Abstract
Central glucagon-like peptide-1 (GLP-1) regulates food intake, glucose homeostasis, and behavioral and neuroendocrine responses to acute stress. Given its pronounced role in acute stress regulation, the GLP-1 system is a prime candidate for mediating the prolonged drive of the hypothalamo-pituitary-adrenocortical axis by chronic stress. To test this hypothesis, we evaluated the necessity and sufficiency of GLP-1 for production of chronic stress-induced changes in HPA axis function. Exogenous GLP-1 or the GLP-1 receptor antagonist, dHG-exendin, were delivered into the 3rd ventricle of control animals or animals exposed to chronic variable stress (CVS) for 7 days. Animals in the CVS groups received GLP-1 or dHG-exendin immediately prior to each stress exposure. Prior to and at the end of the 7-day trial, chronically stressed animals were subjected to a novel stressor to test for HPA axis facilitation. Neither GLP-1 nor dHG-exendin affected CVS-associated increases in adrenal weight or decreases in basal plasma glucose levels. In addition, neither exogenous GLP-1 nor dHG-exendin altered any index of HPA axis activity in unstressed rats. However, GLP-1 enhanced CVS-induced facilitation of corticosterone (but not ACTH) response to an acute stress, whereas dHG-exendin inhibited facilitation. In addition, GLP-1 decreased body weight in chronically-stressed animals. dHG-exendin increased food intake and body weight in unstressed animals, consistent with a tonic role for GLP-1 in body weight regulation. Overall, our data suggest that brain GLP-1 modulates HPA axis activity within the context of chronic stress, perhaps at the level of the adrenal gland.
Keywords: ACTH, chronic variable stress, corticosterone, glucocorticoids, exendin, intracerebroventricular injection, body weight, food intake
Introduction
The hypothalamo-pituitary-adrenocortical (HPA) axis coordinates the release of glucocorticoids in response to stress. The HPA axis responds to real or anticipated homeostatic disruption by stimulating release of ACTH secretagogs (such as corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP)) from hypophysiotrophic neurons in the medial parvocellular region of paraventricular nucleus of hypothalamus (PVN) (2, 37). This neurohemal signal promotes release of adrenocorticotropic hormone (ACTH) by pituitary corticotrophs, which consequently causes a release of adrenal corticosteroids.
Ascending brainstem systems play a major role in excitation of HPA axis stress responses (c.f., (12, 27)). Recent studies from our group suggest that glucagon-like peptide-1 (GLP-1), a neuropeptide that is selectively expressed in the nucleus of solitary tract (NTS) and the ventrolateral medulla (10, 17, 22), plays a major role in HPA activation (17). The PVN is heavily innervated by GLP-1 fibers that form direct synaptic contacts with CRH immunoreactive cell bodies (19, 25, 26, 29), confirming that GLP-1 is in position to directly modulate activity of CRH neurons. Intracerebroventricular infusions of GLP-1 increase plasma ACTH and/or corticosterone (17, 19), indicating that GLP-1 receptor binding is sufficient to trigger HPA activation. Furthermore, pretreatment of GLP-1 antagonist attenuated ACTH and corticosterone responses induced by systemic lithium chloride (LiCl) injection and elevated platform exposure (17), indicating that GLP-1 signaling is also necessary for acute HPA axis stress responses. GLP-1 containing neurons in the NTS are activated by visceral stress (LiCl injection) (25), consistent with a role in central stress integration. Overall, these data support the hypothesis that GLP-1 regulates HPA axis responses to a variety of stressors by stimulating CRH release from PVN neurons.
To date, the GLP-1 system has been studied exclusively in the realm of acute stress. While activation of the HPA axis is typically an adaptive response to an acute stress, chronic activation of the HPA axis can be deleterious and has been linked to a number of different pathologies, including metabolic disease and depression (21). Recent data strongly suggest that different brain circuitries are involved in acute and chronic stress responses. For example, regions such as the paraventricular thalamus regulate HPA axis responses to chronic, but not acute stressors (3). In addition, chronic stress produces a well-documented enhancement of HPA axis responses to new stressors, a process that may involve regions such as the paraventricular thalamus(3) and perhaps central amygdaloid nucleus (8). Consequently, it is critical to determine whether the endogenous GLP-1 system is responsible for deleterious changes in HPA axis function seen following chronic stress. Therefore, the current study was designed to test the role of GLP-1 in the establishment and maintenance of chronic stress-induced HPA hyperactivity, as produced by a well-characterized chronic stress model (chronic variable stress (CVS)).
Materials and Methods
Animals
Male Sprague Dawley rats (275–300g) were acquired from Harlan Labs(Indianapolis, IN). All animals were individually housed in a temperature- and humidity-controlled facility at the University of Cincinnati, on a 6am to 6pm light-dark cycle with free access to standard chow and water. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Surgery
All rats were anesthetized by an intra-peritoneal injection with a cocktail of ketamine (85–95 mg/kg) and xylazine (10–15 mg/kg). Guide cannulas (22 gauge; Plastics One, Inc., Roanoke, VA) were stereotaxically implanted into the 3rd ventricle, in accordance with the atlas of Paxinos and Watson (−2.2 mm anteroposterior (AP) and −7.5 mm dorsoventral (DV) with respect to bregma) (24). Animals were allowed to recover for 7 days following surgery, at which point cannula placement was verified by administering 20 ng of angiotensin II (American Peptide Company, Inc., Sunnyvale, CA). Rats that failed to show a dipsogenic response to angiotensin II (>10ml water consumed in 60 minutes) were removed from the study.
