Abstract
Ambient particulate matter (PM) is an environmental factor that has been associated with increased respiratory morbidity and mortality. The major effect of ambient PM on the pulmonary system is the exacerbation of inflammation, especially in susceptible people. One of the mechanisms by which ambient PM exerts its proinflammatory effects is the generation of oxidative stress by its chemical compounds and metals. Cellular responses to PM-induced oxidative stress include activation of antioxidant defense, inflammation, and toxicity. The pro-inflammatory effect of PM in the lung is characterized by increased cytokine/chemokine production and adhesion molecule expression. Moreover, there is evidence that ambient PM can act as an adjuvant for allergic sensitization, which raises the possibility that long-term PM exposure may lead to increased prevalence of asthma. In addition to ambient PM, rapid expansion of nanotechnology has introduced the potential that engineered NP may also become airborne and may contribute to pulmonary diseases by novel mechanisms that could include oxidant injury. Currently, little is known about the potential adverse health effect of these particles. In this communication, the mechanisms by which particulate pollutants, including ambient PM and engineered NP, exert their adverse effects through the generation of oxidative stress and the impacts of oxidant injury in the respiratory tract will be reviewed. The importance of cellular antioxidant and detoxification pathways in protecting against particle-induced lung damage will also be discussed.
Keywords: Particulate matter, Oxidative stress, Asthma, Dendritic cells, Adjuvant effect, Nanotoxicology
Introduction
Increased vehicular traffic and other combustion processes have resulted in a significant increase in ambient particulate matter (PM) over the last two decades. A sudden surge in the level of PM has been linked to increased morbidity and mortality due to cardiorespiratory events, including asthma, chronic obstructive pulmonary disease (COPD), and atherosclerosis [1–12]. Both in vitro and in vivo studies of the health effects of ambient PM have identified the generation of oxidative stress as one of the major mechanisms by which air pollution particles exert adverse biological effects. Among different size particles, it has also been established that ultrafine particles (UFP), which has an aerodynamic size of < 100 nm, are potentially the most dangerous due to their small size, large surface area, deep penetration and ability to be retained in the lung, and high content of redox cycling organic chemicals [3]. In addition, increased use of engineered nanoparticles (NP) in a wide range of industries has introduced a potential new type of inhaled particulate pollutant [13]. Examples include carbon black, TiO2, ZnO, and cerium oxide nanoparticles [13]. Currently, little is known about the potential adverse health effect of these particles. Therefore, there is an urgent need to understand the potential impact of inadvertent (ambient UFP) or engineered NP exposure on human health. None of these NP is currently being regulated. In this communication, we will review the mechanisms by which particulate pollutants, including ambient and engineered NP, exert their deleterious effects through an ability to generate reactive oxygen species (ROS) and oxidative stress. We will discuss the role of oxidant injury by ambient and engineered NP in the respiratory tract. We will also discuss the importance of cellular antioxidant and detoxification pathways in protecting against particle-induced lung damage.
The role of oxidative stress in the health effects of particulate pollutants
Several mechanisms have been proposed to explain the adverse health effects of particulate pollutants. These include inflammation, endotoxin effects, stimulation of capsaicin/irritant receptors, autonomic nervous system activity, pro-coagulant effects, covalent modification of cellular components and ROS production [3]. Among these, ROS production and the generation of oxidative stress have received the most attention.
Cellular redox homeostasis is carefully maintained by an elaborate antioxidant defense system, which includes antioxidant enzymes, proteins, and low molecular weight scavengers. Excessive ROS production or a weakening of antioxidant defense could lead to oxidative stress [1]. Oxidative stress is a state of redox disequilibrium that is defined as a decrease in the cellular glutathione (GSH)/glutathione disulfide (GSSG) ratio but functionally should be seen as a cellular stress response that activates a number of the redox-sensitive signaling cascades [1]. Not only does the GSH/GSSG redox pair serve as the principal homeostatic regulator of redox balance but also functions as a sensor that triggers these stress responses that, depending on the rate and level of change in this ratio, could be protective or injurious in nature [1, 14].
