Abstract
Neuropsychiatric behaviors are common in people with Alzheimer’s disease (AD) and make both professional and lay caregiving difficult. Light therapy has been somewhat successful in ameliorating disruptive behaviors. This randomized trial tested the effects of morning or afternoon bright light exposure compared with usual indoor light on the presence, frequency, severity, and occupational disruptiveness of neuropsychiatric behaviors in nursing home residents with AD. Light was administered for 1 hr daily (Monday–Friday) for 10 weeks. The Neuropsychiatric Inventory–Nursing Home was used to assess behavior at baseline and end of the intervention. Analyses revealed statistically significant differences between groups on agitation/aggression, depression/dysphoria, aberrant motor behavior, and appetite/eating disorders. The magnitude of change was small and may not represent clinically significant findings. Agitation/aggression and nighttime behaviors commonly occurred and were highly correlated with occupational disruptiveness. Interventions that decrease the presence and/or severity of neuropsychiatric behaviors have the potential to significantly decrease caregiver burden.
Keywords: caregivers, dementia, light therapy, NPI-NH, nursing home
Behavioral disturbances occur frequently in patients with dementing illnesses such as Alzheimer’s disease (AD). Neuropsychiatric behaviors manifest as a result of neurological deterioration that underlies the disease process (Förstl, 2000) and are evident in addition to cognitive impairment and change as the disease progresses (Hatoum et al., 2005; Hope et al., 1998; Yaffe et al., 2002). Examples of common behaviors include aggression and irritability, withdrawn or apathetic moods, changes in appetite or eating behaviors, and nighttime behavioral disturbances (Cummings, 1997; Cummings et al., 1994). This study examines the effect of bright light therapy on neuropsychiatric behavior in persons with AD.
Neuropsychiatric Behaviors and Light Therapy
Neuropsychiatric behaviors are difficult for family and caregivers to manage in the community and frequently necessitate admission to a long-term care facility (Cohen-Mansfield, Marx, & Rosenthal, 1990). In the nursing home, these behaviors typically persist and worsen over time; cause significant distress to patients, families, and staff; and adversely affect the patient’s quality of life (Cummings et al., 1994; De Deyn et al., 2005; Mega, Cummings, Fiorello, & Gornbein, 1996). It may be easier to provide nursing care to patients who have socially acceptable behaviors in the institutional setting (Ancoli-Israel, Martin, Kripke, Marler, & Klauber, 2002; Tan, Wong, & Allen, 2005). Disruptive behaviors can lead to use of antipsychotic medications that have the potential for serious side effects, including injury and psychological distress to patients, families, and staff (Ruths, Straand, & Nygaard, 2001).
Light therapy for the treatment of neuropsychiatric symptoms has been studied to some extent. As a treatment for depression in older nondemented hospitalized adults, Tsai, Wong, Juang, and Tsai (2004) recently explored light therapy and demonstrated a significant reduction in depressive symptoms. Elders with dementia frequently exhibit disturbed rest-activity cycles that are associated with abnormal nighttime and daytime behaviors. Light therapy has shown promising results in ameliorating these abnormalities (Hozumi et al., 1990; Satlin, Volicer, Ross, Herz, & Campbell, 1992). Lyketsos, Veiel, Baker, and Steele (1999) found that institutionalized patients with dementia who exhibited agitated behaviors slept more hours at night after morning bright light exposure. Bright light did not improve agitated behaviors in patients who did not exhibit disturbed sleep-wake cycles. Mishima et al. (1994) found that morning light improved sleep and decreased the frequency of behavioral disturbances in dementia patients exhibiting these problems. Ancoli-Israel et al. (2003) found that bright light improved caregivers’ ratings but had little effect on observational ratings of agitation.
In their systematic review of the efficacy of light therapy for behavioral and psychological symptoms of dementia, Skjerve et al. (2004) were unable to draw conclusions about its use and recommended further research. In a similar comprehensive review, Forbes et al. (2004) found inconclusive evidence for the efficacy of light therapy in improving behavioral disturbances in dementia and emphasized the need for further study. This study was conducted to evaluate the effects of bright light therapy on neuropsychiatric behaviors in AD.
Study Purpose
The purpose of this study was to test the effect of timed morning or afternoon bright light exposure compared with usual indoor light levels on the presence, frequency, severity, and occupational disruptiveness of neuropsychiatric behaviors in nursing home residents with AD. Because this was a secondary aim in a larger study also examining the effect of bright light exposure on a number of sleep- and activity-related variables, we did not specify a priori hypotheses about the effect of light or about morning compared with afternoon light on neuropsychiatric behaviors.
