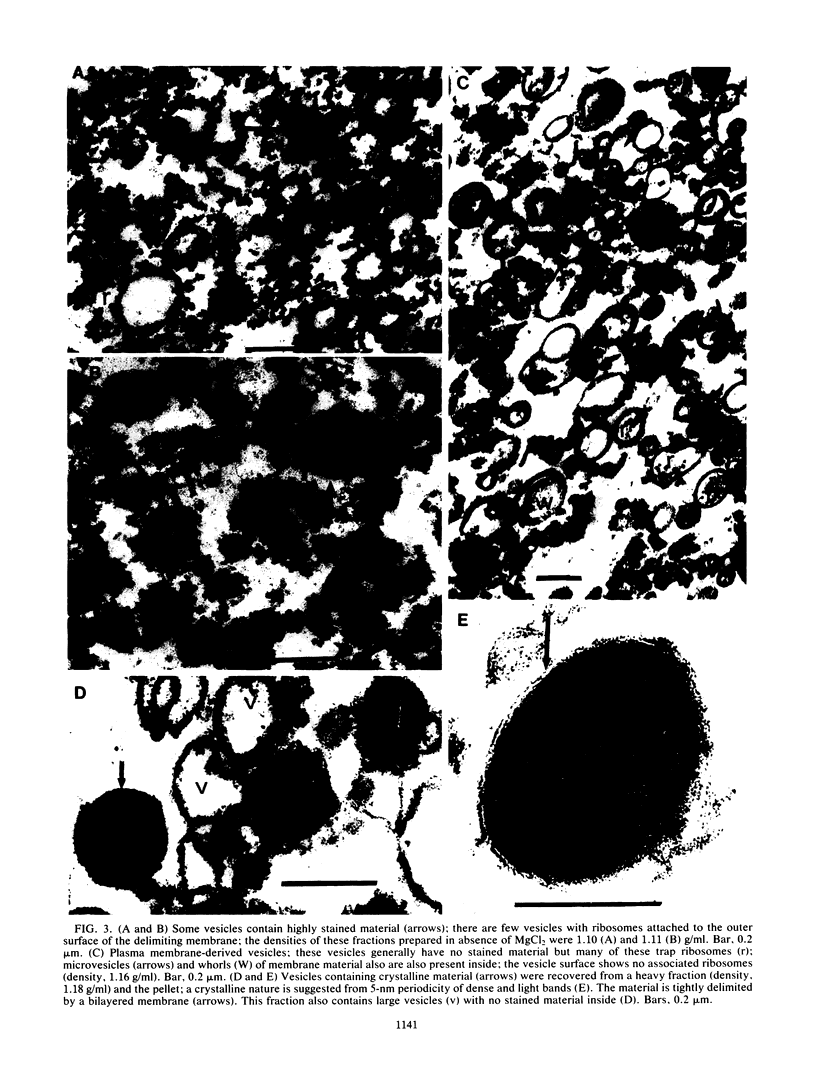
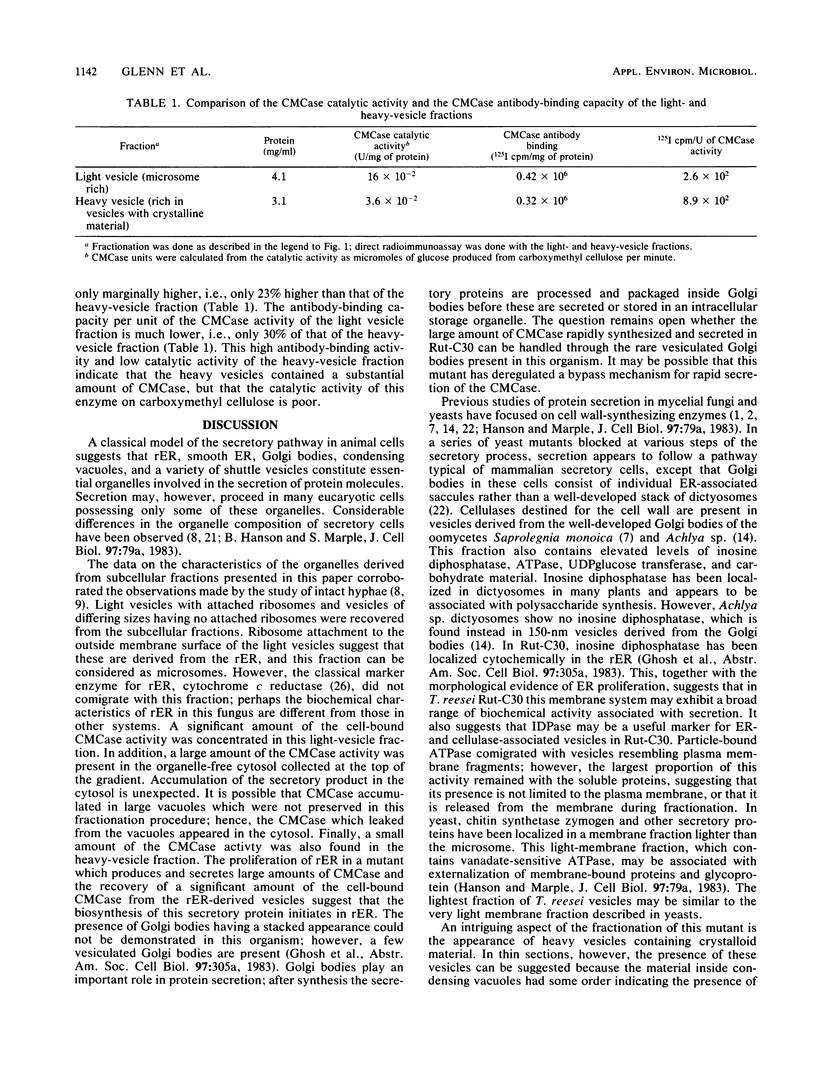
Abstract
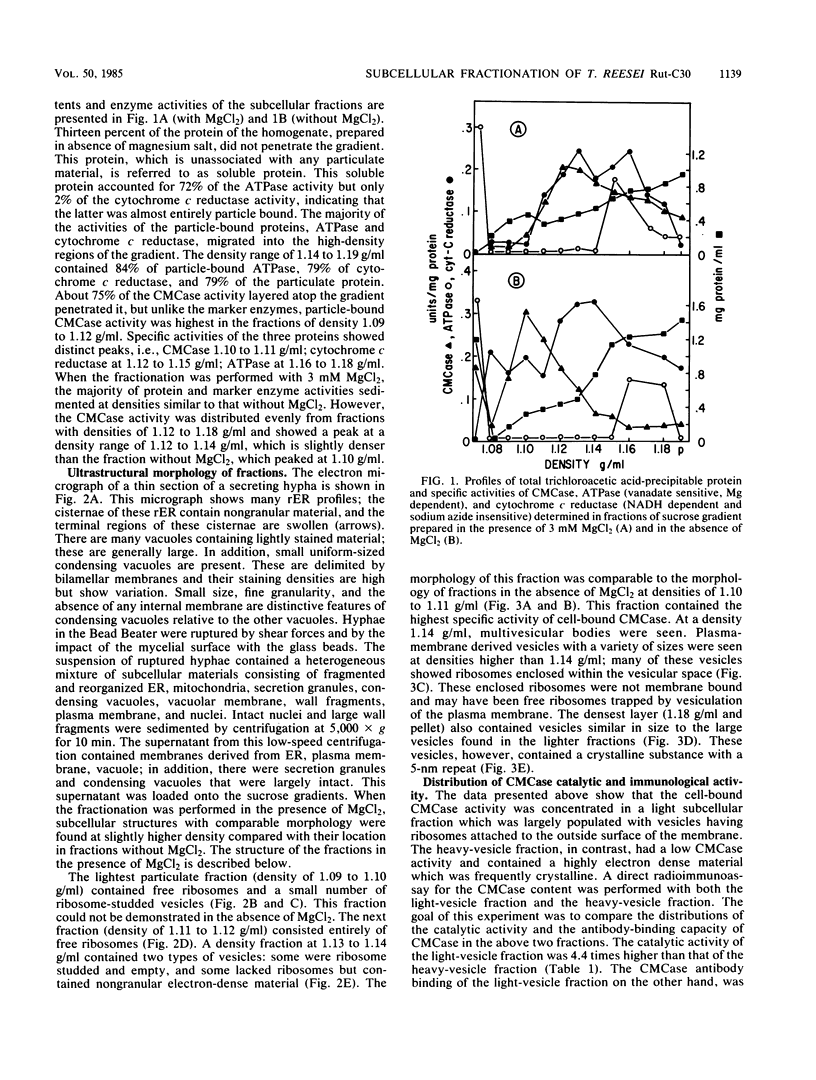
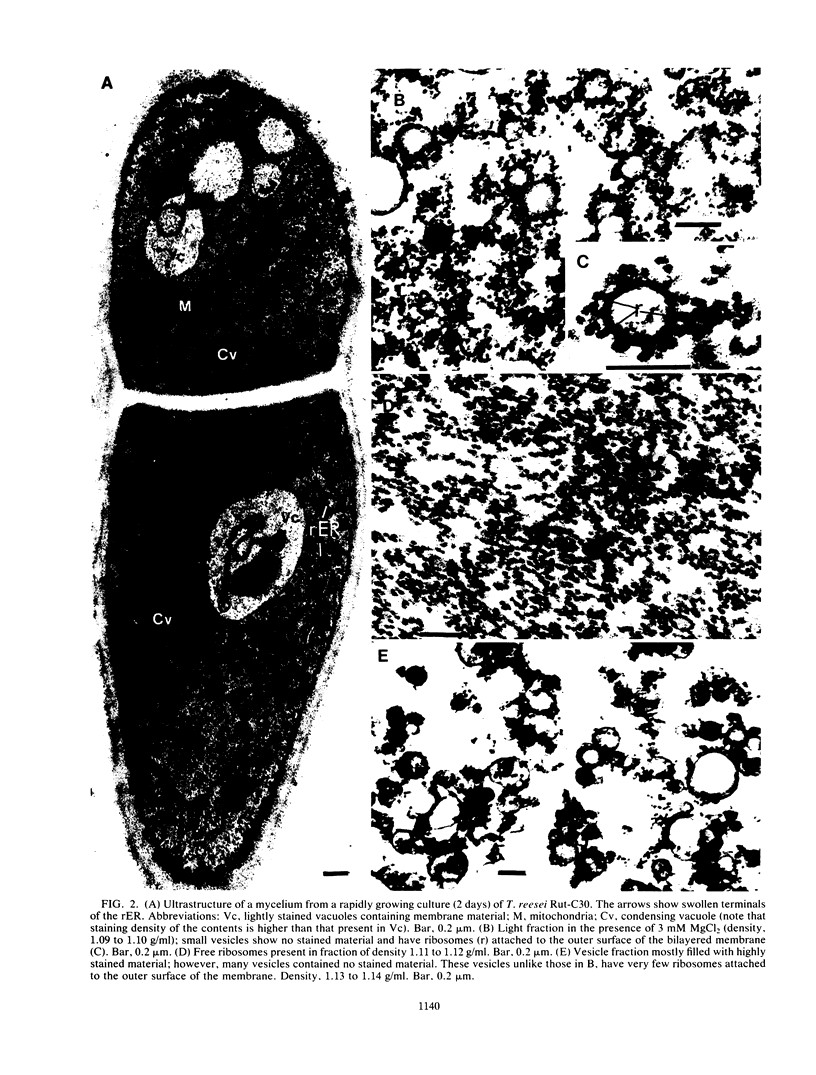
The growing mycelia of Trichoderma reesei Rut-C30 are richly endowed with endoplasmic reticula and a variety of pleomorphic subcellular bodies. Mycelia of the culture growing in presence of avicel pH101 was fractionated in sucrose density gradients, and several morphologically and biochemically distinct fractions were isolated. Mycelia were homogenized in a Bead Beater, and the homogenate was freed of nucleus and wall fragments by low-speed centrifugation before fractionation. Organelle-free cytosol, which did not penetrate the gradient, contained (of the total) 72% of the vanadate-sensitive ATPase, 26% of carboxymethyl cellulase (CMCase), 2% of cytochrome c reductase, and 13% of the protein. Significant fractions separated on a gradient were light vesicles containing heavily stained material inside and ribosomes attached to the outside surface, intact vesicles resembling condensing vacuoles, large vesicles derived from the plasma membrane, and heavy vesicles containing crystalline material. The