Abstract
In adolescence, high levels of drinking over short episodes (binge drinking) is commonly seen in a proportion of the population. Since adolescence is an important neurodevelopmental period, the effects of binge drinking on brain and behavior has become a significant health concern. However, robust animal models of binge drinking in rats are still being developed and therefore further efforts are needed to optimize paradigms for inducing maximal self-administration of alcohol. In the present experiment, one-hour limited-access self-administration sessions were instituted to model excessive drinking behavior in adolescent and adult Wistar rats. In addition to age, the involvement of sex and phase within the light/dark cycle (i.e., drinking in the light or dark) on sweetened 5% ethanol intake were also evaluated over fourteen limited-access sessions using a between-groups design. The results of the experiment showed that over fourteen limited-access sessions, sweetened ethanol intake (g/kg) was significantly higher for adolescents compared to adults. Females were also found to drink more sweetened ethanol as compared to males. Additionally, drinking in the light produced a robust increase in sweetened ethanol intake (g/kg) in adolescents, as compared to adults during the light phase and as compared to both adolescent and adult rats drinking in the dark. Furthermore, the increase in ethanol consumption observed in adolescents drinking during the light phase was dissociable from sweetened solution intake patterns. These results identify that age, sex, and time of day all significantly influence consumption of sweetened ethanol in Wistar rats. Knowledge of these parameters should be useful for future experiments attempting to evaluate the effects of self-administered ethanol exposure in adult and adolescent rats.
Keywords: Adolescent, Ethanol, Sex Differences, Circadian Rhythms, Limited-Access
Introduction
Adolescence is a critical period of maturation involving a host of physiological as well as behavioral changes. From a neurobehavioral standpoint, these changes are not limited to, but primarily involve the development of cognitive, emotional, hormonal and motivational systems (Chambers et al., 2003; Yurgelun-Todd, 2007); components of which are susceptible to the effects of alcohol (Smith, 2003; Spear, 2000; Witt, 1994). In 2004, 17.7% of adolescents (age 12 – 17) were classified as current users of alcohol, with 6% designated as alcohol abusers or alcohol dependent (U.S.Department of Health and Human Services, 2005). Taking into consideration evidence suggesting that early initial alcohol use is predictive of alcohol abuse and dependence in adults (Grant and Dawson, 1997), the current use statistics for adolescent alcohol consumption identifies the necessity for further research in this area. Consequently, developing animal models of adolescent alcohol use is of primary importance in order to study the consequences and impact of alcohol exposure during this critical developmental period at the behavioral, neurobiological, molecular and genetic levels (McBride et al., 2005; Smith, 2003; Spear, 2000; Witt, 1994).
When evaluating the effects of adolescent ethanol exposure, it must be noted that a majority of the previous research has utilized experimenter-delivered as opposed to self-administered ethanol. However, it has been shown that there are distinct differences between the effects of experimenter- and self-administered opiates (Robinson et al., 2002), cocaine (Dworkin et al., 1995; Mark et al., 1999; Wilson et al., 1994), and amphetamine (Stefanski et al., 1999) on the brain and behavior. Some of the differences found between the effects of experimenter- and self-administered drugs of abuse only appear to be restricted to the level of activation (i.e., both methods produce neurochemical changes, but one produces more of a change than the other; (Mark et al., 1999; Robinson et al., 2002), but in some cases, the two administration methods result in a dichotomous response with self-administration producing an effect and experimenter-delivered compounds producing no response (Stefanski et al., 1999; Wilson et al., 1994). This especially seems to be important when evaluating motivational and cognitive systems – systems that have been identified as still undergoing development in adolescence (Chambers et al., 2003; Yurgelun-Todd, 2007). Thus, developing animal models which have a high degree of face validity (i.e., the compound is self-administered) that are conducive to initiating maximal ethanol exposure is critically important to fully evaluate the effects of ethanol exposure on the developing brain and behavior.