Method
Design
This study was a randomized clinical trial designed to compare two experimental groups that received morning or afternoon bright light exposure to a control group that was exposed only to usual indoor light levels. Participants were randomly assigned to one of two experimental groups, one that received morning light (n = 29) or one that received afternoon light (n = 24), or to the control group (n = 17), using a permutated blocking procedure in which the number of patients allocated to each group was forced to be equal after an a priori defined “balancing” number of participants were enrolled in the study (White & Freedman, 1978). Outcome measures were obtained at the end of a baseline week and at the end of the intervention.
Sample
Residents of two large long-term care facilities in San Francisco, California, who experienced rest–activity disruption and were diagnosed with AD were identified by nursing and medical staff. Rest–activity disruptions included insomnia, frequent nighttime awakenings, wandering at night, unusually early morning awakenings, sundowning, and excessive daytime sleepiness. Chart reviews were conducted to confirm that potential participants met the following criteria for inclusion: a diagnosis of AD according to the National Institute of Neurological and Communicative Disorders and Stroke–The Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984), able to perceive light as determined by an ophthalmologist’s examination, and on a stable medication regimen (i.e., not receiving as-needed dosing of antipsychotic or neuroleptic medications; as-needed dosing of short-acting benzodiazepines were permitted for medical or dental procedures). Potential participants were excluded if they had other neurological diagnoses (e.g., Parkinson’s disease) or were regularly taking valerian, melatonin, or sleeping pills. Informed consent was obtained from responsible parties as approved by the Institutional Review Board.
Participants (57 women and 13 men) were, on average, 84 years of age (SD = 10 years, range = 58-98) with a mean Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) score of 7 (SD = 7, range = 0–23). The ethnic make-up of the sample was 80.4% Caucasian, 13.0% African American, 4.4% Latino, and 2.2% Asian.
Measures
The Neuropsychiatric Inventory–Nursing Home version (NPI-NH) is a commonly used instrument that consists of a structured interview format used to measure the presence, frequency, and severity of both behavioral and psychiatric symptoms commonly exhibited in patients with dementia. The NPI-NH was modified from the Neuropsychiatric Inventory, an instrument with reported content and concurrent validity and between-rater, test–retest, and internal consistency reliability (Cummings et al., 1994). The NPI-NH is designed to allow professional caregivers such as nursing home staff to act as informants rather than an informal caregiver such as a family member (Iverson, Hopp, DeWolfe, & Solomons, 2002). The validity of the NPI-NH in assessing neuropsychiatric behaviors in nursing home residents was established by demonstrating that nursing staff could provide interview-based information using the NPI-NH scale that was comparable to assessments of psychiatric symptom disturbances established by trained raters (Wood et al., 2000).
To facilitate ease of instrument administration, the NPI-NH uses a yes/no stem question to assess for 10 types of behaviors or symptoms—agitation/aggression, delusions, hallucinations, depression/dysphoria, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, and aberrant motor behaviors—and two neurovegetative symptoms—nighttime behavior and appetite/eating changes. If the response to the stem question is negative for presence of the behavior, no further responses are required. If the response is positive, indicating the presence of the behavior, additional questions are asked and the frequency and severity of the behavior are rated by the caregiver. Frequency ratings range from 1 (occasionally) to 4 (very frequently), and severity ratings range from 1 (mild) to 3 (severe). Frequency and severity ratings are then multiplied to yield a domain score for each of the 12 items. These domain scores are not normally distributed, as frequency (1–4) and severity (1–3) can only result in scores of 1, 2, 3, 4, 6, 8, 9, and 12. This scoring method effectively overweights more severe scores.
In addition, caregivers rate the amount of distress or work time and effort they incur when caring for a patient exhibiting a particular behavior. These ratings are used to generate an occupational disruptiveness score that ranges from 0 (no distress) to 5 (very severe distress) for each item. Individual item occupational disruptiveness scores are added to yield a total occupational disruptiveness score, and all domain scores are added to yield a total score (Iverson et al., 2002). The instrument was administered in accordance with NPI-NH instructions for administration by a trained member of the research team to nursing staff on each shift to assess behaviors across the 24-hr time period.
Procedures
The study protocol was 11 weeks in duration. Outcome measures were assessed at the end of the baseline week and after the last week of intervention.