Using DEP as a model air pollutant, a hierarchical cellular response model has been developed to explain the role of oxidative stress in mediating the biological effects of PM [14, 15] (Fig. 1). This 3-tier model posits that low levels of oxidative stress induce protective effects that may yield to more damaging effects at higher levels of oxidative stress (Fig. 1). The protective effects (Tier 1) are induced by the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2), which leads to transcriptional activation of > 200 antioxidant and detoxification enzymes that are collectively known as the phase 2 response [16, 17]. Examples of phase 2 enzymes include heme oxygenase 1 (HO-1), glutathione-S-transferase (GST) isoenzymes, NADPH quinone oxidoreductase [18], catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx) [17, 19]. Defects or aberrancy of this protective pathway could determine the susceptibility to particle-induced oxidant injury, e.g., the exacerbation of airway inflammation and asthma by DEP [1]. Thus, it is important to mention that due to the protective Tier 1 response, particle-induced ROS production does not automatically lead to adverse biological outcomes. Should these protective responses fail to provide adequate protection, a further increase in ROS production can result in pro-inflammatory (tier 2) and cytotoxic (Tier 3) effects [3, 14]. Pro-inflammatory effects are mediated by the redox-sensitive MAP kinase and NF-κB cascades that are responsible for the expression of cytokines, chemokines, and adhesion molecules, many of which are involved in the inflammatory process of the lung [1, 3, 14, 19]. Tier 3 cytotoxic effects (aka toxic oxidative stress) involve mitochondria, which are capable of releasing pro-apoptotic factors and inducing apoptosis of lung cells [20, 21]. Taken together, the hierarchical cellular oxidative stress model provides a mechanistic platform against which to understand how PM generates adverse health effects.
Figure 1.
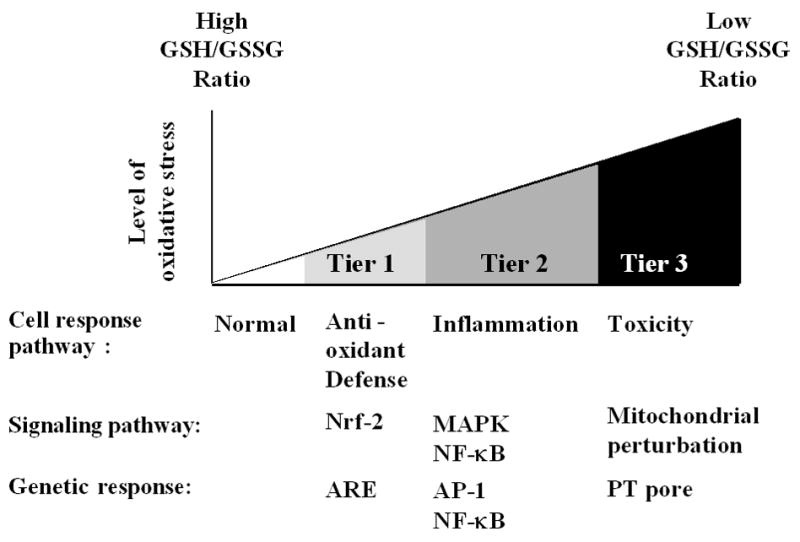
Hierarchical oxidative stress responses. At a low level of oxidative stress (Tier 1), antioxidant enzymes are induced to restore cellular redox homeostasis. At an intermediate level of oxidative stress (Tier 2), activation of MAPK and NF-κB cascades induces pro-inflammatory responses, e.g., cytokines and chemokines. At a high level of oxidative stress (Tier 3), perturbation of the mitochondrial permeability transition pore and disruption of electron transfer result in cellular apoptosis or necrosis.