Drug infusion, restraint challenge, and Chronic Variable Stress (CVS)
After surgery, dummy cannulas were removed and replaced every 3rd day for at least two weeks to acclimate animals to handling and maintain cannula patency. In preparation for the study, the frequency of training was increased to twice/daily (9–11 am 6 and 3–6pm) for 4 days, followed by vehicle intra 3rd ventricular (i3vt) injection twice/daily for 5 days.
Experimental procedures were initiated following the 5th day of vehicle injection. Animals were weight-matched and divided into 6 groups (6 rats in each group): vehicle-handled, GLP-1-handled, dHG-exendin-handled, vehicle-chronic variable stress (CVS), GLP-1-CVS, and dHG-exendin-CVS. On the morning on day 1, prior to any drug treatment or the initiation of CVS, rats in the CVS group received an initial restraint stress test (pre-CVS restraint). An additional group of animals received restraint testing prior to beginning a regimen of vehicle or drug injections in the absence of CVS, to test the ability of GLP-1 or dHG-exedin to alter HPA axis stress responses upon prolonged delivery. Animals were restrained in plastic tubes for 30 min and subsequently returned to their home cages. Blood samples were collected by tail clip (36) at the beginning of the restraint (t=0), at the end of the restraint (t=30), and at 60 and 120 minutes after the initiation of restraint (t=60, 120, respectively). Plasma was collected and kept frozen at −20°C until analyzed.
From the afternoon of day 1, all animals received infusion of either vehicle (sterile-filtered artificial cerebrospinal fluid (aCSF)) (2µl), GLP-1 ((Ser8)-GLP-1 (7–36) amide, 2µg/2µl; American Peptide Company, Inc., Sunnyvale, CA), or the GLP-1 receptor antagonist dHG-exendin (des-His1, Glu9-exendin-4, 100µg/2µl; American Peptide Company, Inc.). GLP-1 and dHG-exendin were dissolved in aCSF and the aliquots were kept frozen at −20°C until injected.
After injection, animals were kept undisturbed in their home cages for 15–30 minutes to ensure drug action. Thereafter, CVS groups were subjected to one of five stressors that were selected randomly. Stressors used were warm swim (31–33°C) for 20 minutes, cold swim (16–18°C) for 5 minutes, hypoxia (8% oxygen) for 30 minutes, cold exposure (4°C) for 1 hour, or shaking (100 rpm) for 1 hour. Control animals did not experience any additional treatment. Drug injection and CVS were performed twice a day (9–11am and 3–6pm) for 7 days. All animals and food hoppers were weighed at the morning injection.
In the morning on day 8, all CVS rats were challenged by restraint stress (post-CVS restraint) for 30 minutes and blood samples were collected by tail clip at 3 time points; t=0, 30, and 60. In addition, animals receiving seven twice-daily infusions of vehicle, GLP-1 or exendin without concomitant CVS received an identical restraint challenge on day 8. Two hours after the initiation of stress, animals were decapitated and trunk blood was collected at t=120. Adrenals and thymi were immediately removed after decapitation, cleaned and weighed.
Plasma analyses
Plasma ACTH levels were measured by radioimmunoassay (RIA) using an antibody generously provided by Dr. William Engeland (University of Minnesota, Minneapolis, MN) and [125I] ACTH (Amersham Biosciences, Piscataway, NJ) as tracer. Plasma corticosterone levels were measured by RIA using a kit (MP Biomedicals, Inc., Costa Mesa, CA). Plasma glucose was measured by the standard glucose oxidase method.
Statistical analyses
All statistical analyses were performed using GB-Stat version 9.0 software (Dynamic Microsystems, Inc., Silver Spring, MD). Organ weight data were analyzed by factorial ANOVA. Food intake and body weight data were analyzed by repeated measures ANOVA, followed by Fisher’s LSD post hoc tests querying differences in group means at the same time points. Plasma ACTH, corticosterone, and glucose data were analyzed by three-way ANOVA. Stress response data of ACTH/corticosterone within the CVS groups were assessed using repeated measures ANOVA. We aimed to test if there were any main effects or interactions between the levels of ACTH/corticosterone and three independent variables (drug treatment, pre-/post- CVS/handling, and time after initiation of stress). Differences at individual time points were examined using Fisher’s LSD post hoc test. Basal and peak glucose level were independently analyzed using repeated measure ANOVA, testing for main effects or interactions of drug treatment, pre-/post-CVS/handling, and stress/handling on glucose levels. Differences between individual treatment groups were examined using Fisher’s LSD post hoc test. Finally, area under the curve (AUC) for corticosterone and ACTH was calculated for each animal and analyzed by two-way ANOVA. Differences with p≤0.05 were regarded as significant.
Results
Organ weights
Two-way ANOVA revealed that CVS significantly increased adrenal weight, as previously documented by our group (34). However, there was no effect of drug on the degree of chronic stress-induced adrenal hypertrophy. There was no effect of CVS or drug treatment on thymus weight.
