Abstract
Human endothelial cells express urotensin II (U-II) as well as its receptor GPR14. Using microfluorimetric techniques, the effect of human U-II on cytosolic Ca2+ concentrations [Ca2+]i in cultured human aortic endothelial cells (HAEC) loaded with Fura-2 was evaluated in static or flow conditions. Under the static state, U-II (100 nM) abolished spontaneous Ca2+ oscillations, which occurred in a population of cultured HAEC. Similarly, U-II reduced thrombin-, but not ATP-induced calcium responses, suggesting that the peptide does not alter the Gq/11/IP3 pathway; rather, it modifies the coupling between protease activated receptors and Gq/11/IP3. Under the flow condition, U-II (1, 10 and 100 nM) produced a dose-dependent increase in [Ca2+]i, which was subjected to desensitization. The result demonstrates a state-dependent effect of U-II in cultured HAEC, which may explain the variable responses to U-II under different experimental conditions.
Keywords: Calcium mobilization, human endothelial cells, G protein-coupled receptor
Introduction
Urotensin II (U-II), a cyclic peptide, was first isolated from the caudal neurosecretory cells of teleost fish, and subsequently in the frog, rodent and human [19, 54]. The human U-II is composed of 11 amino acid residues; the fish and frog U-II consists of 12 and 13 amino acids [20]. The cyclic region, where the biological activity resides, is fully conserved from fish to human [20].
U-II mRNA, or peptide, is expressed in ventral horn neurons of the spinal cord and brainstem in all the species that have been examined including the human [17, 18, 21, 28, 29, 49, 50]. For example, U-II-immunoreactivity of varying intensities is present in a population of ventral horn neurons in the rat spinal cord, hypoglossal nucleus, dorsal motor nucleus of the vagus, facial motor nucleus, nucleus ambiguus, abducens nucleus and trigeminal motor nucleus [28]. Information relative to the physiological or pharmacological action of U-II in the central nervous system is limited. U-II by intracerebroventricular injection causes hypertension and bradycardia, stimulates prolactin and thyrotropin secretion, promotes rapid eye movement sleep episode, and induces a number of behavioral responses indicative of anxiogenic and depressant-like behaviors [24, 31, 36]. A wide distribution of U-II receptors in the brain and spinal cord may contribute to the broad range of central effects elicited by exogenous U-II [39].
Results from several laboratories suggest that U-II is the endogenous ligand for the orphan G-protein coupled receptor GPR14, which has structural similarity with members of the somatostatin/opioid receptor family [5, 42, 44, 47]. In addition to neural tissues, GPR14 mRNA is present in peripheral tissues including the vasculature, heart, and skeletal muscle [43]. Initial studies support a vasoconstrictive action of U-II, which is eight- to 109-fold more potent than endothelin 1 in certain vessels [25]. Subsequent reports show that the vascular response to U-II varied, depending on the species, type of blood vessel, concentration of U-II and route of administration. For example, intravenous infusion of U-II (3 to 300 pmol/min) was found to cause no significant changes in heart rate, mean arterial pressure or cardiac index in healthy male volunteers as compared to saline infusion [4]. In another study where the peptide was infused into the brachial artery, the forearm blood flow was reduced by U-II (1 to 300 pmol/min) in a dose-dependent manner, indicating a vasoconstrictive effect [10]. In human blood vessels in vitro, U-II has been found to cause a vasoconstriction, dilatation or no significant changes [7, 34, 59].
Using calcium flux as an index, the present study was undertaken to investigate the Ca2+ response to human U-II in cultured human aorta endothelial cells (HAEC) under flow or static conditions, which may simulate different experimental states.
Methods
HAEC culture
Human aortic endothelial cells (HAEC) (Clonetics Corp., San Diego, CA) were grown in M199 medium (Invitrogen, Grand Island, NY) containing 20% fetal calf serum (HyClone Laboratories, Logan, UT), 50 μg/ml endothelial cell growth supplement (BD Bioscience, Bedford, MA), and 50 μg/ml heparin (Sigma, St. Louis, MO). The culture medium was supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml). Cells from passages 8-9 were used in the experiments.
Flow vs static peptide administration
HAEC were exposed to laminar shear stress (τ) of 10 dyne/cm2, as calculated by the following formula [9, 38]:
where under our experimental conditions μ is the media viscosity (0.0085 g/cm/s), w is the channel width (1.0 cm), h is the channel height (0.2 cm), and Q is the volumetric flow rate (0.07843 cm3/s).
For static administration, peptides or chemicals were added directly to the organ bath.