Generation of oxidative stress by ambient particulate pollutants
How is oxidative stress generated by ambient PM? The aerodynamic diameters of ambient air particle size vary from 0.005 to 10 μm. Three different types of ambient particles, as defined by size, are characterized in Table 1. Among these, the small size and large surface area of UFP make them carriers for metals and large number of organic carbon compounds. Many of these PM components are capable of ROS generation, e.g. promotion of Fenton and Haber Weiss chemistry to generate ROS and adverse biological effects [1]. In addition, the redox cycling of organic chemical compounds such as quinones could also give rise to the formation of the superoxide radical [1].
Table 1.
Comparison of Coarse, Fine, and Ultrafine Particles
| Parameters | Coarse | Fine | Ultrafine |
|---|---|---|---|
| Size | 2.5–10 μm | 0.10–2.5μm | < 0.10 μm |
| Organic Carbon Content | + | ++ | +++ |
| Metal Content | +++ | ++ | + |
| PAH Content | + | + | +++ |
| Source of ROS | Transition metals | PAH Quinones | PAH Quinones |
Taking advantage of the Versatile Aerosol Concentration Enrichment Systems (VACES), which can collect highly concentrated ambient particles (CAPs) of various sizes, the Southern California Particle Center has conducted studies to identify the relative toxicity of coarse, fine and ultrafine particles in the Los Angeles basin. The toxic potential of these particles could be correlated to their chemical composition and their capacity to induce oxidative stress [15, 22]. In particular, it was demonstrated that the biological activity and oxidant potential of CAPs are determined by the content of redox cycling chemicals [22] (Table 1). While coarse PM include mostly crustal elements, UFP, which are mainly derived from combustion sources, could be shown to include significantly more organic carbon compounds such as polycyclic aromatic hydrocarbons (PAH) and quinones [22] (Table 1). A strong correlation exists between the PM content of redox active chemicals and their capability to induce oxidative stress in macrophages and bronchial epithelial cells [22] (Table 1). Moreover, the intracellular localization of the particles could also play a role in ROS production. For instance, electron microscopy has revealed that UFP are capable of localizing inside damaged mitochondria. Both aromatic organic compounds and quinones contribute to mitochondrial injury and ROS generation [21, 22]. Thus, the extent of cellular toxicity is directly related to these organic chemical compounds. It is possible to assess the pro-oxidant activity of PM by using the dithiothreitol (DTT) assay that reflects the particle content of redox cycling chemical groups such as quinones [22, 23]. This assay is premised on the interaction of redox cycling chemical quinones (Q) with DTT:
| (1) |
| (2) |
| (3) |
| (net) |
The loss of DTT can be followed by its reaction with 5,5′-dithiobis-(2-nitrobenzoic acid). This assay provides a convenient means of comparing the pro-oxidative activity of ambient samples collected in an urban environment. In general, DTT activity is highest in UFP, which also correlates with their ability to induce cellular oxidant stress responses such as HO-1 expression [22].
Studies using fractionated organic DEP extract have demonstrated that quinones and PAHs are representative organic chemical groups that could contribute to the oxidant injury in the lung [23–25]. PAHs can be converted to quinones via biotransformation, e.g. through reactions involving cytochrome P450 1A1, expoxide hydrolase and dihydrodiol dehydrogenase [26]. Quinones produce ROS and may be key compounds in PM toxicity along with transition metals [24, 26]. Redox cycling quinones undergo one-electron reductions by NADPH cytochrome P450 reductase to form semiquinones [27]. The semiquinones can be recycled to the original quinones, leading to the formation of . Not only are quinones byproducts of diesel fuel combustion, but can also be formed by enzymatic conversion of PAHs in lung tissue [28].
