Abstract
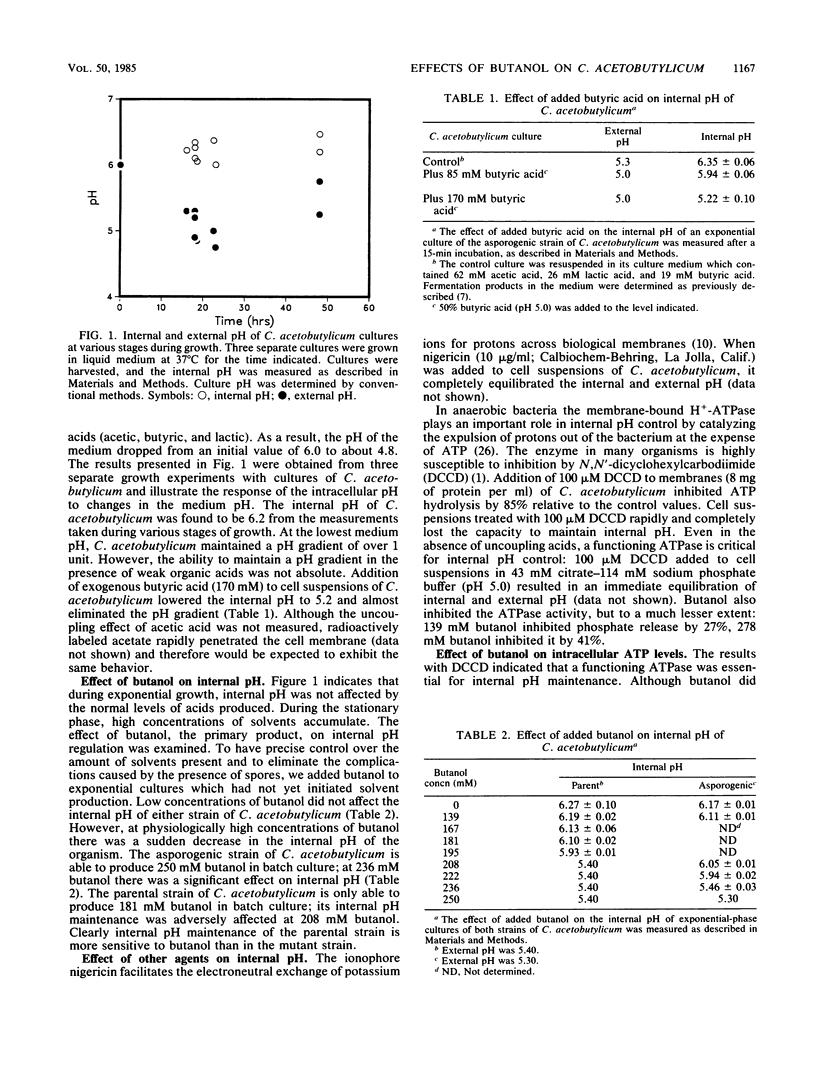
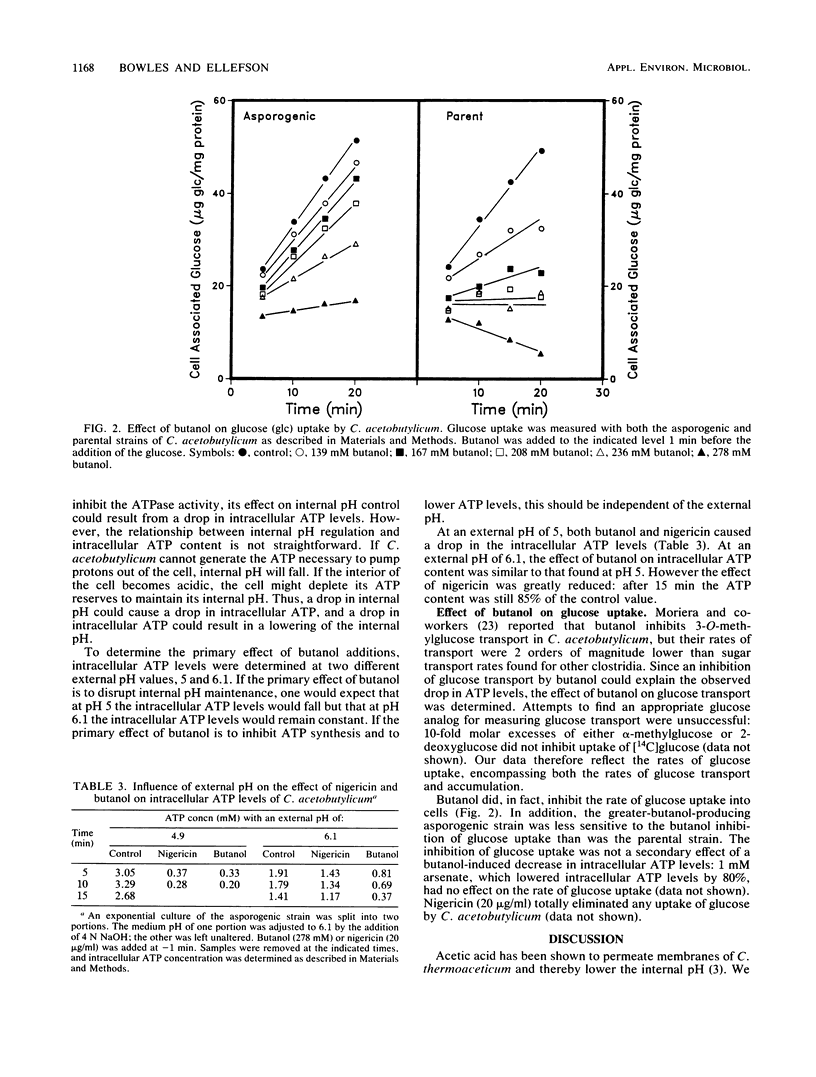
The internal pH of Clostridium acetobutylicum was determined at various stages during the growth of the organism. Even in the presence of significant quantities of acetic, butyric, and lactic acids, an internal pH of 6.2 was maintained. Experiments using N,N'-dicyclohexylcarbodiimide indicated that a functioning H+-ATPase is necessary for internal pH control. Butanol, one of the end products of the fermentation, had numerous harmful effects on C. acetobutylicum. At a concentration high enough to inhibit growth, butanol destroyed the ability of the cell to maintain internal pH, lowered the intracellular level of ATP, and inhibited glucose uptake. Experiments done at two different external pH values suggested that the butanol-mediated decrease in ATP concentration was independent of the drop in internal pH. Glucose uptake was not affected by arsenate, suggesting that uptake was not ATP dependent. The effects of butanol on C. acetobutylicum are complex, inhibiting several interrelated membrane processes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R., Morris J. G. Carbohydrate transport in Clostridium pasteurianum. Biosci Rep. 1982 Jan;2(1):47–53. doi: 10.1007/BF01142198. [DOI] [PubMed] [Google Scholar]
- Booth I. R., Morris J. G. Proton-motive force in the obligately anaerobic bacterium Clostridium pasteurianum: a role in galactose and gluconate uptake. FEBS Lett. 1975 Nov 15;59(2):153–157. doi: 10.1016/0014-5793(75)80364-4. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Datta R., Zeikus J. G. Modulation of acetone-butanol-ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol. 1985 Mar;49(3):522–529. doi: 10.1128/aem.49.3.522-529.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Chen J. S. Acidic Conditions Are Not Obligatory for Onset of Butanol Formation by Clostridium beijerinckii (Synonym, C. butylicum). Appl Environ Microbiol. 1983 Aug;46(2):321–327. doi: 10.1128/aem.46.2.321-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves D. J., Gronlund A. F. Carbohydrate transport in Clostridium perfringens type A. J Bacteriol. 