Ca2+ measurement
Cytosolic Ca2+concentrations [Ca2+]i were measured by the microfluorimetric technique, as previously described [14]. Cultured HAEC were loaded with the fluorescent Ca2+ indicator Fura-2 AM (3 μM) by incubation of the cells in Hank’s balanced salt solution (HBSS) plus Fura-2 AM for 45 min, and HBSS alone for an additional 15-60 min to allow de-esterification of the dye. Coverslips were mounted in a diamond-shaped recording chamber (model RC-25, Warner Instrument Inc., Hamden, CT) that provides laminar solution flow. The recording chamber was mounted on the stage of a TE2000U Eclipse Nikon inverted microscope equipped with a Photometrics CoolSnap HQ CCD camera (Roper Scientific, Tucson, AZ). The volume of the chamber was 500 μl. For laminar flow experiments, the coverlips were perfused with HBSS at 2.5 ml/min using a Minipuls 3 peristaltic pump (Gilson Inc, Middleton, WI). Fura-2 fluorescence (emission = 520 nm), following alternate excitation at 340 nm and 380 nm, was acquired at a frequency of 0.2 Hz using a MetaFluor software.
Statistics
Statistical significance between groups was evaluated using one-way ANOVA followed by Bonferroni test, p< 0.05 being considered significantly different.
Chemicals
ATP and thrombin were from Sigma Aldrich (St. Louis, MO), and human urotensin II from Phoenix Pharmaceuticals, Inc. (Burlingame, CA).
Results
[Ca2+] in flow stimulated HAEC
The basal value of [Ca2+]i in cultured HAEC was 68 ± 4.2 nM (n= 85). Saline perfusion at a flow rate of 0.07843 cm3/s (equivalent to 10 dyne/cm2 of shear stress) rapidly raised the [Ca2+]i to 283 ± 5.7 nM (n= 50). Addition of U-II (1, 10, 100 nM) to perfusing saline produced a rapid rise in [Ca2+]i by an additional 72 ± 4 nM (n=16), 168 + 5 nM (n=12) and 463 ± 8.4 nM (n=15), respectively (Fig. 1). In a Ca2+-free saline, U-II (100 nM) induced a transitory elevation in [Ca2+]i by 348 ± 6.4 nM (n=9) (Fig. 1).
Fig. 1.
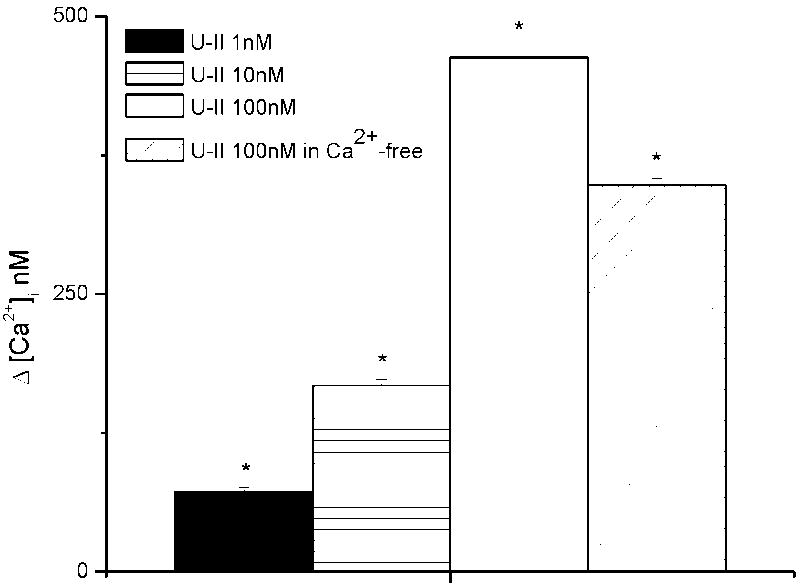
Ca2+ responses induced by urotensin-II (U-II) in human aortic endothelial cells. Addition of U-II (1, 10, 100 nM) to perfusing saline increased [Ca2+]i by an additional 72 ± 4 (n=16), 168 ±5 (n=12) and 463 ± 8.4 nM (n=15), respectively. In a Ca2+-free saline, U-II (100 nM) induced a transitory increase in [Ca2+]i by 348 ± 6.4 nM (n=9). The asterisk denotes statistically significant difference as compared to control.
In cultured HAEC exposed to two consecutive superfusion of U-II (100 nM), the second superfusion consistently caused a much smaller increase in [Ca2+]i as compared to that produced by the first application; a representative experiment is shown in Fig. 2A. The first and second administration produced an averaged increase in [Ca2+]i of 463 ± 8 nM (n=23) and 216 ± 7 nM (n=23), respectively (Fig. 2B).
Fig. 2.