In addition, it is necessary to point out that other PM chemicals and transition metals may also contribute to ROS overproduction. For example, it has been demonstrated that metals associated with PM can exert pro-inflammatory effect in the respiratory system and the generation of ROS by transition metals (e.g. Fe, Ni, Cu, Co, and Cr) may play an important role in this effect [29–31]. One study conducted by Becker et al showed that PM10 stimulated IL-8 and ROS production normal human bronchial epithelial (NHBE) cells and IL-6 production in alveolar macrophages. Further analyses of the principal components indicated that while Fe and Si in the PM correlated with IL-6 release, Cr correlated with IL-8 increase [32]. Soluble metals on inhaled PM have been shown to induce cellular oxidative stress in airway epithelial cells. Using metal chelator and antioxidants such as N-acetyl cysteine (NAC), a number of metals have been identified to be responsible for the pro-oxidant and pro-inflammatory effect of PM. High vanadium (V) content in residual oil fly ash (ROFA) has been implicated in the activation of NF-κB and increased production of IL-6 in NHBE cells [33]. PM with high Cu content induced cytokine release and NF-κB activation in human bronchial epithelial cell line (BEAS-2B) [33]. In animal studies, short-term exposure to CAPs aerosol led to significant increase of thiobarbituric reactive substances (TBARS) and oxidized proteins in rat lung indicating the presence of oxidative stress. This was accompanied by increased polymorphonuclear cells (PMN) in the BAL from thses animals. A strong association has been identified between increased TBARS and Al, Si, and Fe content of CAPs. There was also a correlation between PMN count in BAL and CAPs-associated Cr, Zn, and Na [34]. Soluble fraction of ROFA has been shown to be capable of generating metal-dependent hydroxyl radicals in a cell free system and cause lung inflammation in rats [35]. The pro-oxidative and pro-inflammatory effects of PM-associated metals have also been demonstrated in human studies. One report showed that instillation of metal-rich ambient PM2.5 from smelter area into the lungs of healthy subjects resulted in airway inflammation characterized by increased ROS and cytokine production (IL-6 and TNFα) as well as monocyte infiltration. It has been suggested that transition metals may be responsible for these effects [36]. Studies conducted in Utah Valley has provided another piece of evidence demonstrating the role of metals in the biological effects of ambient PM [37]. The closure and opening of a steel mill in the area had significant impact on the PM levels as well as its composition. When the steel mill was operating, the PM in the area contained significantly larger amount of metals including Fe, Cu, Zn, Pb, Ni and V. Aqueous extracts from the metal-rich PM had stronger ability to generate ROS and to increase IL-8 and IL-6 release by BEAS-2B cells compared with that collected when the steel mill was closed. Human exposure to the aqueous PM extracts containing high metal content caused inflammation in the lower respiratory tract as evidenced by significantly increased IL-8 and TNF levels in the BAL fluid. It has been suggested that the inflammatory injury correlated with the metal content and redox potential of the PM [37].
All considered, it could be concluded that redox-active organic chemicals could be major PM toxicants that are responsible for ROS generation and the induction of oxidative stress. Metals may synergize with organic PM components in this process leading to further escalation of oxidative stress [38].
The impact of particulate pollutants on asthma
Epidemiological evidence has shown a good correlation between increased ambient PM levels and cardiorespiratory morbidity and mortality [22, 39]. There is growing recognition that susceptible people could be more prone to these adverse health effects and that the protective effect of tier 1 of the hierarchical oxidative stress model may be helpful in understanding this susceptibility. This is best explained by studies looking at pro-oxidative and pro-inflammatory PM effects in the lung.
PM is capable of generating acute airway inflammation that can lead to asthma flares after a sudden surge in ambient PM 2.5 levels [39–41]. These acute exacerbations are characterized by increased symptom score as well as the requirement for more frequent medication and hospitalization [39, 40]. In addition to these acute effects, which are likely caused by an exacerbation of already existing airway inflammation and airway hyperreactivity, there is increasing evidence that particulate pollutants act as an adjuvant for allergic sensitization to common environmental allergens [25, 42–46]. This raises the possibility that long-term PM exposures may lead to increased prevalence of asthma and allergic diseases. While this notion is compatible with the increased prevalence of asthma in polluted urban environments, this topic is still controversial because of the complicated pathogenesis of this disease, including in the existence of heterogeneous asthma phenotypes that may be differently affected by environmental stimuli [47].
