection in surgical disease and experimental injury models may occur via a complex exchange of paracrine signals. During injury, local hypoxia, inflammation, and reactive oxygen species may activate transplanted stem cells, resident stem cells, and injured cell types. Stem cells may subsequently confer protection by beneficial effects on the extracellular matrix, resident stem cells, and injured neighboring cells. VEGF, HGF, and FGF2 are key signaling factors that promote angiogenesis. TGF β, IL-10, and IL-13 may be important paracrine components which suppress local inflammation. Anti-apoptotic signals may play a role in autocrine stem cell survival as well as salvage of neighboring injured cells. HGF and IGF-1 also mobilizes circulating progenitor cells and resident stem cells from the surrounding tissue into the dead tissue.
Acknowledgments
This work was supported in part by NIH R01GM070628, NIH R01HL085595, NIH K99/R00 HL0876077, NIH F32HL085982, AHA Grant-in-aid, and AHA Postdoctoral Fellowship 0725663Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 2.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, et al. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1) J Mol Cell Cardiol. 2007;42(1):142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39(2):363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Markel TA, Crisostomo PR, Wang M, Herring CM, Meldrum DR. Activation of Individual Tumor Necrosis Factor Receptors Differentially Affects Stem Cell Growth Factor and Cytokine Production. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00230.2007. In press. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with Adult Progenitor Cells Improves Recovery and Decreases Native Myocardial Proinflammatory Signaling after Ischemia. Shock. 2006;25(5):454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R880–884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 7.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 8.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007 doi: 10.1016/j.surg.2007.04.013. In press. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99(7):776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]