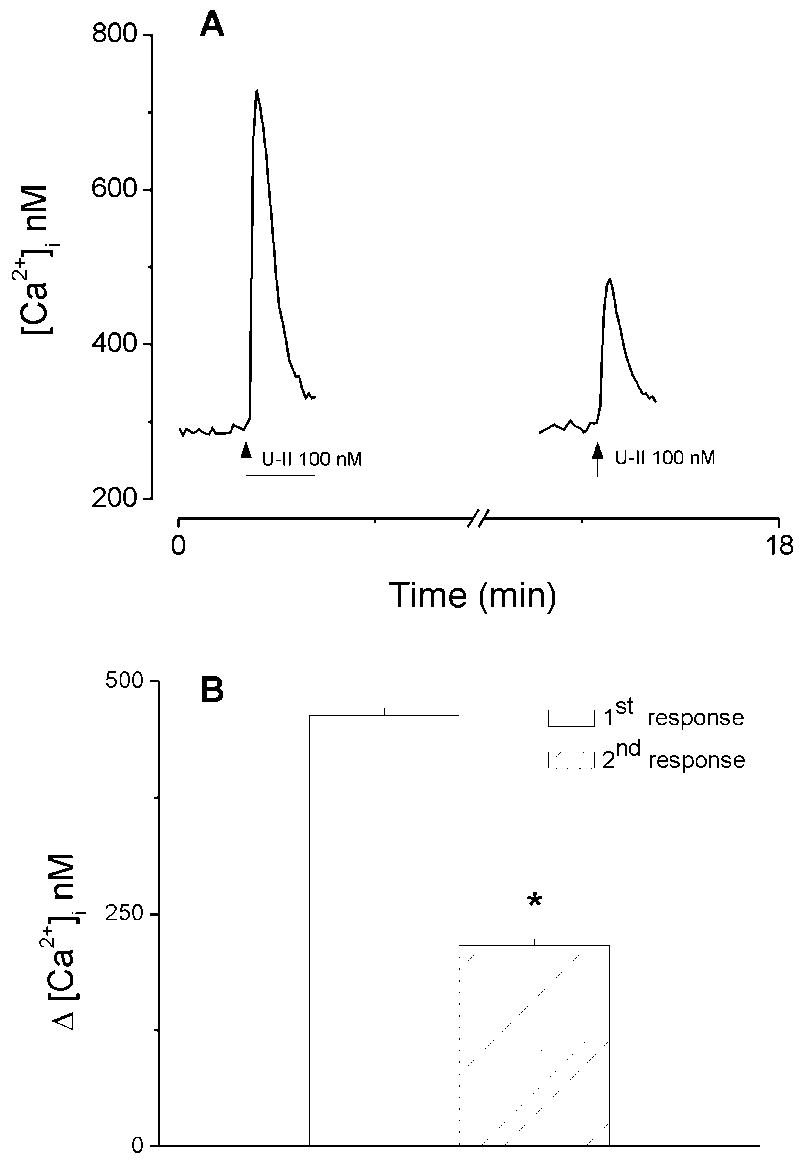
Ca2+ responses induced by two consecutive administrations of urotensin-II (U-II). A, Actual traces of two consecutive responses produced by superfusion of U-II (100 nM); the second superfusion consistently caused a much smaller increase in [Ca2+]i as compared to that produced by the first application. B, Comparison of the first and second response produced by U-II (100 nM): the first administration produced an increase in [Ca2+]i by 463 ± 8 nM, whereas the second administration produced an increase by 216 ± 7 nM (n=23). The asterisk denotes statistically significant difference as compared to the first response.
[Ca2+]i in static HAEC
Under static conditions, U-II (100 nM) added directly to cultured HAEC did not result in a significant change of [Ca2+]i in any of the cells tested (n= 76). Spontaneous Ca2+oscillations occurred in 14 out of 161 HAEC examined (8.7%). Addition of U-II (100 nM) abolished oscillations in all of the 14 cells analyzed; a representative example of actual recordings from three cells displaying oscillations is shown in Fig. 3.
Fig. 3.
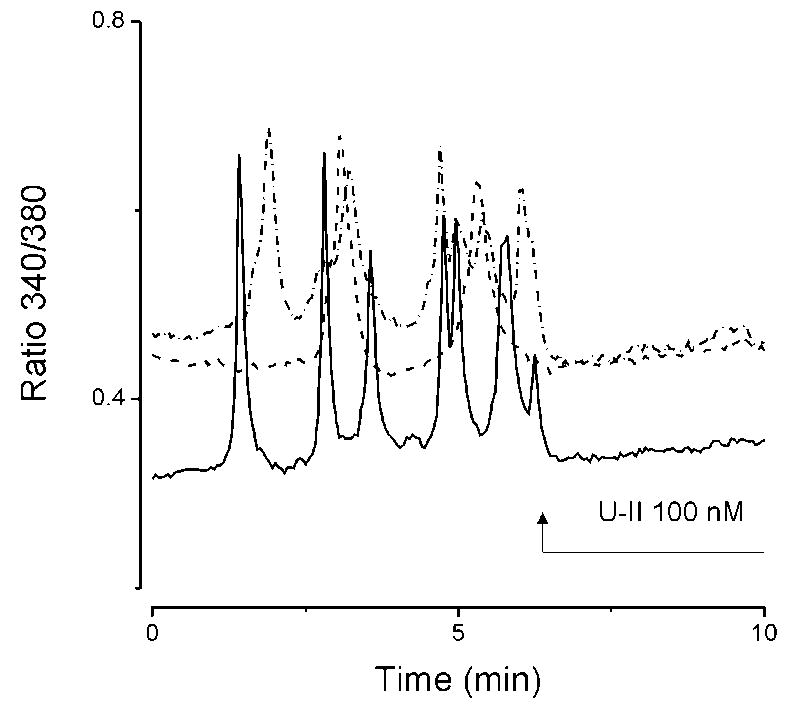
Effects of urotensin II (U-II) on Ca2+ oscillations. U-II (100 nM) abolished spontaneous Ca2+ oscillations in HAEC. Actual recordings from three different cells (solid line, dashed line and dot-dash line) exhibiting Ca2+ oscillations are shown.