The control group received usual indoor light (150–200 lux) and participated in their regularly scheduled activities in the usual location. Participants in the experimental conditions received either morning (9:30-10:30 a.m.) or afternoon (3:30–4:30 p.m.) bright light exposure (>2,500 lux in gaze direction) Monday through Friday for 10 weeks. During this time, participants in the experimental groups (3–8 patients together) participated in activities in a brightly lit area, either outdoors or in an indoor space with windows to let in ample natural light. APOLLO Brite Lite IV (Orem, Utah) light boxes were used when necessary to supplement the ambient light. These boxes (23 in. × 12 in. × 4 in.) provide 10,000 lux exposure at 26 in. and 2,500 lux exposure at 4 ft. A Cal LIGHT 400 (Auburn Hills, Michigan) calibrated precision light meter was used to monitor light levels in gaze direction for each participant at the beginning of each intervention day. When sessions were held exclusively in natural light, additional readings were taken periodically to ensure that each participant received sufficient exposure, and if necessary, research staff moved participants to ensure that they received more than 2,500 lux. The experimental groups participated in activities similar to those provided to the control group participants. Attendance and approximate percentage of intervention missed (e.g., eyes closed or sleeping, toileting time, etc.) were recorded for each participant. Nursing staff were not unaware of participants’ study group assignment.
Analysis of Data
Outcome measures derived from the NPI-NH included presence, frequency, severity, domain score (frequency times severity), and occupational disruptiveness score for each of the 12 behaviors at baseline and at the end of the intervention. The total of domain scores and total occupational disruptiveness scores were also computed. Outcomes measures were obtained from certified nursing assistants (CNAs) 67% of the time; registered nurses, 22%; and licensed vocational nurses (LVNs), 4%; 7% of outcomes measures were missing. If answers differed between shifts, the highest scores were recorded.
Repeated measures analysis of variance (ANOVA) was used to test between-and within-participants effects. Thus, we tested for the main effect of group, the main effect of time, and the interaction of Group × Time (treatment effect). Repeated measures ANOVA was run on each of the 12 behavioral domains, total score, and occupational disruptiveness scores. Post hoc pairwise contrasts for differences from baseline to end of intervention for each of the three possible pairs (i.e., morning vs. afternoon light, afternoon light vs. control, morning light vs. control) were performed only on those domains for which the omnibus F test was significant. To control for the inflation of Type I error, we used a Bonferroni correction (i.e., p = .05/3) and only considered a post hoc p less than .0167 as significant. Pearson correlations were used to examine the relationships of behavioral scores to occupational disruptiveness scores.
Results
The amount of light exposure (dose) received by each participant was calculated by dividing the actual hours of intervention received by the total number of possible intervention hours (50 hr over the 10-week intervention period). Participants received, on average, 76% (SD = 17, range = 28–100) of the possible amount of light exposure. There was no significant difference in dose between the morning and afternoon light groups. Participants missed part or all of an intervention light treatment session for a variety of reasons including toileting, falling asleep, acute illness, or refusal to attend.