Variables that have been previously investigated in adolescent self-administration paradigms include sex, age, ethanol fluid concentration, isolate-housing and use of different sipper-tube types; however, these studies provided 24-hour access to the ethanol solutions (Bell et al., 2006; Brunell and Spear, 2005; Doremus et al., 2005; Ehlers et al., 2007; Fullgrabe et al., 2007; Lancaster et al., 1996; Siciliano and Smith, 2001). In the present experiment, male and female adolescent and adult rats were compared for ethanol self-administration behavior during one hour limited-access sessions, which have been proposed to model binge-like behavior (Martinetti et al., 2006). Additionally, limited-access drinking was evaluated during two time points in the animal’s circadian cycle; with half the animals having access to ethanol solutions in the light phase and the other half access during the dark phase to identify which time point promoted the highest level of alcohol consumption. Using lines of rats selected for ethanol preference, it has been previously shown that there were circadian differences in alcohol consumption (Bell et al., 2006), however this issue has not been systematically studied in non-selected Wistar rats. Therefore, the present study was designed to evaluate the contribution of age, sex, and cycle on sweetened ethanol intake and preference ratios during limited-access self-administration sessions in Wistar rats.
Materials and Methods
Subjects
Sixty male and forty female Wistar rats (Charles River, Wilmington, MA) were used in the present study. For the ethanol consumption portion of the experiments, twenty animals of each sex were adolescents (P23 on arrival) and twenty were adults (P65 - 69 on arrival). An additional adolescent sucrose control group comprised of twenty adolescents (P23 on arrival) was also evaluated. Those animals designated as the experimental group were same-sex pair-housed within a temperature-controlled (21.5 °C) vivarium with half the animals (i.e., ten adolescent and ten adult animals from each sex) being maintained on a 12-hour light/dark cycle (lights on at 6 a.m.) and the other half on a reverse 12- hour light/dark cycle (lights on at 8 p.m.). In addition, half of the animals comprising the sucrose control group were maintained on a standard light cycle whereas the other half were maintained on a reverse light cycle (as described above). Upon arrival, animals were weighed and handled daily. Normal and reverse light-cycle groups were matched for weights based on their arrival weight prior to group assignment. Ad libitum food and water were provided for the duration of the experiment. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Procedure
Male and female adolescent rats were 29 days old and adults were approximately 75 days old at the onset of the experimental procedures. Fluid consumption tests were conducted five days a week, two hours after the onset of the light or dark phase of the light/dark cycle for the light and dark phase drinking groups, respectively. Animals were weighed in the vivarium, transported to the testing room 30 minutes prior to the initiation of the two-bottle choice sessions and transferred to plastic cages [25 (w) × 20 (h) × 45 cm (l)] with wire bar cage tops 10 minutes prior to the start of the session. Solutions were presented using 100 ml graduated cylinders (Nalgene Labware, Rochester, NY) fitted with curved ball-point sipper tubes (Ancare, Bellmore, NY). Animals were given one hour of access to the solutions without food availability, after which the cages were cleaned with 70% ethanol. Following the limited-access sessions, animals were returned to the vivarium. Blood samples (0.5 μl) immediately following ethanol consumption sessions were taken on three separate occasions (the 3rd, 8th and 13th exposure to the final concentration of sweetened ethanol solution) from the tip of the tail to assess blood alcohol levels (BALs). Following centrifugation, plasma alcohol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
Solutions
Two bottles were presented; one always contained water and the other contained a sweetened solution (SS). The sweetened solution was prepared (w/v) using tap water, 0.125% saccharin (Sigma Chemical Co., St. Louis, MO) and 3% sucrose (C & H sugar, Crockett, CA). 95% ethanol (Gold Shield Chemicals; Hayward, CA) was added to the sweetened solution until the appropriate concentration (w/v) was reached. The order and number of consumption sessions was as follows: SS (2 days), SS + 1% ethanol (1 day), SS + 2.5% ethanol (1 day) and SS + 5% ethanol (16 days). Once the final sweetened ethanol concentration (i.e., SS + 5% ethanol) was reached, the animals were given two days to habituate to the SS + 5% ethanol solution followed by a 14-day exposure period. Bottle position alternated daily to avoid position preference. Food was not available during the 1 hour limited-access session. It must be noted that when the fluid consumption sessions initiated, the animals would have had 6 days to adjust to their respective light cycle. However, at the beginning of the 14-day exposure period, the animals would have had 2 weeks to acclimate and allow for shifts in their circadian cycle.