Plasma ACTH
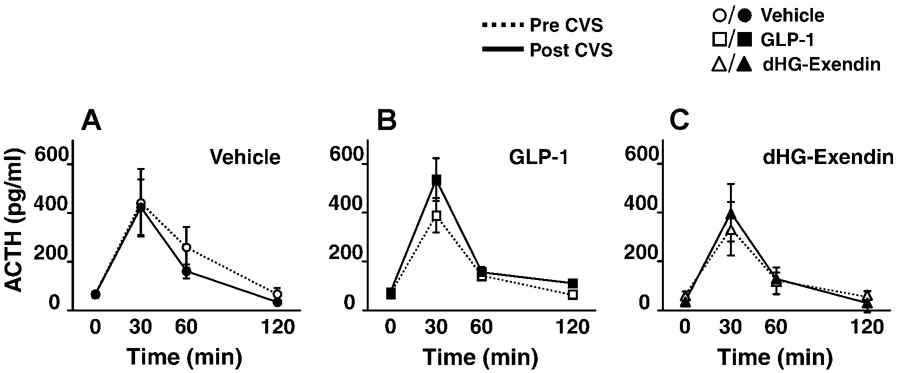
Resting AM ACTH levels, as determined from the initial blood sample on day 8, was not affected by either CVS or drug. In both the pre- and post-CVS restraint stress tests, plasma ACTH was elevated at 30 minutes in all groups. However, drug treatment did not affect initial ACTH responses to restraint, and there was no significant effect of CVS on the magnitude of ACTH stress responses (Fig. 2A–C). There were no significant differences in the pre- or post-CVS integrated ACTH responses (AUC) (data not shown).
Figure 2.
Plasma ACTH levels following restraint challenge prior to and after CVS (D–F) (mean ±S.E.M., n=4–6). Plasma ACTH levels were increased by restraint stress prior to and following CVS. ACTH responses were not altered by CVS or drug treatment, and no interaction effects were evident.
Plasma corticosterone
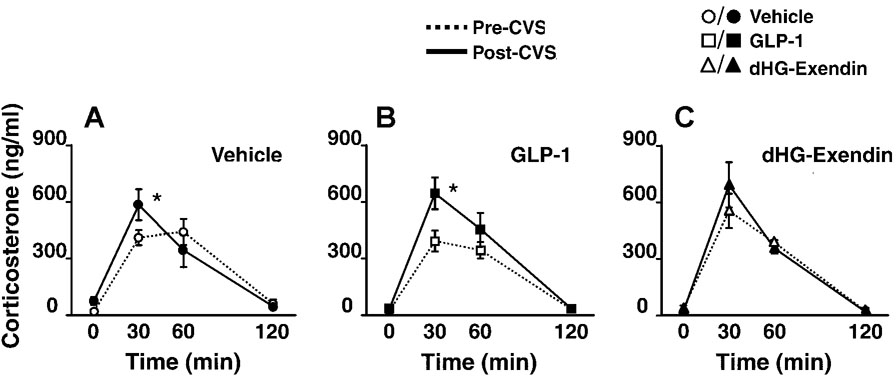
Resting AM corticosterone levels were not affected by CVS or drug treatment, as determined from the initial blood sample on day 8. In contrast, a significant effect of drug treatment was observed in the CVS groups. Post-hoc analysis revealed that both vehicle-CVS and GLP-1-CVS groups showed exaggerated peak corticosterone responses (t=30) in post-CVS compared to pre-CVS testing (Fig. 3A,B), consistent with facilitation of the HPA axis, as described by Akana and colleagues (1). In contrast, peak response magnitude was not affected by CVS in the dHG-exendin-CVS group (Fig. 3C).
Figure 3.
Plasma corticosterone levels following restraint challenge prior to and after CVS (A–C) (mean ±S.E.M., n=4–6). Exaggerated corticosterone responses were observed in vehicle (A) and GLP-1 (B) treated animals following CVS relative to pre-CVS values, marked by accentuated peak corticosterone secretion at t=30. In contrast, dHG-exendin treated animals did not show accentuated stress response profiles at t=30 (C). *=p<0.05.
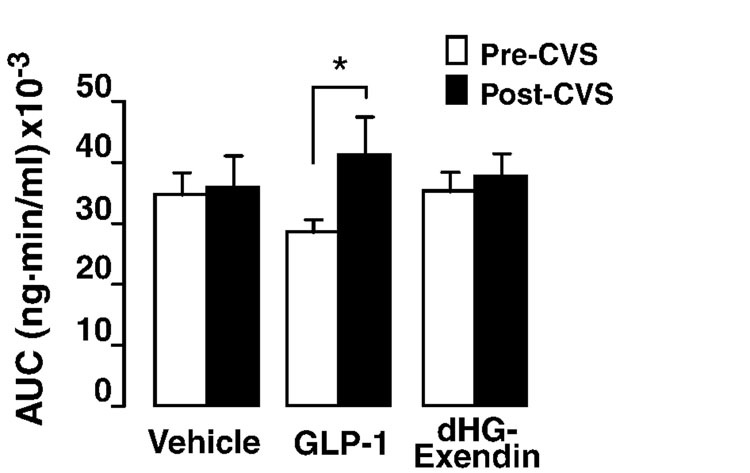
There was a significant effect of one-week of CVS on the integrated corticosterone response (AUC) (Fig. 4). Post hoc test revealed a significant difference between pre- and post-CVS in the GLP-1 treated group (Fig. 4), consistent with enhanced facilitation relative to the vehicle-CVS and dHG-exendin-CVS groups.