Table 1 illustrates the number of participants who exhibited behaviors on the NPI-NH as well as the means and standard deviations for domain, occupational disruptiveness, and total scores for the 12 behaviors at baseline and end of the intervention for the three groups. This table only includes those participants who evidenced the presence of the behaviors.
Table 1.
Mean Domain, Occupational Disruptiveness, and Total Scores on the Neuropsychiatric Inventory–Nursing Home for Participants With Positive Responses to the Screening Question
| Baseline
|
End of Intervention
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presence of Behavior
|
Domain
|
Disruptiveness
|
Presence of Behavior
|
Domain
|
Disruptiveness
|
|||||||
| Behavior/Symptom | % | n | M | SD | M | SD | % | n | M | SD | M | SD |
| Delusions | ||||||||||||
| Morning | 45 | 13 | 4.6 | 3.6 | 1.9 | 1.6 | 45 | 13 | 3.7 | 2.6 | 1.2 | 1.6 |
| Afternoon | 33 | 8 | 2.6 | 1.6 | 1.0 | 1.4 | 33 | 8 | 5.9 | 3.9 | 1.4 | 1.1 |
| Control | 53 | 9 | 5.2 | 2.9 | 0.8 | 1.1 | 35 | 6 | 2.5 | 2.1 | 0.3 | 0.8 |
| Hallucinations | ||||||||||||
| Morning | 35 | 10 | 4.4 | 3.4 | 1.4 | 1.5 | 24 | 7 | 5.7 | 2.2 | 1.1 | 1.4 |
| Afternoon | 29 | 7 | 4.0 | 2.5 | 1.1 | 0.9 | 25 | 6 | 6.0 | 2.5 | 1.2 | 1.2 |
| Control | 35 | 6 | 3.8 | 2.8 | 0.5 | 0.6 | 24 | 4 | 2.5 | 2.4 | 0.8 | 1.5 |
| Agitation/aggression | ||||||||||||
| Morning | 86 | 25 | 5.3 | 3.5 | 2.0 | 1.5 | 66 | 19 | 5.5 | 3.3 | 1.5 | 1.2 |
| Afternoon | 83 | 20 | 3.7 | 2.4 | 1.4 | 1.1 | 75 | 18 | 4.8 | 2.6 | 1.8 | 1.2 |
| Control | 77 | 13 | 5.8 | 3.4 | 0.9 | 1.3 | 41 | 7 | 4.3 | 2.5 | 1.3 | 1.4 |
| Depression/dysphoria | ||||||||||||
| Morning | 41 | 12 | 3.3 | 1.8 | 0.9 | 1.1 | 38 | 11 | 3.0 | 1.8 | 1.2 | 1.3 |
| Afternoon | 46 | 11 | 2.4 | 1.9 | 1.2 | 1.2 | 46 | 11 | 4.9 | 3.9 | 1.2 | 1.2 |
| Control | 65 | 11 | 2.9 | 1.8 | 0.7 | 0.9 | 35 | 6 | 1.7 | 0.5 | 0.3 | 0.5 |
| Anxiety | ||||||||||||
| Morning | 59 | 17 | 4.8 | 3.6 | 1.2 | 1.4 | 31 | 9 | 4.8 | 3.8 | 1.4 | 1.7 |
| Afternoon | 42 | 10 | 2.6 | 1.7 | 0.9 | 1.1 | 46 | 11 | 5.3 | 3.4 | 1.3 | 1.5 |
| Control | 41 | 7 | 1.9 | 1.1 | 0.6 | 0.5 | 18 | 3 | 1.0 | 0.0 | 0.0 | 0.0 |
| Elation/euphoria | ||||||||||||
| Morning | 17 | 5 | 7.2 | 3.7 | 1.0 | 1.4 | 17 | 5 | 5.0 | 3.2 | 1.0 | 1.2 |
| Afternoon | 33 | 8 | 4.0 | 2.9 | 0.9 | 1.1 | 29 | 7 | 3.6 | 2.1 | 0.4 | 0.8 |
| Control | 41 | 8 | 2.0 | 1.8 | 0.6 | 0.8 | 18 | 3 | 5.0 | 1.7 | 0.7 | 0.6 |
| Apathy/indifference | ||||||||||||
| Morning | 62 | 18 | 7.2 | 4.1 | 1.1 | 1.5 | 55 | 16 | 6.3 | 3.2 | 1.1 | 1.3 |
| Afternoon | 71 | 17 | 6.6 | 4.6 | 0.9 | 1.3 | 63 | 15 | 5.7 | 3.8 | 1.1 | 1.3 |
| Control | 41 | 7 | 2.7 | 1.7 | 0.4 | 0.8 | 29 | 5 | 5.3 | 3.2 | 0.3 | 0.5 |
| Disinhibition | ||||||||||||
| Morning | 41% | 12 | 4.4 | 3.2 | 1.5 | 1.6 | 24 | 7 | 1.9 | 1.9 | 1.7 | 1.4 |
| Afternoon | 58% | 14 | 3.0 | 3.1 | 0.7 | 0.6 | 29 | 7 | 3.7 | 2.6 | 0.9 | 1.1 |
| Control | 41% | 7 | 3.7 | 3.9 | 1.0 | 1.4 | 24 | 4 | 4.0 | 2.7 | 0.5 | 0.6 |
| Irritability/lability | ||||||||||||
| Morning | 66% | 19 | 3.3 | 2.3 | 1.3 | 1.3 | 41 | 12 | 3.7 | 2.4 | 1.2 | 0.9 |
| Afternoon | 75% | 18 | 3.8 | 3.1 | 1.2 | 1.0 | 54 | 13 | 4.7 | 2.6 | 2.0 | 1.4 |
| Control | 82% | 14 | 4.1 | 3.0 | 0.8 | 1.1 | 47 | 8 | 3.9 | 3.0 | 0.6 | 0.7 |
| Aberrant motor behavior | ||||||||||||
| Morning | 48% | 14 | 5.8 | 4.1 | 1.7 | 1.7 | 21 | 6 | 7.0 | 4.2 | 1.7 | 1.6 |