The adolescent SS control groups were exposed to an identical schedule of limited-access consumption sessions as described for the ethanol consumption groups. However, in contrast to the ethanol groups, the SS (0.125% saccharin and 3% glucose) was presented for the entire experiment, along with water as described above.
Data Analysis
Ethanol intake adjusted for weight (grams of ethanol / kilograms of body weight; g/kg), water consumption and ethanol preference (i.e., amount of ethanol consumed / total amount of solution consumed) were analyzed using a four-way mixed-model analysis of variance (ANOVA). The between-subjects factors were light cycle, age and sex and the within-subject factor was session in which ethanol intake (g/kg), water consumption or ethanol preference were evaluated. Post-hoc independent sample t-tests were used to compare individual group performance if an interaction was identified with the ANOVA. To minimize the probability of a
Type I error occurring due to multiple comparisons, a Bonferroni correction was used in which the α = 0.05 was divided by the number of post-hoc comparisons to establish a corrected α. SS consumption adjusted for body weight (g/kg) was analyzed using a two-way mixed model ANOVA with session as the within-subject factor and cycle as the between subjects factor. Pearson correlation tests were used to assess the relationship between ethanol intake (g/kg) and BALs.
Results
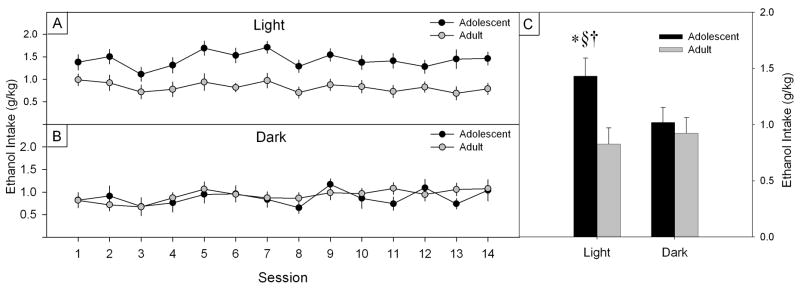
As illustrated in Figure 1, evaluation of ethanol intake (g/kg) using the three-way ANOVA identified main effects of age (F (1, 72) = 30.413, p < 0.001), sex (F (1, 72) = 10.717, p < 0.01) and light cycle (F (1, 72) = 6.242, p < 0.05), and as seen in Figure 2, an Age x Cycle interaction (F (1, 72) = 16.188, p < 0.001). Independent sample t-tests confirmed that ethanol intake for adolescent animals in the light was significantly higher than that for the adults in the light (t (22) = 11.90, p < 0.001), and higher than both the adolescent and the adult ethanol consumption in the dark (t (26) = 9.31, p < 0.001 and t (26) = 9.16, p < 0.001, respectively).
Figure 1.
Mean (± S.E.M.) ethanol intake (g/kg) from the fourteen one-hour limited-access sessions for adolescent and adult (A), male and female (B) and light or dark phase (C) Wistar rats. Inset of each figure corresponds to the mean (+S.E.M.) ethanol intake (g/kg) of the fourteen sessions (*** = p < 0.001, ** = p < 0.01 and * = p < 0.05).
Figure 2.
Left panel: Mean (±S.E.M.) ethanol intake (g/kg) for adolescent and adult Wistar rats during the fourteen one-hour limited-access light (A) or dark (B) phase consumption sessions. Right panel (C): Mean (+ S.E.M.) ethanol intake of the fourteen sessions for adolescent and adult rats drinking during the light or dark phase of the light/dark cycle (* = p < 0.001 when compared to Adult/Light, § = p < 0.001 when compared to Adolescent/Dark, and † = p < 0.001 when compared to Adult/Dark groups).