In addition to the epidemiological evidence, experimental data also suggest an association between particulate pollutants and asthma [1, 48]. DEP, one of the major sources of ambient UFP, has been used as a model particulate pollutant to elucidate the mechanisms by which ambient PM may contribute to asthma. This includes some evidence that the generation of oxidative stress by organic DEP chemicals could be responsible for the pro-inflammatory and adjuvant effects of these particles in the respiratory tract [23, 49–51]. Redox-active PM chemicals can exert non-specific pro-inflammatory as well as allergic inflammatory effects in the nose depending on whether the challenge is performed in a non-atopic or an atopic individual. While nonspecific inflammation could play a role in acute asthma flares, the adjuvant effects of PM involve the targeting of specific cellular elements in the immune system [1].
In vitro studies have identified macrophages and bronchial epithelial cells as important cellular targets for PM in the lung [50–53]. Exposure of these cells to ambient PM and organic DEP chemicals can induce the generation of ROS and oxidative stress, which can result in increased cytokine and chemokine production [54, 55]. Examples include increased TNF-α and IL-6 production in macrophages and IL-8 production in bronchial epithelial cells [54]. Additional evidence supporting the role of oxidative stress in PM-induced airway inflammation comes from animal studies [56–58]. For example, intratracheal instillation of DEP leads to increased PMN infiltration, increased mucus, nitric oxide production, and increased airway hyperreactivity (AHR) in mice, all of which play important role in the pathogenesis of asthma [59–65]. These effects can be suppressed by pre-treating the animals with SOD or with the nitric oxide synthase inhibitors [60, 63, 64]. In addition, thiol antioxidants such as NAC and bucillamine are capable of suppressing the adjuvant effects of aerosolized DEP on ovalbumin (OVA)-induced allergic responses in mice [66]. NAC also abrogated AHR induction by incinerator particles [67]. Using in vivo chemiluminescent imaging, Gurgueira et al have demonstrated that 5-hr exposure to CAPs aerosol significantly increased ROS production in the lung and heart of Sprague-Dawley rats compared with the animals exposed to the filtered air. Increased oxidative stress was accompanied by mild, but significant, damage to both organs [68]. This study has provided most direct in vivo evidence that PM induce ROS generation and cause oxidative tissue damage. In humans, experimental DEP exposures result in increased CO in exhaled air; CO is the catalytic product of HO-1, which acts as a sensitive marker for PM-induced oxidative stress [69–72].
The adjuvant effects of ambient PM and DEP have been demonstrated in a number of human and animal studies [42–46]. Combined DEP and ragweed nasal challenge significantly enhances ragweed-specific IgE and IL-4 production in humans [44]. In addition, intranasal instillation of DEP also increased the expression of several CC chemokines including RANTES, MIP-1α, and MCP-1 in the human nose [43]. Gilliland et al reported that individuals with GST M1 null genotype exhibit increased nasal allergic and allergen-specific IgE response to nasal DEP challenge, thereby demonstrating the possible linkage of these responses to an oxidative stress mechanism [73]. This finding suggests that the antioxidant and anti-inflammatory effects of phase II enzymes could play an important role in protecting against the pro-inflammatory and pro-allergic effects of PM [74–76].
In animal studies, DEP has been shown to enhance OVA-induced eosinophilic airway inflammation, OVA-specific IgG1 and IgE production, goblet cell proliferation, and local expression of several Th2 cytokines and chemokines [77]. Similar results have also been reported in animals receiving intratracheal instillation of the dust mite allergen, Der f, in the presence of DEP [78, 79]. Furthermore, when Balb/c mice were exposed to an aerosolized leachate of residual oil fly ash, their offspring demonstrated a significant increase in airway hyperresponsiveness, eosinophilic inflammation and IgE production in response to sensitization with a suboptimal dose of OVA [80]. Cultured splenocytes from these offspring demonstrated an increased IL-4/IFNγ ratio, suggesting a skewing towards Th2 immunity [80].