Figure 4.
Integrated plasma corticosterone responses to restraint, expressed as the area under curve (AUC). CVS-GLP-1 treated animals had a significant difference in corticosterone response to restraint challenge between pre- and post-CVS, whereas no differences in the integrated response magnitude were observed between any other groups. *=p<0.05.
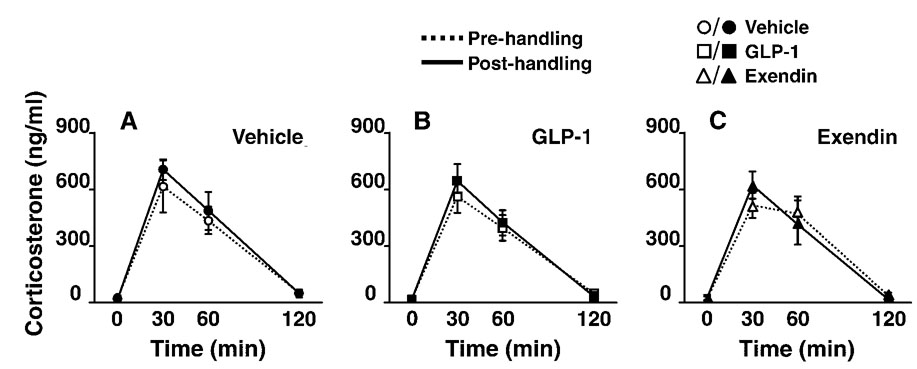
In contrast to the effects of GLP-1 and dHG-exendin on corticosterone responses following CVS, neither drug significantly affected restraint-induced corticosterone secretion following chronic delivery to otherwise unstressed rats who received only the handling involved in the injection procedure (Fig. 5). In addition, the magnitude of the corticosterone response to acute stress did not differ between days 1 and 8 in the vehicle-treated groups.
Figure 5.
Plasma corticosterone levels following restraint challenge prior to (Pre) and after (Post) a one-week, bidaily vehicle, GLP-1 or dHG-exendin injection/handling regimen (A–C) (mean ±S.E.M., n=4–6). Corticosterone responses to restraint were not affected by the intervening series of daily handling and icv injections (vehicle group). In addition, neither GLP-1 nor dHG-exendin affected subsequent responses to restraint.
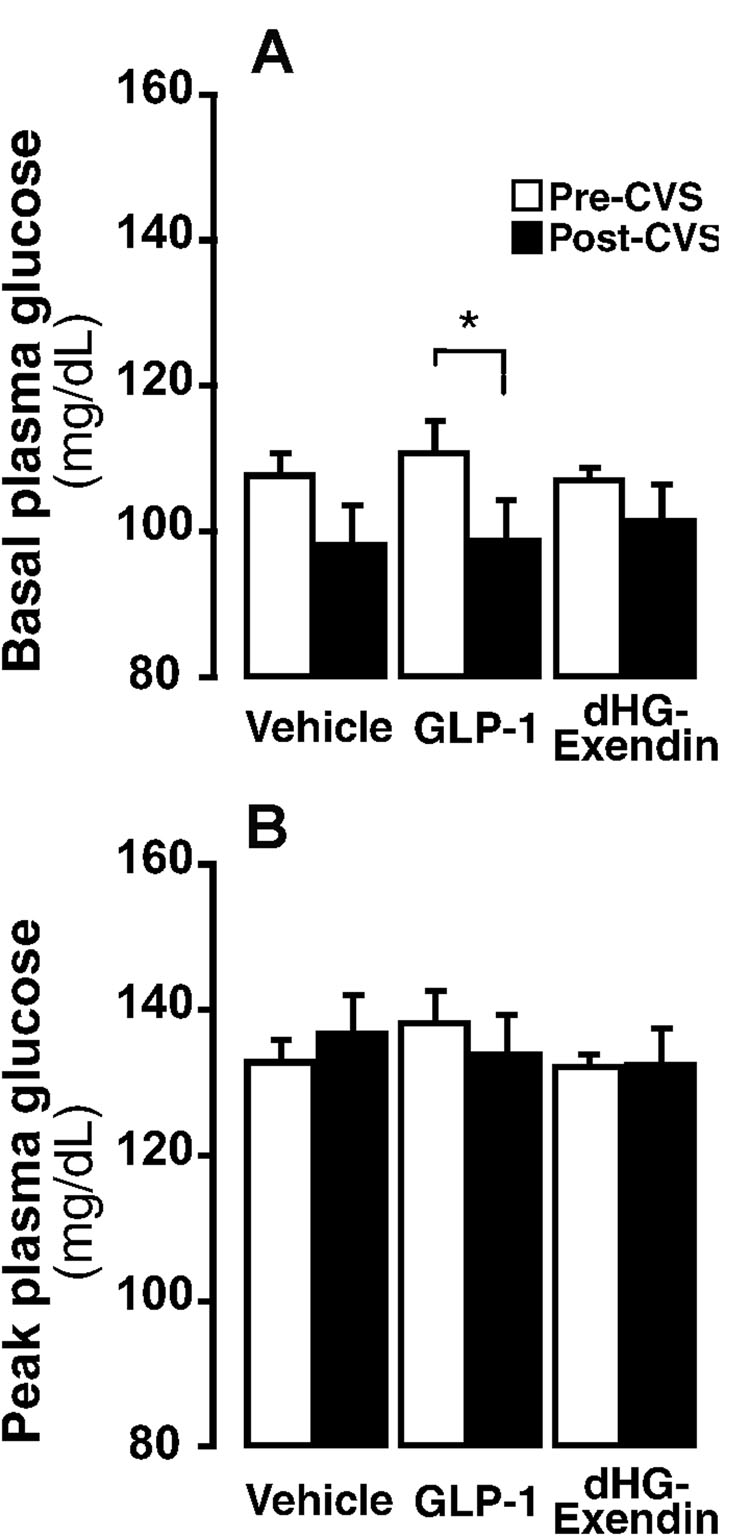
Basal plasma glucose
Drug injections did not alter basal glucose levels prior to CVS (Fig 6A). However, CVS caused a significant decrease in resting glucose across treatment groups (Fig. 6A). Post-hoc analysis indicated a significant pre- and post-stress difference only in the GLP-1 treated group, suggesting that stress-induced decreases were most pronounced in this treatment group. Following acute stress, plasma glucose level was significantly increased in all groups (Fig. 6B). Stress-induced hyperglycemia was not affected by drug or CVS exposure.