Sweetened solution consumption data for adolescent rats in displayed in Figure 3. The two-way ANOVA conducted to evaluate control SS consumption (g/kg) during the light or dark in adolescent rats identified a within-subject main effect of session (F (13, 234) = 2.875, p < 0.001), indicating that consumption for changed over time. The between-groups analysis showed no effect of cycle on SS consumption (F (1, 18) = 4.692, p > 0.05.
Figure 3.
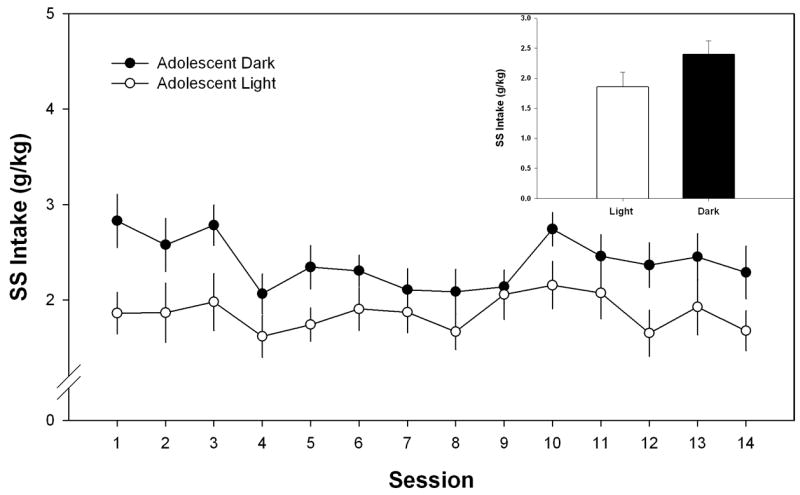
Mean (± S.E.M.) sweetened solution (SS) intake (g/kg) from the fourteen one-hour limited-access sessions for adolescent Wistar rats during the light or dark phase of their circadian cycle. Inset of each figure corresponds to the mean (+S.E.M.) SS intake (g/kg) of the fourteen sessions.
When evaluating water consumption using the ANOVA, as displayed in Table 1, the between-subjects analysis, identified a main effect of Age (F (1, 72) = 22.682, p < 0.001), with adults consuming more water than adolescents, and Cycle (F (1, 72) = 18.743, p < 0.001) in which water consumption was higher during the dark cycle.
Table 1.
Average ethanol solution intake, water intake and ethanol solution preference ratios of the 14 one-hour limited-access consumption sessions that occurred during the light or dark cycles for adolescent and adult male and female Wistar rats. 5E + SS = sweetened 5% ethanol (w/v) solution. Data are the mean ± SEM (
| Adolescent | Adult | Male | Female | Light | Dark | |
|---|---|---|---|---|---|---|
| 5E + SS preference (%) | 83 ± 3.4* | 74 ± 4.8 | 79 ± 4 | 78 ± 4.2 | 84 ± 3.4† | 73 ± 4.8 |
| 5E + SS intake (mls) | 5.1 ± 0.5 | 5.5 ± 0.7 | 6.0 ± 0.6 | 4.7 ± 0.6 | 5.5 ± 0.6 | 5.1 ± 0.6 |
| Water intake (mls) | 1.0 ± 0.5 | 1.9 ± 0.4 | 1.6 ± 0.3 | 1.3 ± 0.2 | 1.05 ± 0.2 | 1.9 ± 0.4 |
= p ≤ 0.001 when compared to Adult and
= p < 0.001 when compared to the Dark groups).
Ethanol preferences are displayed in Table 1. The ANOVA identified a main effect of age (F (1, 72) = 11.734, p = 0.001) and cycle (F (1, 72) = 14.447, p < 0.001), as well as a marginal Age x Cycle interaction (F (1, 72) = 3.956, p = 0.051). The average preference ratios for the adolescents and adults during either the light or dark phase are presented in Table 2.
Table 2.