The immunological basis for the adjuvant effects of PM is still improperly understood. Several cell types are involved in allergen sensitization and asthma pathogenesis, including antigen-presenting cells (APC), T-helper 2 (Th2) lymphocytes, IgE-secreting plasma cells, mast cells, eosinophils, neutrophils, mucus-secreting goblet cells, smooth muscle and endothelial cells. DEP can directly impact a number of the cells that play a role in the afferent or efferent immune response [25, 81–87]. Traditional adjuvants exert their effects on the afferent or early phase of the immune response, which implies possible effects on antigen presenting cells (APC) [88, 89]. Consequently, a lot of attention is currently being directed at the possible contribution of dendritic cells (DC). DC plays a crucial role in initiating T-cell activation and is the main APC that are responsible for allergen processing and presentation in asthma. Airway DC continuously sample their environment for antigens and allergens [90–92]. After allergen capture and receipt of a danger signal, DC upregulate CCR7 expression, enter the afferent lymphatic vessels, and carry the allergen to the draining lymph nodes, where it is presented by MHC in the presence of co-stimulatory molecules. Allergen-specific T-cells are selected for antigen specificity and induced to proliferate. Depending on the cytokine milieu and other variables, DC could initiate a primary Th2 response in regional lymph nodes [90–95]. Following immune excitation, memory/effector CD4+ Th2 cells then leave the draining lymph nodes and extravasate at sites of inflammation during the challenge phase. Once in the tissues, Th2 cells interact with IgE-bearing local DC to increase IL-4, IL-5, IL-9, and IL-13 production [90–92, 96–102]. These cytokines are important for inducing tissue eosinophilia, airway hyper-reactivity, and the production of chemokines that attract further inflammatory cells.
What is the evidence that PM can impact this scenario of events? First, it has been reported that certain PM is capable of skewing immune response towards Th2 differentiation by interfering with DC function. A recent study demonstrated that diesel-enriched PM could increase antigen uptake by DC while also enhancing the surface expression of co-stimulatory molecules [103]. In co-stimulation assays of PM-exposed DC and alloreactive CD4+ T cells, DEP directed a Th2-like pattern of cytokine production (e.g. enhanced IL-13 and IL-18 and suppressed IFNγ production) [103]. Several studies now seem to indicate that oxidative stress is capable of shifting the immune response from Th1 to Th2 dominance [104]. In this regard, we have demonstrated that organic DEP extracts are capable of inducing oxidative stress effects in myeloid DC that leads to interference in IL-12 production, a key cytokine for T-helper 1 immunity [105]. This, in turn, results in decreased IFNγ production in antigen-specific T cells, which means that the overall decrease in Th1 immunity could promote Th2 skewing of the immune response [106]. Similar effects on IFNγ production have been demonstrated in intact animals [106]. One possible explanation for the perturbation of DC function by oxidative stress is the activation of Nrf2-mediated pathway, which exerts negative regulatory effects on the NF-κB signaling [105]. The NF-κB pathway plays an important role in IL-12 production, costimulatory receptor expression and DC maturation. Thus, one scenario is that a decrease in Th1 immunity may promote the adjuvant effects of DEP.
In addition to adjuvant effects, PM exposure induces acute asthma exacerbations independent of their effects on allergic sensitization [107]. For instance, it is capable of inducing AHR in naïve mice in the absence of allergen [62, 108]. It has also been demonstrated that DEP alone can induce increased AHR in asthmatic individuals [109]. While these effects may be related to PM effects on the immune system, the particles and their components may directly contribute to increased AHR during asthma attack [110–112]. One possible mechanism is nitric oxide generation, as evidenced by the ability of nitric oxide synthase inhibitors to interfere with DEP-induced AHR in mice [60]. Shedding of airway epithelial cells is another possibility, based on the ability of DEP to induce acute epithelial damage in vivo and in vitro [51, 113–115].