Effects of U-II on ATP- and thrombin-induced [Ca2+]i in static state
IP3 has been shown to be one of the signaling pathways involved in Ca2+oscillations [49]. ATP and thrombin are known to mobilize Ca2+ in endothelial cells through the IP3 pathway. The following experiments were conducted to test the hypothesis that U-II abolishes Ca2+ oscillations by modulating the IP3 pathway. Under static conditions, ATP (10 μM) caused a fast and transitory increase of [Ca2+]i (ΔF/F0, Fig. 4A1, black trace, n=27). Pretreating the HAEC with U-II (100 nM) did not significantly alter the ATP-induced increase in [Ca2+]i either in Ca2+-containing (Fig. 4A1, red trace) or Ca2+-free saline (Fig. 4A2, red trace, n=35). U-II was added to the chamber one minute before ATP and for the duration of ATP administration.
Fig. 4.
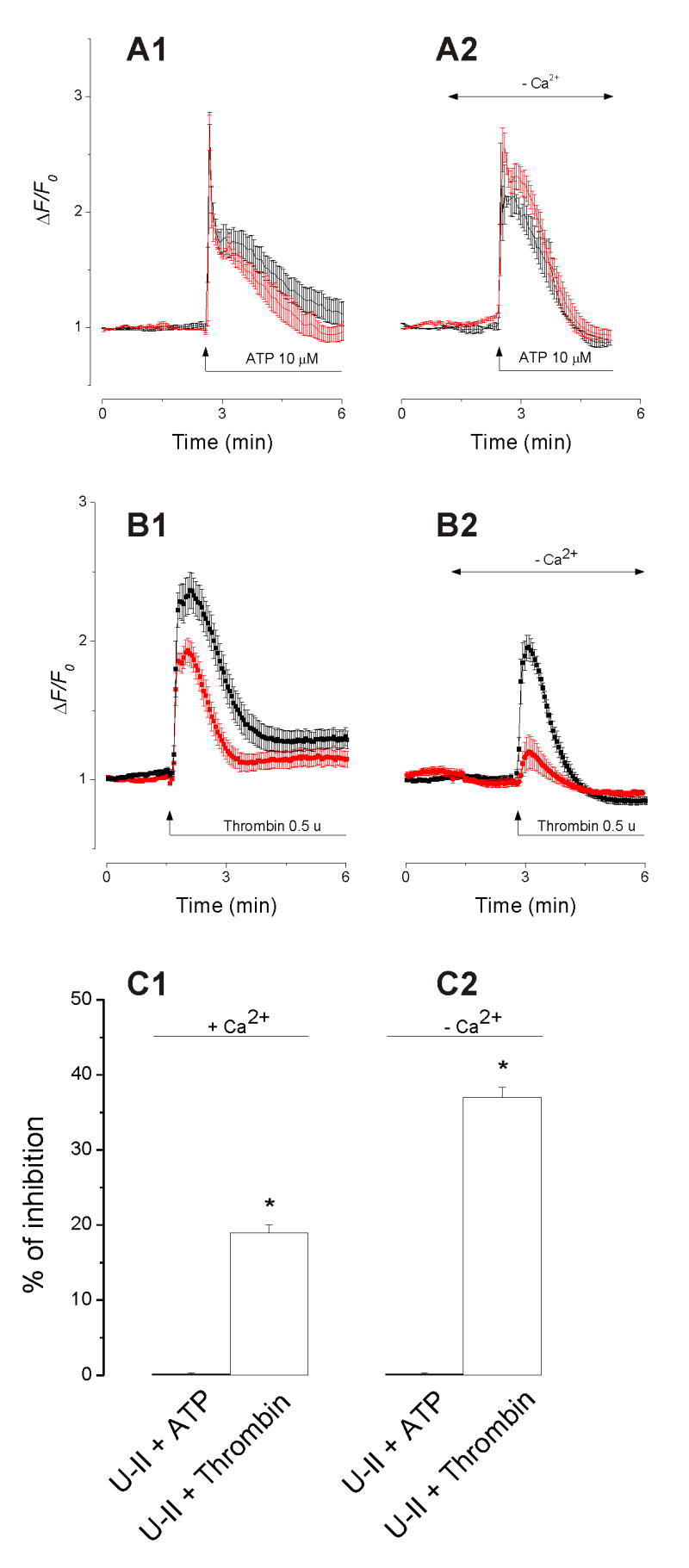
Effect of urotensin-II (U-II, 100 nM) on ATP- and thrombin-induced increase in [Ca2+]i. A1 and A2, administration of U-II (red trace) did not significantly affect the ATP-induced increase in [Ca2+]i (black trace) in Ca2+-containing or in Ca2+-free saline; traces represent mean ΔF/F0 ± S.E.M. B1 and B2, administration of U-II (red trace) reduced the thrombin-induced (black trace) increase in [Ca2+]i in Ca2+-containing and Ca2+-free saline. C1 and C2, comparison of the effect of U-II on ATP- and thrombin-induced increase in [Ca2+]i in saline with and without Ca2+.