Figure 6.
Basal (A) and peak stress induced (30 min) plasma glucose levels prior to and after CVS exposure. Overall, basal plasma glucose levels (A) were not affected by drug treatment, but were significantly decreased by CVS (p≤0.05). The GLP-1 group showed significant decrease in basal glucose following CVS (p≤0.05). There were no drug or CVS effects on peak glucose levels (t=30) (B). *=p<0.05.
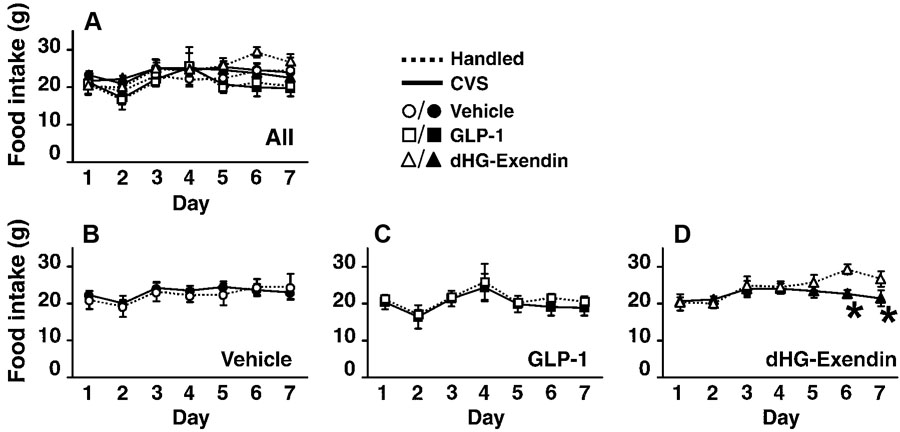
Daily food intake
Food intake data are presented in Fig. 7. All data are graphed in Fig. 7A; for clarity, Fig 7B–D shows the same data, separated for easier visualization of comparisons between handled and CVS groups. Food intake was significantly increased over the course of the experiment (Fig. 7A). There was a significant effect of drug treatment. Although the main effect of CVS was not significant, there was a significant CVS by time interaction. Within the handled groups, dHG-exendin-treated animals consumed significantly greater amounts of chow than vehicle (day 5–7) and GLP-1 (day 6) groups (Fig. 7D). There was no significant difference between vehicle and GLP-1 groups at any time point. In contrast, within CVS groups, the GLP-1 group ate significantly less than did the vehicle (day 5–7) and dHG-exendin (day 2) groups (Fig. 7A). There was no significant difference between vehicle and dHG-exendin-treated CVS groups at any time point. Within vehicle and GLP-1 groups, there was no difference between handled and CVS groups (Fig. 7B, C). However in dHG-exendin groups, handled animals ate significantly more than CVS group on day 6 and 7 (Fig. 7D).
Figure 7.
Daily food intake (mean ±S.E.M., n=4–6). Figure 7A shows daily food intake of all 6 groups. Food intake in each drug treatment groups were compared in figures 7B–D to clearly depict the effects of chronic stress and drug treatment. CVS did not affect food intake within the control (B) or GLP-1 (C) treated animals, but decreased intake in dHG-exendin treated animals (D). *=p<0.05.
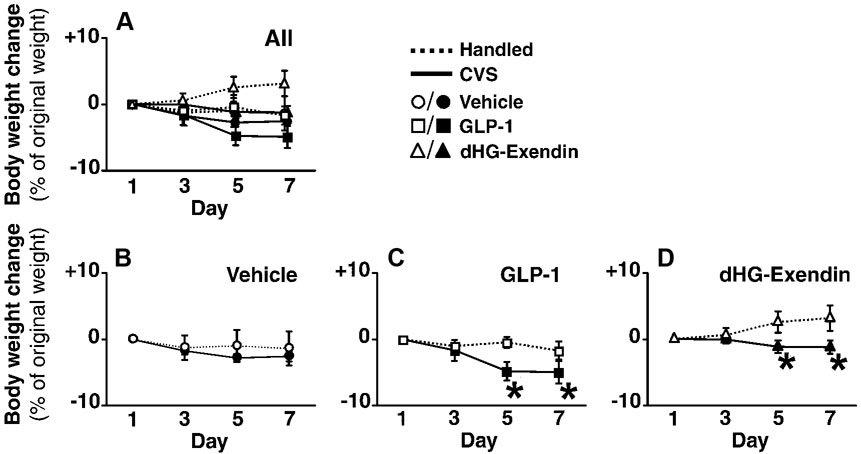
Body weight
Body weight data are presented in Fig. 8. As above, all data are graphed in Fig. 8A; with Fig. 8B–D illustrating comparisons between handled and CVS groups. Body weight increased in handled groups, but decreased in CVS-treated animals (Fig. 8A; broken lines and solid lines, respectively). There were main effects of drug, CVS, and time. Significant interactions were found between drug and time, and CVS and time, but not between drug and CVS.