Average ethanol (EtOH) intake, water intake and ethanol preference ratios of the 14 light or dark cycle one-hour limited-access consumption sessions for adolescent and adult Wistar rats. 5E + SS = sweetened 5% ethanol (w/v) solution. Data are the mean ± SEM.
| Adolescent/Light | Adult/Light | Adolescent/Dark | Adult/Dark | |
|---|---|---|---|---|
| 5E + SS preference (%) | 90 ± 2.6 | 75 ± 4.2 | 75 ± 4.3 | 71.12 ± 5.3 |
| 5E + SS intake (mls) | 6.0 ± 0.6 | 5.1 ± 0.6 | 4.3 ± 0.5 | 5.9 ± 0.7 |
| Water intake (mls) | 0.7 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.3 | 2.36 ± 0.5 |
The relationship between ethanol intake (g/kg) and BALs (n = 240) was assessed using Pearson correlation tests. As illustrated in Figure 4, the Pearson test identified highly significant positive correlations between the BALs and g/kg of ethanol for all the groups when analyzed individually or in unison (overall trendline: y = 47.523x − 2.2177, r = .76, p < 0.001).
Figure 4.
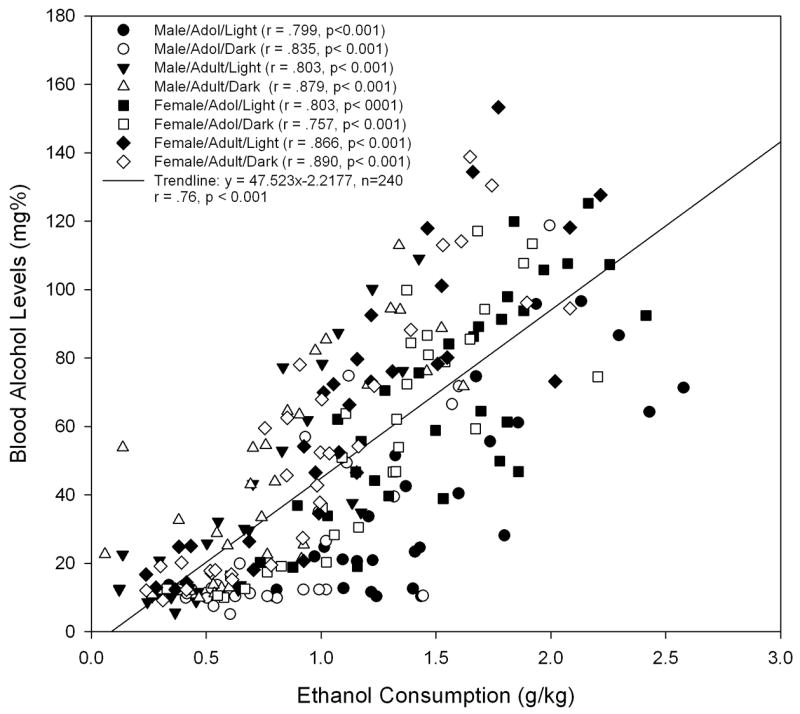
The BALs for separate groups of Wistar rats were collected on three occasions and plotted against ethanol intake (g/kg) for that session. Significant associations were found for not only the individual groups (n = 30, ps < 0.001), but also the entire sample (n = 240, p < 0.001).