| Afternoon | 38% | 9 | 5.8 | 4.5 | 1.1 | 0.9 | 50 | 12 | 6.1 | 3.6 | 1.5 | 1.5 |
| Control | 41% | 7 | 4.7 | 3.3 | 1.1 | 1.1 | 35 | 6 | 6.5 | 3.8 | 1.3 | 1.2 |
| Nighttime behavior | ||||||||||||
| Morning | 62% | 18 | 5.5 | 3.7 | 1.7 | 1.4 | 52 | 15 | 4.8 | 3.0 | 0.7 | 1.0 |
| Afternoon | 58% | 14 | 4.9 | 3.7 | 1.1 | 1.3 | 67 | 16 | 5.4 | 2.9 | 0.9 | 1.4 |
| Control | 59% | 10 | 2.6 | 1.5 | 0.7 | 1.2 | 18 | 3 | 2.7 | 2.9 | 0.3 | 0.9 |
| Appetite/eating disorders | ||||||||||||
| Morning | 28% | 8 | 4.9 | 3.5 | 1.0 | 1.2 | 48 | 14 | 5.6 | 3.6 | 1.1 | 1.5 |
| Afternoon | 42% | 10 | 8.3 | 4.1 | 1.0 | 1.6 | 38 | 5 | 4.4 | 2.4 | 1.6 | 1.2 |
| Control | 35% | 6 | 3.2 | 2.6 | 1.0 | 0.6 | 24 | 4 | 4.5 | 3.1 | 0.5 | 1.0 |
| Total | ||||||||||||
| Morning | 29.4 | 20.7 | 8.7 | 8.1 | 26.3 | 13.9 | 7.2 | 7.4 | ||||
| Afternoon | 27.0 | 15.7 | 6.5 | 5.3 | 27.5 | 16.5 | 7.2 | 6.5 | ||||
| Control | 24.1 | 15.8 | 5.1 | 8.2 | 19.6 | 10.8 | 4.4 | 4.3 | ||||
Repeated measures ANOVA on NPI-NH domain scores revealed statistically significant omnibus F-test results in this sample for agitation/aggression, F(2, 55) = 3 .66, p = .032; depression/dysphoria, F(2, 55) = 3 .36, p = .042; aberrant motor behavior, F(2, 55) = 4.15, p = .021; and appetite/eating disorders, F(2, 54) = 4.94, p = .011. Post hoc pairwise contrasts revealed a significant difference between morning and afternoon light on agitation/aggression scores from baseline to the end of the intervention. Although participants in both morning and afternoon light conditions evidenced increased agitation/aggression scores at the end of intervention assessment compared with baseline, this increase was greater in the morning light group, t = –2.70, p = .009. A significant difference between morning light and usual indoor light (control group) was also found on agitation/aggression scores from baseline to end of the intervention. Scores increased for participants exposed to morning light, whereas scores decreased for participants in the control group, t(55) = –2.52, p = .015. Participants in the afternoon group differed significantly from those receiving usual indoor light on depression/dysphoria scores from baseline to the end of the intervention. Scores increased for participants exposed to afternoon light, whereas scores decreased for participants in the control group, t(55) = 2.57, p = .013. Finally, there was a significant difference between morning light and usual indoor light on aberrant motor behavior scores from baseline to the end of the intervention. Scores decreased for participants exposed to morning light, whereas scores increased for participants in the control group, t(55) = 2.78, p = .007. There were no significant post hoc findings for appetite/eating disorders. Repeated measures ANOVA on total NPI-NH and occupational disruptiveness scores revealed no statistically significant omnibus F-test results.
Figure 1 illustrates domain scores for the six most frequently exhibited behaviors in this sample of institutionalized participants with AD. The three most frequent behaviors were agitation/aggression, apathy/indifference, and nighttime behavior. These behaviors were consistently first, second, and third, respectively, at both measurement time points. The next three most frequent behaviors were aberrant motor behavior, irritability/lability, and appetite/eating disorders.
Figure 1. Six Most Frequently Occurring Behaviors.
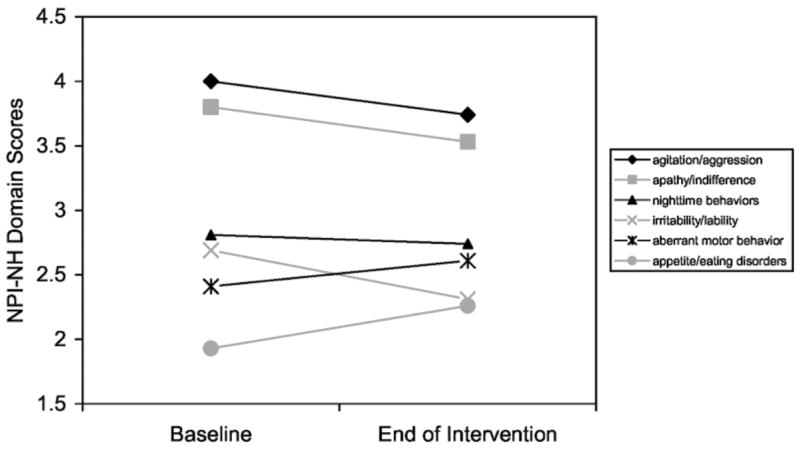
Note: NPI-NH = Neuropsychiatric Inventory–Nursing Home version.