U-II (100 nM, red trace) reduced the thrombin-induced increase in [Ca2+]i (Fig. 4B1 and 4B2, black trace, n=33). This effect was more evident in Ca2+-free saline (Fig. 4B2, n=29), as U-II inhibited thrombin-induced [Ca2+]i increase by 19 ± 1% in Ca2+-containing saline and by 37 ± 1.3 % in Ca2+-free saline (Fig. 4C1 and 4C2). The traces represent the mean ΔF/F0 ± S.E.M.
Discussion
Endothelial cells have a major role in regulating the diameter of the blood vessels and their adaptation to hemodynamic demands [45]. Urotensin II, the most potent vasoconstrictor agonist yet identified, was first reported to produce an endothelium-dependent relaxation and endothelium-independent contractions of rat aorta [32]. Significant differences in the vascular response to U-II have been reported [15, 26]. For example, U-II is an endothelium-dependent vasodilator in mesenteric and coronary arteries in the rat, as well as in the capillaries of the ear, but not in the basilar artery [11, 52]. The relaxant responses are attributed to a release of nitric oxide and endothelium-derived hyperpolarizing factors [3, 11, 62].
Intracellular calcium acts as a second messenger and serves a critical role in regulating the activity of endothelial cells. The vascular endothelium responds to several hormones and chemical signals via changes in cytosolic Ca2+, with subsequent activation of Ca2+-dependent signaling mechanisms [35]. U-II reportedly mobilizes Ca2+ by different mechanisms in different types of cell. For example, the effect of U-II was abolished by thapsigargin, indicating the participation of endoplasmic reticulum Ca2+ pools in rhabdomyosarcoma cell line [27] as well as in frog motor nerve terminals [12]. In rat, rabbit and cat blood vessels [2, 32, 55, 56, 61] and in rat cultured astrocytes [16], the effect of U-II was inhibited by the phospholipase C inhibitor U-73122, indicating the involvement of phospholipase-C/IP3 pathways. In contrast, U-II elevated [Ca2+]i largely by facilitating Ca2+ entry through plasmalemmal Ca2+ channels in rat spinal motoneurons [30].
With respect to the HAEC, our result indicates that U-II induced an elevation of [Ca2+]i under flow but not under static state. Similarly, rat aortic adventitial segments exposed to U-II release nitric oxide upon continuous shaking [41]. Elevation of endothelial cell [Ca2+]i may be achieved by Ca2+ entry via Ca2+ channels in the plasma membrane and/or by Ca2+ release from intracellular stores [1]. In shear stress, U-II caused a concentration-dependent elevation of [Ca2+]i mediated by Ca2+entry through plasmalemmal Ca2+ channels as well as Ca2+ release from intracellular Ca2+stores. In large arteries, the average wall shear stress is between 1 to 20 dyne/cm2. At curves and bifurcations, peak wall shear stress may be as high as 100 dyne/cm2. Immediate (milliseconds to seconds) responses to shear stress include increases in ionic conductance [40, 48], intracellular Ca2+ [57, 58] and IP3 [8, 46]. As a corollary, U-II may facilitate the shear stress-induced increase of [Ca2+]i and/or IP3. In the case of consecutive administration of U-II to HAEC, the response to the second administration of U-II was smaller than the first response, implying the occurrence of desensitization. This result is similar to that reported in rat vasculature [15], but different from that of spinal neurons [30]. An alternative interpretation would be that the internal pool of Ca2+ contributing to the overall U-II-induced Ca2+ increase was only partially refilled at the time interval between applications.
Ca2+ oscillations, which are probably initiated by Ca2+ release from intracellular pools rather than Ca2+ entry from the extracellular medium, have been demonstrated in a population of cultured endothelial cells [45]. A second novel observation made in our study is that U-II not only did not raise [Ca 2+]i but abolished Ca2+ oscillations in HAEC under static conditions.
In endothelial cells, IP3 is the most common pathway leading to an elevation of [Ca2+]i. At concentrations up to 10 μM, ATP acting on P2Y purinergic receptors raised [Ca2+]i and activated Gq/G11 phospholipase C pathways [53, 60]. Thrombin is another potent agonist that elevates [Ca2+]i in endothelial cells by different mechanisms, including Ca2+ influx [23]. Thrombin signaling in the endothelium is mediated by a family of G protein–coupled receptors known as protease-activated receptors (PARs) [22]. In aortic endothelial cells, activation of PAR-2 or P2Y receptors elevates Ca2+ through phospholipase C/IP3 pathways subsequent to activation of Gq/11 [37]. Under static conditions, pretreatment of HAEC with U-II (100 nM) did not affect ATP-induced [Ca2+]i elevation either in normal or Ca2+-free saline, indicating that the peptide does not interfere with phospholipase C/IP3 pathways. In contrast, U-II pretreatment significantly reduced thrombin-induced [Ca2+]i mobilization. Since the ATP response is not affected, U-II may directly modulate PAR-2, thereby affecting the coupling with Gq protein in HAEC.