1969 Dec;100(3):1256–1263. doi: 10.1128/jb.100.3.1256-1263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Stephens G. M., Morris J. G. Production of Solvents by Clostridium acetobutylicum Cultures Maintained at Neutral pH. Appl Environ Microbiol. 1984 Dec;48(6):1166–1170. doi: 10.1128/aem.48.6.1166-1170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., Peck M. W., Rodger G., Morris J. G. On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum. Biochem Biophys Res Commun. 1981 Mar 16;99(1):81–88. doi: 10.1016/0006-291x(81)91715-0. [DOI] [PubMed] [Google Scholar]
- Kim B. H., Bellows P., Datta R., Zeikus J. G. Control of Carbon and Electron Flow in Clostridium acetobutylicum Fermentations: Utilization of Carbon Monoxide to Inhibit Hydrogen Production and to Enhance Butanol Yields. Appl Environ Microbiol. 1984 Oct;48(4):764–770. doi: 10.1128/aem.48.4.764-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Butanol Production by a Butanol-Tolerant Strain of Clostridium acetobutylicum in Extruded Corn Broth. Appl Environ Microbiol. 1983 Mar;45(3):966–973. doi: 10.1128/aem.45.3.966-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C. Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J Bacteriol. 1983 Mar;153(3):1461–1470. doi: 10.1128/jb.153.3.1461-1470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Catabolism of fructose and mannitol in Clostridium thermocellum: presence of phosphoenolpyruvate: fructose phosphotransferase, fructose 1-phosphate kinase, phosphoenolpyruvate: mannitol phosphotransferase, and mannitol 1-phosphate dehydrogenase in cell extracts. J Bacteriol. 1971 Jan;105(1):226–231. doi: 10.1128/jb.105.1.226-231.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling V., Jungermann K. Properties and function of clostridial membrane ATPase. Biochim Biophys Acta. 1976 Jun 8;430(3):434–444. doi: 10.1016/0005-2728(76)90019-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Vollherbst-Schneck K., Sands J. A., Montenecourt B. S. Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1984 Jan;47(1):193–194. doi: 10.1128/aem.47.1.193-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hugo H., Gottschalk G. Distribution of 1-phosphofructokinase and PEP:fructose phosphotransferase activity in Clostridia. FEBS Lett. 1974 Sep 15;46(1):106–108. doi: 10.1016/0014-5793(74)80345-5. [DOI] [PubMed] [Google Scholar]