Figure 8.
Body weight change, presented as % of original weight (mean ±S.E.M., n=4–6). Figure 8A shows body weight change of all 6 groups. Body weight change in each drug treatment groups were compared in figures 8B–D to clearly depict the effects of chronic stress and drug treatment. Stress did not affect the body weight in vehicle treated animals (B), but negatively affected body weight in both GLP-1 (C) and dHG-exendin (D) treated animals. *=p<0.05.
In agreement with the food intake data, dHG-exendin significantly increased body weight, whereas no differences were evident between vehicle and GLP-1 groups (Fig. 8D). On the other hand, within the CVS groups, GLP-1 significantly decreased body weight relative to controls, whereas there was no difference between vehicle and dHG-exendin groups (Fig. 8C).
When handled and CVS groups in each drug treatment were compared, there was no effect of stress on the vehicle-treated group (Fig. 8B). However, GLP-1 decreased body weight in the handled CVS group, whereas dHG-exendin increased body weight in handled controls (Fig. 8C,D).
Discussion
Our results indicate that GLP-1 is involved in chronic stress-induced facilitation of corticosterone responses to a novel stressor. Chronic GLP-1 significantly decreased both body weight and resting glucose levels in animals exposed to chronic stress, suggesting that elevated GLP-1 activity may amplify the effects of chronic stress on the organism. However, central GLP-1 administration itself does not precipitate chronic stress-like effects on long-term consequences of HPA hyperactivity, such as adrenal hypertrophy/hyperplasia and stress facilitation. Furthermore, the effects of chronic stress on adrenal weight still occurs in animals undergoing chronic dHG-exendin treatements, suggesting that the exogenous GLP-1 system is not necessary for the development of chronic stress-induced adrenal hypertrophy.
GLP-1 signaling and stress facilitation
Substantial evidence indicates that GLP-1 signaling contributes to endocrine and behavioral responses to stress. GLP-1 immunoreactive axons innervate parvocellular CRH neurons (10, 22, 26, 29). Visceral stress (injection of lithium chloride) causes cellular activation in GLP-1 expressing neurons of the nucleus of the solitary tract (NTS) (25), and a direct activation of central GLP-1 receptors generate HPA axis responses (17, 19). Thus, it is reasonable to hypothesize that chronic GLP-1 infusion would result in enhanced chronic drive of the HPA axis, marked by adrenal hypertrophy associated with cumulative increases in ACTH release, whereas chronic blockade of GLP-1 signal by dHG-exendin would prevent these changes. However, we observed no difference in adrenal weight in GLP-1 treated handled controls, suggesting that chronic GLP-1 administration alone is not sufficient to mimic the pronounced HPA changes associated with CVS models. Furthermore, all CVS groups increased adrenal weight regardless of drug treatment, indicating that GLP-1 antagonist dHG-exendin did not block HPA axis activation associated adrenal hypertrophy elicited by CVS. Finally, resting corticosterone and ACTH were not altered following drug treatment with or without concomitant stress exposure, and one week of drug treatment was not sufficient to alter responses of animals to an acute stressor. Thus, chronic drive of the HPA axis appears to involve brain circuits that complement or replace the acute actions of the GLP-1 system.
The lack of an effect of GLP-1 on CVS-induced adrenal hypertrophy and HPA activity is unlikely to be due to receptor desensitization. Exogenous GLP-1 produced slight but persistent decreases in body weight in CVS rats, indicating that GLP-1 retained efficacy throughout the delivery period. This interpretation is further supported by previous work indicating persistent anorexic effects of high-dose GLP-1 treatment (9).
Although chronic GLP-1 itself does not elicit frank HPA activation, our results from CVS treated animals indicate that GLP-1 signaling interacts with HPA axis facilitation. For example, exposure to chronic stress typically enhances HPA responsiveness to novel, heterotypic stressors in the face of greater glucocorticoid feedback signals (4). In our experiments, chronic administration of GLP-1 enhanced HPA axis facilitation following CVS, resulting in a disproportionately high corticosterone release upon exposure to a novel stressor (restraint). In particular, the peak corticosterone response to stress (t=30) was elevated in the GLP-1-CVS group relative to the initial stress test performed in this group on day 1. Moreover, the cumulative corticosterone response (as denoted by AUC) was significantly increased in this group. Collectively, these data indicate that exogenous GLP-1 is sufficient to enhance responsivity of the HPA axis following stress. It remains to be determined whether conditions that activate endogenous GLP-1 release (i.e., visceral illness (18)) produce similar enhancements in stress facilitation.