A possible explanation for the differences observed between U-II-induced effects in shear stress vs static state is that the affinity of U-II to its receptors may vary in different microenvironment. Alternatively, there is evidence that peptides may be active when internalized into the cytoplasm [6, 13, 33]. Hence, we cannot exclude a possible differential regulation of calcium homeostasis in endothelial cells by activated intracellular U-II receptors.
In conclusion, our result shows that, depending on the condition under which the experiment is conducted, U-II can exert multiple effects on human aortic endothelial cells.
Acknowledgments
Supported by NIH Grants NS18710, HL51314, HL67033, HL77288, and HL74925 from the Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- 2.Aiyar N, Johns DG, Ao Z, Disa J, Behm DJ, Foley JJ, Buckley PT, Sarau HM, vanderKeyl HK, Elshourbagy NA, Douglas SA. Cloning and pharmacological characterization of the cat urotensin-II receptor (UT) Biochem Pharmacol. 2005;69:1069–1079. doi: 10.1016/j.bcp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Abdelrahman AM, Pang CCY. Involvement of the nitric oxide/L-arginine and sympathetic nervous systems on the vasodepressor action of human urotensin II in anesthetized rats. Life Sci. 2002;71:819–825. doi: 10.1016/s0024-3205(02)01743-5. [DOI] [PubMed] [Google Scholar]
- 4.Affolter JT, Newby DE, Wilkinson LB, Winter MJ, Balment RJ, Webb DJ. No effect on central or peripheral blood pressure of systemic urotensin II infusion in humans. Br J Clin Pharmacol. 2002;54:617–621. doi: 10.1046/j.1365-2125.2002.t01-1-01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 6.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol. 2006;291:C995–C1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bennett RT, Jones RD, Morice AH, Smith CF, Cowen ME. Vasoconstrictive effects of endothelin-1, endothelin-3, and urotensin II in isolated perfused human lungs and isolated human pulmonary arteries. Thorax. 2004;59:401–407. doi: 10.1136/thx.2003.011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhagyalakshmi A, Berthiaume F, Reich KM, Frangos JA. Fluid shear stress stimulates membrane phospholipids metabolism in cultured human endothelial cells. J Vasc Res. 1992;29:443–449. doi: 10.1159/000158963. [DOI] [PubMed] [Google Scholar]
- 9.Bird RB, Stewart WE, Lightfoot EN. Transportation Phenomena. New York: John Wiley & Sons, Inc; 1960. [Google Scholar]
- 10.Bohm F, Pernow J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br J Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottrill FE, Douglas SA, Hiley CR, White R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br J Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brailoiu E, Brailoiu GC, Miyamoto MD, Dun NJ. The vasoactive peptide urotensin II stimulates spontaneous release from frog motor nerve terminals. Br J Pharmacol. 2003;138:1580–1588. doi: 10.1038/sj.bjp.0705204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brailoiu E, Filipeanu CM, Tica A, Toma CP, de Zeeuw D, Nelemans SA. Contractile effects by intracellular angiotensin II via receptors with a distinct pharmacological profile in rat aorta. Br J Pharmacol. 1999;126:1133–1138. doi: 10.1038/sj.bjp.0702421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 15.Camarda V, Rizzi A, Calo G, Gendron G, Perron SI, Kostenis E, Zamboni P, Mascoli F, Regoli D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:141–149. doi: 10.1007/s00210-001-0503-0. [DOI] [PubMed] [Google Scholar]
- 16.Castel H, Diallo M, Chatenet D, Leprince J, Desrues L, Schouft MT, Fontaine M, Dubessy C, Lihrmann I, Scalbert E, Malagon M, Vaudry H, Tonon MC, Gandolfo P. Biochemical and functional characterization of high-affinity urotensin II receptors in rat cortical astrocytes. J Neurochem. 2006;99:582–595. doi: 10.1111/j.1471-4159.2006.04130.x. [DOI] [PubMed] [Google Scholar]
- 17.Chartrel N, Leprince J, Dujardin C, Chatenet D, Tollemer H, Baroncini M, Balment RJ, Beauvillain JC, Vaudry H. Biochemical characterization and immunohistochemical localization of urotensin II in the human brainstem and spinal cord. J Neurochem. 2004;91:110–118. doi: 10.1111/j.1471-4159.2004.02698.x. [DOI] [PubMed] [Google Scholar]
- 18.Chatenet D, Dubessy C, Boularan C, Scalbert E, Pfeiffer B, Renard P, Lihrmann I, Pacaud P, Tonon MC, Vaudry H, Leprince J. Structure-activity relationships of a novel series of urotensin II analogues: identification of urotensin II antagonists. J Med Chem. 2006;49:7234–7238. doi: 10.1021/jm0602110. [DOI] [PubMed] [Google Scholar]
- 19.Conlon JM. Singular contributions of fish neuroendocrinology to mammalian regulatory peptide research. Regul Pept. 2000;93:3–12. doi: 10.1016/s0167-0115(00)00172-5. [DOI] [PubMed] [Google Scholar]