Table 2 depicts the correlation of each domain score with its corresponding occupational disruptiveness score. Agitation/aggression, anxiety, and nighttime behavior were significantly correlated with staff perceptions of disruptiveness at both measurement time points. Hallucinations, disinhibition, and irritability/lability were significantly correlated with staff perceptions of disruptiveness at baseline only; delusions, aberrant motor behavior, and appetite/eating disorders were significantly correlated with staff perceptions of disruptiveness at the end of the intervention only.
Table 2.
Pearson Correlations of Domain With Occupational Disruptiveness Scores on the Neuropsychiatric Inventory–Nursing Home for Participants With Positive Responses to the Screening Question
| Baseline
|
End of Intervention
|
|||
|---|---|---|---|---|
| Behavior/Symptom | r | n | r | n |
| Delusions | .34 | 30 | .48* | 27 |
| Hallucinations | .59* | 23 | .46 | 17 |
| Agitation/aggression | .47* | 58 | .45* | 44 |
| Depression/dysphoria | .19 | 34 | .33 | 28 |
| Anxiety | .50* | 34 | .45* | 23 |
| Elation/euphoria | .20 | 21 | .19 | 15 |
| Apathy/indifference | .22 | 42 | .23 | 36 |
| Disinhibition | .53* | 33 | .16 | 18 |
| Irritability/lability | .40* | 51 | .28 | 33 |
| Aberrant motor behavior | .29 | 30 | .48* | 24 |
| Nighttime behavior | .70* | 42 | .63* | 34 |
| Appetite/eating disorders | .32 | 24 | .43* | 23 |
| Total | .65 | 69 | .69 | 59 |
p < .05.
Discussion
Repeated measures ANOVA revealed statistically significant changes in four of the neuropsychiatric behavioral domains: agitation/aggression, depression/dysphoria, aberrant motor behavior, and appetite/eating disorders. Although these changes were statistically significant, the actual changes in scores were less than 1 point on a 12-point scale. The scoring method inherently overweights more severe scores, and in general our participants’ scores were in the lower third (<4) of the possible total range. The degree of change on an assessment instrument score necessary to reliably infer improvement or worsening of a clinical condition is often problematic in research. Iverson et al. (2002) found that a large change (±22 points) in total NPI-NH score was necessary to reliably conclude neuropsychiatric symptom improvement or deterioration. This large change was necessary to exceed the level of change that could be attributed to measurement error alone. It therefore seems doubtful that the statistically significant differences we found represent clinically meaningful changes that would affect the care of these patients. One could conclude from our findings that bright light therapy did not clinically affect neuropsychiatric behaviors. Several alternative interpretations are also possible.
In this study, the CNA assigned to work with the participant was generally chosen as the nursing staff member likely to be most familiar with the participant’s behaviors and best able to assess level of associated occupational disruptiveness. However, issues associated with staff ratings, particularly those of CNAs, may have offset the advantage of familiarity. In some instances, staffing changes from baseline to the end of the intervention made it necessary to query different caregivers at the two data collection time points and impossible to determine the extent and impact of interrater variability on resulting scores. Wood et al. (2000) compared assessments on the NPI-NH completed by LVNs, CNAs, and research staff and found that LVN assessments consistently correlated more closely with research staff assessments than with those of CNAs, who did two thirds of assessments in this study. Furthermore, accuracy of assessments on patients with more frequent or severe symptoms may have differed from assessments on those with less frequent or severe symptoms. Wood et al. also reported that the correlations between assessments by research staff and LVNs were higher for residents with more severe levels of neuropsychiatric symptoms. Despite our attempts to gather accurate data, it is possible that our CNAs’ assessments differ from assessments that might have resulted if research staff or staff with more advanced professional training had been queried. Finally, staff commented informally and scored behaviors as nondisruptive, noting that it was “part of their job” to attend to such behavior. Although it might seem intuitive that staff on a unit with 10 patients awake at night and exhibiting agitated, irritable, or aberrant motor behaviors would report that these behaviors created more work, this was often not reflected in their actual ratings. Cultural and gender differences in the way nursing staff respond to the NPI-NH questions may have also occurred.