Pre-stress injection of the GLP-1 receptor antagonist dHG-exendin attentuated stress-induced facilitation of corticosterone responses to a novel stressor (restraint). Consistent with previous studies of stress facilitation, peak responses are typically increased following novel stress exposure, whereas shut-off occurs on the normal timescale (3). Following dHG-exendin treatment, CVS failed to increase peak responsiveness (30 min time-point). Thus, antagonism of endogenous GLP-1 receptors was sufficient to inhibit CVS-induced facilitation of the peak corticosterone response.
Chronic stress-induced facilitation of corticosterone release was not accompanied by a facilitated ACTH response. There are two possible explanations for this dissociation. First, the protocol used here is optimized for measuring corticosterone; peak ACTH release typically occurs at 10–20 minutes after stress onset. Indeed, previous reports indicate that facilitation of ACTH release is particularly robust at 15 minutes post-stress (3), significantly earlier than the 30 min time point measured in the current study. Given the differences in time-to-peak, it is not unusual to see such dissociations between ACTH and corticosterone, and it is possible that facilitation of ACTH release may have been missed in this analysis. Second, facilitation of the corticosterone response may be associated with chronic-stress induced enhancement of sympathetic nervous system activity, which may be sufficient to enhance adrenal sensitivity to ACTH (14). In support of this possibility, it is known that acute central GLP-1 administration activates sympathetic preganglionic neurons, increasing heart rate and blood pressure (39). These neurons receive direct projections from GLP-1-synthesizing cell groups in the brainstem and GLP-1-receptive hypothalamic neurons, consistent with a role of endogenous GLP-1 in autonomic activation (13, 39). Thus, the facilitatory effect of GLP-1 on stress responses may be also mediated by enhancement of adrenal sensitivity to ACTH by chronic intermittent activation of autonomic nervous system.
Overall, the role of GLP-1 in chronic stress appears to be manifest following novel stress exposure, implying interactions with circuits modulating stress facilitation. Notably, GLP-1 fibers and GLP-1 receptors are localized in the thalamic paraventricular nucleus (15, 22), which is critical for stress facilitation (3). Thus, while the GLP-1 system is not responsible for enhancement of resting HPA axis activity following chronic stress, it is well-positioned to tune responsiveness to new stressors in the wake of prolonged stress exposure. Stress facilitation is an important biological process that insures that the HPA axis can respond to new stressors despite elevations in glucocorticoids and alterations in endocrine and immune function (1). The overshoot of the HPA axis response may contribute to behavioral or endocrine abnormalities seen in stress related disease states such as depression, symptoms of which are modeled by chronic variable stress regimens (e.g., anhedonia, helplessness, sleep disturbances) (5, 11, 38).
The failure to observe facilitation of corticosterone responses in the dHG-exendin group occurs within the context of higher pre-test corticosterone values relative to the GLP-1 and vehicle group. This necessitates consideration of the possibility that the lack of observable facilitation is due to a ‘ceiling effect’. We consider this possibility highly unlikely. First, the values obtained in the corticosterone and ACTH assays lie within the recent historical range of values observed in our lab following restraint (7, 35), and are considerably lower in magnitude and duration than values obtained following other stressors, such as lithium chloride injection or hypoxia (17, 23). Second, in other recent studies, we have observed CVS-induced facilitation of corticosterone responses to levels substantially above peak values observed in the current experiments (35). Finally, any samples with values falling outside of the linear range of the assay are re-diluted and rerun so as to read on the standard curve, thereby eliminating the possibility of ceiling effects secondary to assay saturation. Thus, our accumulated data suggest that restraint is in the ‘mid-range’ of stressor intensity, at least in terms of HPA axis responses, and hence it is unlikely that the capacity for corticosterone release is saturated in this experimental model.
GLP-1 regulates CVS-induced anorexia and bodyweight
The anorectic properties of GLP-1 are well documented (30, 33). In addition, high dose of central GLP-1 injection can generate visceral illness and cause conditioned taste aversions, which can also suppress food intake (28, 32). Therefore, food intake and body weight were monitored to allow assessment of possible metabolic changes in chronically treated rats.
Body weight measures indicated a chronic stress-dependent effect of GLP-1 administration on body weight, manifest only when animals were stressed. Thus, chronic twice-daily GLP-1 delivery did not produce a marked anorexia. Importantly, the body weight change was not accompanied by reduced food intake, suggesting that GLP-1 amplifies the effect of CVS on body weight by modulating energy expenditure. In contrast, dHG-exendin increased both food intake and body weight over time, but only in 18 unstressed animals. These data indicate that blockade of the endogenous GLP-1 increases food intake and body weight, consistent with the hypothesis that the endogenous GLP-1 system regulates energy balance. However, chronic stress was still able to reduce food intake in dHG-exendin-treated animals, suggesting that CVS-induced weight loss is mediated by factors other than (or in addition to) GLP-1.
Stress, GLP-1, and Glucose Homeostasis
Since central GLP-1 can regulate insulin release and blood glucose, we also measured plasma glucose levels. Time-course analysis revealed that acute stress elicited large increases in blood glucose that were not different between the various treatment groups. However, basal blood glucose levels were significantly decreased following chronic stress, an effect that was most pronounced in GLP-1 treated rats.