- 20.Coulouarn Y, Jégou S, Tostivint H, Vaudry H, Lihrmann I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- 21.Coulouarn Y, Lihrmann I, Jégou S, Anouar Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA, Vaudry H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 23.D’Amore P, Shepro D. Stimulation of growth and calcium influx in cultured, bovine, aortic endothelial cells by platelets and vasoactive substances. J Cell Physiol. 1977;92:177–183. doi: 10.1002/jcp.1040920206. [DOI] [PubMed] [Google Scholar]
- 24.Do-Rego JC, Chatenet D, Orta MH, Naudin B, Le Cudennec C, Leprince J, Scalbert E, Vaudry H, Costentin J. Behavioral effects of urotensin-II centrally administered in mice. Psychopharmacol. 2005;183:103–117. doi: 10.1007/s00213-005-0140-2. [DOI] [PubMed] [Google Scholar]
- 25.Douglas SA, Ashton DJ, Sauermelch CF, Coatney RW, Ohlstein DH, Ruffolo MR, Ohlstein EH, Aiyar NV, Willette RN. Human urotensin-II is a potent vasoactive peptide: pharmacological characterization in the rat, mouse, dog and primate. J Cardiovasc Pharmacol. 2000a;36:S163–S166. doi: 10.1097/00005344-200036051-00051. [DOI] [PubMed] [Google Scholar]
- 26.Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, Ohlstein EH, Willette RN. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000b;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas SA, Naselsky D, Ao Z, Disa J, Herold CL, Lynch F, Aiyar NV. Identification and pharmacological characterization of native, functional human urotensin-II receptors in rhabdomyosarcoma cell lines. Br J Pharmacol. 2004;142:921–932. doi: 10.1038/sj.bjp.0705743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dun SL, Brailoiu GC, Yang J, Chang JK, Dun NJ. Urotensin II-immunoreactivity in the brainstem and spinal cord of the rat. Neurosci Lett. 2001;305:9–12. doi: 10.1016/s0304-3940(01)01804-3. [DOI] [PubMed] [Google Scholar]
- 29.Egginger JG, Camus A, Calas A. Urotensin-II expression in the mouse spinal cord. J Chem Neuroanat. 2006;31:146–154. doi: 10.1016/j.jchemneu.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Filipeanu CM, Brailoiu E, Dun SL, Dun NJ. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J Neurochem. 2002;83:879–884. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 31.Gartion J, Parker F, Harrison DC, Douglas SA, Ashmeade TE, Riley GJ, Hughes ZA, Taylor SG, Munton RP, Hagan JJ, Hunter JA, Jones DN. Central effects of urotensin-II following ICV administration in rats. Psychopharmacol. 2001;155:426–433. doi: 10.1007/s002130100715. [DOI] [PubMed] [Google Scholar]
- 32.Gibson A. Complex effects of Gillichthys urotensin II on rat aortic strips. Br J Pharmacol. 1987;91:205–212. doi: 10.1111/j.1476-5381.1987.tb09000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 34.Hillier C, Berry C, Petrie MC, O’Dwyer PJ, Hamilton C, Brown A, McMurray J. Effects of urotensin II in human arteries and veins of varying caliber. Circ. 2001;103:1378–1381. doi: 10.1161/01.cir.103.10.1378. [DOI] [PubMed] [Google Scholar]
- 35.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertens. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 36.Huitron-Resendiz S, Kristensen MP, Sánchez-Alavez M, Clark SD, Grupke SL, Tyler C, Suzuki C, Nothacker HP, Civelli O, Criado JR, Henriksen SJ, Leonard CS, de Lecea L. Urotensin II Modulates Rapid Eye Movement Sleep through Activation of Brainstem Cholinergic Neurons. J Neurosci. 2005;25:5465–5474. doi: 10.1523/JNEUROSCI.4501-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh Y, Sendo T, Oishi R. Physiology and pathophysiology of proteinase-activated receptors (PARs): role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharmacol Sci. 2005;97:14–19. doi: 10.1254/jphs.fmj04005x3. [DOI] [PubMed] [Google Scholar]
- 38.James NL, Harrison DG, Nerem RM. Effects of shear on endothelial cell calcium in the presence and absence of ATP. FASEB J. 1995;9:968–973. doi: 10.1096/fasebj.9.10.7615166. [DOI] [PubMed] [Google Scholar]
- 39.Jegou S, Cartier D, Dubessy C, Gonzalez BJ, Chatenet D, Tostivint H, Scalbert E, Leprince J, Vaudry H, Lihrmann I. Localization of the urotensin II receptor in the rat central nervous system. J Comp Neurol. 2006;495:21–26. doi: 10.1002/cne.20845. [DOI] [PubMed] [Google Scholar]