Some behaviors are more amenable to observation (e.g., agitation/aggression, nighttime behavior), and others are less so (e.g., apathy/indifference). Our data may more accurately reflect the presence of those easily observable behaviors and less accurately reflect those less easily observed. Agitation/aggression and nighttime behavior were frequently occurring behaviors and were also strongly correlated with occupational disruptiveness scores at both data collection time points. Ancoli-Israel et al. (2003) found that caregivers’ ratings of agitated behaviors improved with bright light treatment despite the fact that light had little effect on observational ratings of agitation. It appears that the relationship of neuropsychiatric behaviors to perceived occupational disruption depends on a variety of caregiver factors in addition to the characteristics of patients’ behaviors.
It was not feasible for staff to be unaware of the participants’ experimental condition, and this may have biased ratings on the NPI-NH. The NPI-NH interviews were conducted with members of the night shift (who were not present during the actual implementation of the experimental protocols). These night shift staff members may have received anecdotal information from other shifts but were not able to observe participants’ daytime responses. Day and evening shift personnel were also asked the NPI-NH interview questions to obtain the maximum possible information about each participant. We interviewed staff members from all shifts to make sure that any behaviors exhibited across the 24-hr day were captured in the data. Open-treatment designs have been used in other studies. Satlin et al. (1992) concluded that, based on their results, nurse ratings were not influenced by the presence of the light treatment and were corroborated by the activity monitor data. The NPI-NH data were collected at two time points during the 11-week protocol, during the baseline week and during the 10th week of the intervention. Given the waxing and waning of many neuropsychiatric symptoms, it is possible that behaviors occurring at a specific data collection time point did not reflect a broader behavioral profile for a given participant over a more extended period of time. For example, light may have had an impact on behaviors during the first week of the intervention, but that effect may have no longer been present at the end of the intervention. In future research, it would be enlightening to use observational assessments in conjunction with the NPI-NH over shorter periods of time to help to answer the question of whether the instrument is useful for tracking change over time or is more appropriately used as a means to identify participants with neuropsychiatric symptoms.
Medications or changes in medication regimens might also account for the lack of clinically significant findings. Although a stable medication regimen was a criterion for inclusion in the study, we did not track medication additions, deletions, or dose changes over the course of the study. It is therefore impossible to determine what effect, if any, medication changes may have had on the behavioral symptoms.
Further research is necessary to examine the impact of bright light on neuropsychiatric behaviors. Results from the larger study reported elsewhere demonstrated that 1 hr of bright light administered either in the morning or in the afternoon facilitated entrainment of the rest–activity rhythm to the 24-hr day (Dowling, Mastick, Hubbard, Luxenberg, & Burr, 2005). This effect of light either did not affect neuropsychiatric behaviors or was not detected by the NPI-NH. The ability of light to entrain the rest–activity rhythm is potentially clinically significant because it should be easier for caregivers to provide care to patients with socially acceptable day–night rhythms. Although this finding may be less important in an institutional setting, it could be extremely important in the community setting where a caregiver’s primary reason for institutionalization is disruption of the rest–activity rhythm in the care recipient. Results from observational instruments, like the Neuropsychiatric Inventory, might be more valid in a community setting because informal caregivers may be able to provide a more consistent account of patient behaviors as they generally spend more total time with the care recipient than do professional caregivers who are caring for multiple patients (Lange, Hopp, & Kang, 2004). Future research should also evaluate the ability of light to affect neuropsychiatric symptoms in patients with milder cognitive impairment and other forms of dementia. In addition, studies using different intensities, durations of exposure, and timing of administration of light will provide useful guidance for clinicians.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Nursing Research Grant NR002968. Carla Graf, RN, MS, assisted with data analysis and manuscript preparation. Erin M. Hubbard, MA, assisted with data collection, data analysis, and manuscript preparation. Jay S. Luxenberg, MD, assisted with study design, implementation, data analysis, and manuscript preparation. We wish to acknowledge and thank the administration, staff, and patients at the Jewish Home and Laguna Honda Hospital in San Francisco, California, for their assistance and cooperation. Our thanks are also extended to Theresa Berta, MD, H. Rachel Davids, MD, Annie Davidson, BS, and Shirley Wu, BA, who participated in recruitment, data collection, and analyses; to Judy Mastick, RN, MN, who provided thoughtful comments and critique of the manuscript; and to Bruce Cooper, PhD, who assisted with data analysis.