light-vesicle fraction contained a large portion of the cell-bound CMCase activity. The particle-bound ATPase and cytochrome c reductase activities were concentrated in heavy fractions. The fractionation in the presence of MgCl2 improved the preservation of subcellular bodies derived from the endoplasmic reticula. Although the CMCase activity of the light-vesicle fraction was 4 times higher than the activity in the heavy-vesicle fraction, the CMCase antibody-binding capacities of both fractions were about the same. This discrepancy between the catalytic activity and the antibody-binding capacity suggests that the heavy vesicles might have contained considerable amount of inactive CMCase compared with that present in the light vesicles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeson C. E., Bowman B. J. Isolation and characterization of the Neurospora crassa endoplasmic reticulum. J Bacteriol. 1983 Oct;156(1):362–368. doi: 10.1128/jb.156.1.362-368.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J., Slayman C. W. Isolation and characterization of plasma membranes from wild type Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12336–12342. [PubMed] [Google Scholar]
- Eveleigh D. E., Montenecourt B. S. Increasing yields of extracellular enzymes. Adv Appl Microbiol. 1979;25:57–74. doi: 10.1016/s0065-2164(08)70146-1. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hill T. W., Mullins J. T. Hyphal tip growth in Achlya. II. Subcellular localization of cellulase and associated enzymes. Can J Microbiol. 1980 Sep;26(9):1141–1146. doi: 10.1139/m80-188. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 1976;(6):21–33. [PubMed] [Google Scholar]
- Marriott M. S. Enzymic activity of purified plasma membranes from the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1975 Aug;89(2):345–352. doi: 10.1099/00221287-89-2-345. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Ovtracht L. Dynamics of the Golgi apparatus: membrane differentiation and membrane flow. Int Rev Cytol Suppl. 1977;(5):61–188. [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Osumi M., Nagano M., Yamada N., Hosoi J., Yanagida M. Three-dimensional structure of the crystalloid in the microbody of Kloeckera sp.: composite crystal model. J Bacteriol. 1982 Jul;151(1):376–383. doi: 10.1128/jb.151.1.376-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]