- 40.Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channel in vascular endothelial cells as mechanotrasducers? Nature. 1987;325:811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 41.Lin L, Ding WH, Jiang W, Zhang YG, Qi YF, Yuan WJ, Tang CS. Urotensin-II activates L-arginine/nitric oxide pathway in isolated rat aortic adventitia. Peptides. 2004;25:1977–1984. doi: 10.1016/j.peptides.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, Pong SS, Zeng Z, Zhang Q, Howard AD, Williams DL, Jr, Davidoff M, Wang R, Austin CP, McDonald TP, Bai C, George SR, Evans JF, Caskey CT. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem Biophys Res Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- 43.Matsushita M, Shichiri M, Imai T, Iwashina M, Tanaka H, Takasu N, Hirata Y. Co-expression of urotensin II and its receptor (GPR14) in human cardiovascular and renal tissues. J Hypertens. 2001;19:2185–2190. doi: 10.1097/00004872-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Mori M, Sugo T, Abe M, Shimomura Y, Kurihara M, Kitada C, Kikuchi K, Shintani Y, Kurokawa T, Onda H, Nishimura O, Fujino M. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14) Biochem Biophys Res Commun. 1999;265:123–129. doi: 10.1006/bbrc.1999.1640. [DOI] [PubMed] [Google Scholar]
- 45.Nilius B. Signal transduction in vascular endothelium: the role of intracellular calcium and ion channels. Verh K Acad Geneeskd Belg. 1998;60:215–250. [PubMed] [Google Scholar]
- 46.Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol triphosphate levels in human endothelial cells. Biochem Biophys Res Commun. 1990;10:281–287. doi: 10.1016/0006-291x(90)91271-s. [DOI] [PubMed] [Google Scholar]
- 47.Nothacker HP, Wang Z, McNeill AM, Saito Y, Merten S, O’Dowd B, Duckles SP, Civelli O. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nature Cell Biol. 1999;1:383–385. doi: 10.1038/14081. [DOI] [PubMed] [Google Scholar]
- 48.Olsen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 49.Pelletier G, Lihrmann I, Vaudry H. Role of androgens in the regulation of urotensin II precursor mRNA expression in the rat brainstem and spinal cord. Neurosci. 2002;115:525–532. doi: 10.1016/s0306-4522(02)00413-x. [DOI] [PubMed] [Google Scholar]
- 50.Pelletier G, Lihrmann I, Dubessy C, Luu-The V, Vaudry H, Labrie F. Androgenic down-regulation of urotensin II precursor, urotensin II-related peptide precursor and androgen receptor mRNA in the mouse spinal cord. Neurosci. 2005;132:689–696. doi: 10.1016/j.neuroscience.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Politi A, Gaspers LD, Thomas AP, Hofer T. Models of IP3 and Ca2+ oscillations: frequency encoding and identification of underlying feedbacks. Biophys J. 2006;90:3120–3133. doi: 10.1529/biophysj.105.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi JS, Schulingkamp R, Parry TJ, Colburn R, Stone D, Haertlein B, Minor LK, Andrade-Gordon P, Damiano BP. Urotensin-II induces ear flushing in rats. Br J Pharmacol. 2007;150:415–423. doi: 10.1038/sj.bjp.0707006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pirotton S, Communi D, Motte S, Janssens R, Boeynaems JM. Endothelial P2-purinoceptors: subtypes and signal transduction. J Auton Pharmacol. 1996;16:353–356. doi: 10.1111/j.1474-8673.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 54.Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossowski WJ, Cheng BL, Taylor JE, Datta R, Coy DH. Human urotensin II-induced aorta ring contractions are mediated by protein kinase C, tyrosine kinases and Rho-kinase: inhibition by somatostatin receptor antagonists. Eur J Pharmacol. 2002;438:159–170. doi: 10.1016/s0014-2999(02)01341-9. [DOI] [PubMed] [Google Scholar]
- 56.Saetrum-Opgaard O, Nothacker H, Ehlert FJ, Krause DN. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur J Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz G, Callewaert G, Droogmans G, Nilus B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol (London) 1992;458:527–538. doi: 10.1113/jphysiol.1992.sp019432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen J, Luscinskas FW, Connolly A, Dewey CF, Gimbrone MA. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992;262:C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- 59.Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, Kirk A, Richardson M, Maclean MR. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- 60.Viana F, de Smedt H, Droogmans G, Nilius B. Calcium signalling through nucleotide receptor P2Y2 in cultured human vascular endothelium. Cell Calcium. 1998;24:117–127. doi: 10.1016/s0143-4160(98)90079-3. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe T, Koba S, Katagiri T, Pakala R, Benedict CR. Lysophosphatidylcholine potentiates the mitogenic effect of various vasoactive compounds on rabbit aortic smooth muscle cells. Japan Heart J. 2002;43:409–416. doi: 10.1536/jhj.43.409. [DOI] [PubMed] [Google Scholar]
- 62.Zhang AY, Chen YF, Zhang DX, Yi FX, Qi J, Andrade-Gordon P, deGaravilla L, Li PL, Zou AP. Urotensin II is a nitric oxide-dependent vasodilator and natriuretic peptide in the rat kidney. Am J Physiol Renal Physiol. 2003;285:F792–F798. doi: 10.1152/ajprenal.00342.2002. [DOI] [PubMed] [Google Scholar]