Discussion
In the present experiment, ethanol intake was measured during the light or dark phase of the 24-hour cycle using a limited-access consumption model in male and female adolescent and adult Wistar rats. Ethanol intake (g/kg) over the fourteen sessions was consistently higher for adolescent animals when compared to adults. These data are in accordance to earlier findings showing that adolescent rats consumed more alcohol during 24-hour access sessions (Brunell and Spear, 2005; Doremus et al., 2005; Lancaster et al., 1996; Yoshimoto et al., 2002). However, others have found that increased intake is only observed when female adolescents are compared to adults and not between male adults and adolescents (Siciliano and Smith, 2001). Considering that adolescent animals have been shown to have lower serum ethanol concentrations that last for a shorter period of time when compared to older animals (Little et al., 1996), it is possible that differences in the metabolic clearance of ethanol contributed to the dissociable consumption behavior observed between adolescents and adults in the present experiment. However, other evidence suggests that elimination rates have are comparable for animals in early adolescence and early adulthood (Silveri and Spear, 2000). It must also be noted that adolescent rats had a higher ethanol solution preference ratio (see Table 1) in the present study, which could be indicative of decreased sensitivity to the aversive properties of ethanol and could be driving the increased consumption as is thought to be the case in alcohol-preferring (Froehlich et al., 1988) and Sardinian alcohol-preferring (Brunetti et al., 2002) lines of rats. However, it must be noted that alcohol-preferring rats have been shown to be more sensitive to the reinforcing properties of ethanol than Wistar rats in that smaller doses of intracranial self-administered ethanol are able to support self-administration behavior in the alcohol-preferring line (Rodd et al., 2004), suggesting that taste reactivity is not the only factor involved in the alcohol-preferring strains propensity to self-administer ethanol.
Female rats consumed more ethanol (g/kg) than males over the fourteen limited-access consumption sessions. These results are also consistent with previous reports of female versus male ethanol consumption during long-access sessions (Adams, 1995; Almeida et al., 1998; Juarez and de Tomasi, 1999; Lancaster et al., 1996; Lancaster and Spiegel, 1992). The increased consumption of ethanol observed in the present study could be attributable to sex differences in the rate of ethanol elimination. In contrast to human alcohol dehydrogenase profiles, female rats have been shown to have higher gastric levels of the enzyme (Mezey et al., 1992) which could metabolize ethanol faster and require more ethanol consumption to reach a preferred level of reinforcement. However, this hypothesis is not consistent with the fact that female rats have been shown to have higher BALs compared to males following intraperitoneal ethanol administration (Rivier, 1993). In the present study, under limited-access conditions, females and males did not show differential preference for ethanol solutions.
There were also differences between ethanol consumption depending on whether animals had the limited-access sessions during the light or dark phases of their light/dark cycles. Levels of drinking in the light were found to be consistently higher than drinking in the dark. Preferences for the ethanol solution were also higher during the light phase. Recently a drinking in the dark model (DID) has been proposed as a method to produce robust ethanol consumption in selected strains of mice (Kamdar et al., 2007; Rhodes et al., 2007) and alcohol-preferring rats (Bell et al., 2006). In the present study, however, it has clearly been shown that ethanol intake (g/kg) is enhanced during the light phase in Wistar rats. In both the rat and mouse DID models, the ethanol solutions are unsweetened, which contrasts with the sweetened solution used in the present study. However, in regards to developing self-administration models of adolescent ethanol exposure, a primary goal is to induce high quantities of ethanol intake in order to evaluate the impact of such exposure on the brain and behavior. Thus, in terms of maximal intake, the beginning of the light phase appears to be the most conducive for Wistar rats under these experimental conditions.
The most dramatic, and perhaps the most interesting, finding in the present study is the Cycle x Age interaction that was observed for ethanol intake (g/kg) which was found to be the highest in adolescent animals that were allowed to self-administer ethanol solutions during the light phase of the light/dark cycle. Not only was ethanol intake (g/kg) higher in the light phase for adolescent animals compared to adults drinking during the light phase, but it was higher than both adolescent and adults drinking during the dark phase as well. In addition, there was a trend towards an increased preference for ethanol in adolescents drinking during the light phase of their light/dark cycle. While the exact factors underlying the increase in consumption for adolescent animals remain to be established, increased exploratory behavior during the light phase has also been reported for adolescent Wistar rats when compared to adults (Andrade et al., 2003). There is the possibility that the adolescent animals could be more sensitive to the 10 minute isolation period prior to the onset of consumption sessions, however, one would expect both adolescent groups to be affected if that were the case. The increased light phase drinking behavior in adolescents also appears to be in accordance with clinical circadian cycle evidence suggesting that adolescents have shifts in their sleep/wake cycle with sleep coming later for adolescents (Andrade et al., 1992; Carskadon, 1990; Carskadon et al., 1993).