Contributor Information
Glenna A. Dowling, University of California, San Francisco
Carla Graf, University of California, San Francisco.
Erin M. Hubbard, Institute on Aging, Research Center, San Francisco, California
Jay S. Luxenberg, Jewish Home, San Francisco, California
References
- Ancoli-Israel S, Martin JI, Gehrman P, Shochat T, Corey-Bloom J, Marler M, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer disease. American Journal of Geriatric Psychiatry. 2003;11:194–203. [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS, Rosenthal AS. Dementia and agitation in nursing home residents: How are they related? Psychology and Aging. 1990;5:3–8. doi: 10.1037//0882-7974.5.1.3. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48(Suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Katz IR, Brodaty H, Lyons B, Greenspan A, Burns A. Management of agitation, aggression, and psychosis associated with dementia: A pooled analysis including three randomized, placebo-controlled double-blind trials in nursing home residents treated with risperidone. Clinical Neurology and Neurosurgery. 2005;107:497–508. doi: 10.1016/j.clineuro.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2005;20:738–743. doi: 10.1002/gps.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes D, Morgan DG, Bangma J, Peacock S, Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia. Cochrane Database of Systematic Reviews 2004. 2004;(2):Art. No.: CD003946. doi: 10.1002/14651858.CD003946.pub2. DOI: 001002/14651858.CD003946.pub2. [DOI] [PubMed] [Google Scholar]
- Förstl H. Neuropathology of behavioral and psychological symptoms of dementia. International Psychogeriatrics. 2000;12(Suppl S1):77–81. [Google Scholar]
- Hatoum HT, Lin SJ, Arcona S, Thomas SK, Koumaras B, Mirski D. The use of the Occupational Disruptiveness Scale of the Neuropsychiatric Inventory-Nursing Home version to measure the impact of Rivastigmine on the disruptive behavior of nursing home residents with Alzheimer’s disease. Journal of the American Medical Directors Association. 2005;6:238–245. doi: 10.1016/j.jamda.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. International Journal of Geriatric Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hozumi S, Okawa M, Mishima K, Hishakawa Y, Hori H, Takahashi K, et al. Phototherapy for elderly patients with dementia and sleep-wake rhythm disorders—A comparison between morning and evening light exposure. Japanese Journal of Psychiatry and Neurology. 1990;44(4):813–814. [Google Scholar]
- Iverson GL, Hopp GA, DeWolfe K, Solomons K. Measuring change in psychiatric symptoms using the Neuropsychiatric Inventory: Nursing Home version. International Journal of Geriatric Psychiatry. 2002;17:438–443. doi: 10.1002/gps.617. [DOI] [PubMed] [Google Scholar]
- Lange RT, Hopp GA, Kang N. Psychometric properties and factor structure of the Neuropsychiatric Inventory Nursing Home version in an elderly neuropsychiatric population. International Journal of Geriatric Psychiatry. 2004;19:440–448. doi: 10.1002/gps.1108. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Veiel LL, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K, et al. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatrica Scandinavica. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Ruths S, Straand J, Nygaard HA. Psychotropic drug use in nursing homes—Diagnostic indications and variations between institutions. European Journal of Clinical Pharmacology. 2001;57:523–528. doi: 10.1007/s002280100348. [DOI] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer’s disease. American Journal of Psychiatry. 1992;149:1028–1032. doi: 10.1176/ajp.149.8.1028. [DOI] [PubMed] [Google Scholar]
- Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM. Improvement in behavioral symptoms and advance of activity acrophase after short-term bright light treatment in severe dementia. Psychiatry and Clinical Neurosciences. 2004;58:343–347. doi: 10.1111/j.1440-1819.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- Tan LL, Wong HB, Allen H. The impact of neuropsychiatric symptoms of dementia on distress in family and professional caregivers in Singapore. International Psychogeriatrics. 2005;17:253–263. doi: 10.1017/s1041610205001523. [DOI] [PubMed] [Google Scholar]
- Tsai YF, Wong TK, Juang YY, Tsai HH. The effects of light therapy on depressed elders. International Journal of Geriatric Psychiatry. 2004;19:545–548. doi: 10.1002/gps.1125. [DOI] [PubMed] [Google Scholar]
- White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. British Journal of Cancer. 1978;37:849–857. doi: 10.1038/bjc.1978.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the Neuropsychiatric Inventory in nursing home residents: Characterization and measurement. American Journal of Geriatric Psychiatry. 2000;8:75–83. doi: 10.1097/00019442-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]