Several studies indicate that stress modulates peripheral energy homeostasis. It is well known that plasma glucose can be increased by acute stress or glucocorticoid exposure, presumably through activation of sympathoadrenal system (6, 16) Glucocorticoid infusion also increases metabolic rate in human (6, 31). However, the effect of chronic stress on blood glucose level is poorly understood. Previous work has shown that repeated restraint stress increases basal energy expenditure in rats (20). Therefore, stress-induced increases in daily energy expenditure may explain lower basal glucose availability observed in the current study. It is also possible that the drop in basal glucose is a reaction to glucose responses experienced following each stressor; thus, reduced glucose may either compensate for the cumulative exposure to glucose, or alternatively, occur in anticipation of the initiation of another stress response.
The plasma glucose data indicate that the effects of GLP-1 and dHG-exendin on stress facilitation, food intake and body weight are not related to frank glucose dyshomeostasis in any simple way.
Conclusion
Overall, our data indicate that central GLP-1 neurons act within the context of chronic stress to modify novel stress-induced facilitation of corticosterone release and body weight. Endogenous GLP-1 is involved in stress-induced facilitation of the HPA axis, but is not responsible for generating tonic changes in HPA secretion and consequent effects on stress-sensitive organ systems, such as the adrenal. Therefore, it is likely that the GLP-1 system is involved in central pathways responsible for adjusting HPA sensitivity to chronic stress. The fact that GLP-1 can augment stress-induced weight loss implies that the GLP-1 system may be involved in multiple aspects of central stress integration, including alterations in energy homeostasis
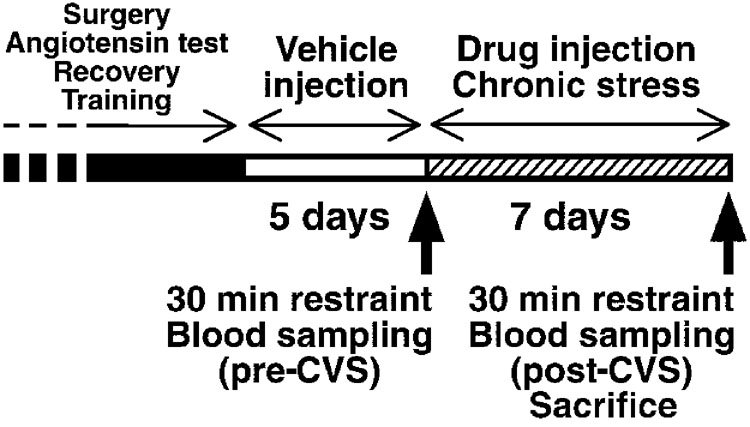
Figure 1.
Experimental design. Pre- and post-CVS acute restraint tests were carried out in the morning of on day 1 (prior to the first stress) and day 8 of CVS/handling, respectively.
Acknowledgments
The authors acknowledge the expert technical assistance of Ben Packard, Kenneth Jones and Amanda Robertson. This work was supported by MH069680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 2.Antoni FA. Hypothalamic control of adrenocorticotropin secretion: Advances since the discovery of 41-residue corticotropin-releasing factor. Endocrine Rev. 1986;7:351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 5.Bielajew C, Konkle AT, Kentner AC, Baker SL, Stewart A, Hutchins AA, Santa-Maria Barbagallo L, Fouriezos G. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–280. doi: 10.1080/10253890310001602829. [DOI] [PubMed] [Google Scholar]
- 6.Brillon DJ, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol. 1995;268:E501–E513. doi: 10.1152/ajpendo.1995.268.3.E501. [DOI] [PubMed] [Google Scholar]
- 7.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputsthalamic-pituitary-adrenal axis activity: implications. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis HR, Jr, Mullins DE, Pines JM, Hoos LM, France CF, Compton DS, Graziano MP, Sybertz EJ, Strader CD, Van Heek M. Effect of chronic central administration of glucagon-like peptide-1 (7–36) amide on food consumption and body weight in normal and obese rats. Obes Res. 1998;6:147–156. doi: 10.1002/j.1550-8528.1998.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ. Glucagon and the glucagon-like peptides. Pancreas. 1990;5:484–488. doi: 10.1097/00006676-199007000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Gronli J, Murison R, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Chronic mild stress affects sucrose intake and sleep in rats. Behav Brain Res. 2004;150:139–147. doi: 10.1016/S0166-4328(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118:33–38. doi: 10.1016/j.regpep.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology. 1994;59:97–109. doi: 10.1159/000126645. [DOI] [PubMed] [Google Scholar]
- 15.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund Pk. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271`:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 16.Kai K, Morimoto I, Morita E, Okada Y, Yamamoto S, Kanda K, Uriu K, Eto S. Environmental stress modifies glycemic control and diabetes onset in type 2 diabetes prone Otsuka Long Evans Tokushima Fatty (OLETF) rats. Physiol Behav. 2000;68:445–452. doi: 10.1016/s0031-9384(99)00187-0. [DOI] [PubMed] [Google Scholar]
- 17.Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 20.Laugero KD, Moberg GP. Energetic response to repeated restraint stress in rapidly growing mice. Am J Physiol Endocrinol Metab. 2000;279:E33–E43. doi: 10.1152/ajpendo.2000.279.1.E33. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 22.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]
- 25.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropinreleasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 27.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog. Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 28.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- 30.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 31.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 32.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 33.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 34.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 37.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 38.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]