However, one could argue that the increased sweetened ethanol consumption could actually be attributable to altered SS intake patterns as opposed to ethanol consumption patterns. To that effect, an adolescent control condition was included to exclusively assess SS intake patterns in adolescent animals exposed to limited-access sessions during their light or dark cycle. It was shown that there were statistically no differences between light and dark SS consumption in male adolescent Wistar rats, although the dark cycle group consistently consumed more of the SS solution than the light cycle group. Therefore, the increased ethanol consumption observed for adolescent animals during the light did not seem to be related to patterns of SS intake.
In humans, the criteria for binge drinking has recently been defined as achieving a BAL of .08 g% within a 2-hour time period (NIAAA Advisory Council, 2004). When trying to model this behavior in animals, the ideal goal would be to approach comparable BALs within a similar time frame. Considering that the average intake of adolescent animals drinking during the light was approximately 1.5 g/kg over a one-hour period of time, if one extrapolates using the data from Figure 4, the average consumption of adolescents would yield a BAL of approximately .07 g%. Thus, the BALs achieved in adolescent animals in the present study are close to the definition of a binge in humans, although further work is needed to try and more closely model the human condition.
In summary, ethanol intake was evaluated in adolescent and adult male and female Wistar rats given exposure to a sweetened 5% ethanol solution during either the light or dark phases of their light/dark cycle. It was shown that adolescents consumed more than adults, females had higher intake than males and that ethanol consumption was increased during the light compared to the dark phase of their 24-hour cycle. Most importantly for developing animal models of adolescent ethanol exposure, adolescent animals had higher ethanol intake (g/kg) when limited-access sessions occurred during the light phase of their light/dark cycle. It was further shown that the increased sweetened ethanol intake observed during the light cycle in adolescent rats was dissociable from the sweetened solution intake itself. Therefore, this model could prove useful to delineate the effects of ethanol exposure on the developing adolescent brain and behavior and to determine if altered neuroadaptations induced by adolescent ethanol exposure persist into adulthood.
Acknowledgments
Support for this research was provided by a National Institute on Alcohol Abuse and Alcoholism grant awarded to CLE (AA014339).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. doi: 10.1016/0741-8329(95)00032-m. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MM, edito-Silva AA, Menna-Barreto L. Correlations between morningness-eveningness character, sleep habits and temperature rhythm in adolescents. Braz J Med Biol Res. 1992;25:835–839. [PubMed] [Google Scholar]
- Andrade MM, Tome MF, Santiago ES, Lucia-Santos A, de Andrade TG. Longitudinal study of daily variation of rats’ behavior in the elevated plus-maze. Physiol Behav. 2003;78:125–133. doi: 10.1016/s0031-9384(02)00941-1. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Brunetti G, Carai MA, Lobina C, Melis S, Serra S, Vacca G, Gessa GL, Colombo G. Differences in ethanol-induced conditioned taste aversion in Sardinian alcohol-preferring and Sardinian alcohol-nonpreferring rats. Alcohol. 2002;26:167–172. doi: 10.1016/s0741-8329(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Juarez J, de Tomasi B. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG. Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, nonpreferring, and Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:836–843. doi: 10.1111/j.1530-0277.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Mezey E, Sharma S, Rennie L, Potter JJ. Sex differences in gastric alcohol dehydrogenase activity in Sprague-Dawley rats. Gastroenterology. 1992;103:1804–1810. doi: 10.1016/0016-5085(92)91438-a. [DOI] [PubMed] [Google Scholar]
- NIAAA Advisory Council. NIAAA Council approves definition of binge drinking. NIAAA Newsletter. 2004;3:5. [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav. 2001;74:637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. Modeling Adolescent Development and Alcohol Use in Animals. Alcohol Research and Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- U.S.Department of Health and Human Services. National Survey on Drug Use and Health, 2004. Washington, DC: 2005. [Google Scholar]
- Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcohol Clin Exp Res. 2002;26:63S–65S. doi: 10.1097/01.ALC.0000026977.19902.61. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007 doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]