Two recent reviews have summarized the potential mechanisms of PM-lung interaction and particle translocation to other tissues with a focus on the UFP [116, 117]. It has been suggested that the unique physical and chemical properties of UFP play important roles in particle deposition in the lung and translocation to the extra-lung tissues. When inhaled UFP deposit on the epithelial surface of the peripheral lungs, their contact with surfactant layer and epithelial lining fluid (ELF) leads to their interactions with proteins and other biomolecules in the ELF. The large number concentration of UFP, compared with that of micron-sized PM, allows them to deposit over a large surface area of alveoli. This may result in a scattered chemotractant signal that leads to less recognition and phagocytosis of UFP by alveolar macrophages. In addition, PM may form complexes with proteins in the ELF. While proteins on the surface of micron-sized PM are immobilized and therefore allow rapid phagocytosis by alveolar macrophages, the extremely small size of UFP may make UFP-protein complexes protein-specific and less accessible to the cells of defense system such as macrophages in the lung epithelium. Modifications of UFP may also allow DCs to process these particles, take up antigenic material and carry it to the immune system, where it elicits an immune response [116, 117].
Potential health effects of engineered nanoparticles
In addition to the inadvertent generation of air pollution particles by the burning of fossil fuel products, rapid expansion of nanotechnology may lead to adverse health effects. Nanotechnology broadly refers to the manipulation and manufacture of materials and devices in the size range 1 –100 nm. These engineered nanomaterials include nanoparticles, nanospheres, nanotubes, and nanofibers. While engineered NPs are in the same size range as ambient UFP, they have their own unique physical and chemical characteristics as well as functionality (Table 2). Nanomaterials are widely used in a wide range of industries, including food, clothing, automobile manufacture, electronic, cosmetics, medicine, and agriculture [3].
Table 2.
Comparison of Ambient ultrafine particles (UFP) and nanomaterials
| Particle Types | Ambient UFP | Nanoparticle |
|---|---|---|
| Source | Anthropogenic | Engineered |
| Size | < 100 nm | < 100 nm |
| Uniformity | No | Yes |
| Organic Chemicals | High | Low |
| Transition Metals | High | Varies |
| Oxidative Stress | Yes | Varies |
| Toxicity | Yes | Varies |
| Portal-of-entry | Lung | Lung, Skin, Blood |
Increasing production and usage of nanomaterials in consumer products may lead to human exposures. Among the different exposure routes (e.g. inhalation, skin contact, ingestion, and injection), particle inhalation is an important exposure mechanism. This could happen during manufacturing, shipping or handling of nanoparticles, especially of these are produced in bulk or powder form. However, exposure could also happened through the wear and tear of the finished product, e.g., shedding of particles from car tires that include a nanomaterials such as carbon nanotubes. While to date there has been no examples of lung pathology in humans due to the inhalation of engineered nanoparticle, copious experimental evidence has been provided for the generation of pulmonary inflammation and interstitial fibrosis by metal oxide nanoparticles and carbon nanotubes, respectively. Thus, the potential exists that nanoparticles could lead to lung disease in humans. While the understanding of the toxic potential of NPs is very limited, nanotoxicology is a new area of science that is looking at the possibility that the novel physicochemical properties of nanomaterials could give rise to hereto-unseen adverse biological outcomes [3, 118–120].
Among the possible mechanisms of NP-induced injury, ROS generation remains an important consideration [3, 48]. It may be useful therefore to compare engineered to ambient NP in terms of similarities as well as differences in the generation of oxidant injury (Table 2, Fig. 2). For example, similar to ambient UFP, some engineered NP are capable of abiotic ROS generation. The proposed NP properties that could lead to this outcome are depicted in Figure 3. The first is the formation of electron hole pairs in TiO2 NP by UV activation. The distribution of some of the hole pairs to the particle surface could participate in the electron donor or capture interactions that generate superoxide or hydroxyl radicals (OH·), respectively (Fig. 3, upper left quadrant). A second mechanism could be that an excited energy state in a semiconductor NP could lead to an electron jumping from the conduction band to O2 to generate (Fig. 3, upper right quadrant). Examples of such materials include fullerenes and TiO2. A third mechanism is the dissolution of NP and release of metal ions (e.g. ZnO → Zn2+) that catalyze ROS generation (Fig. 3, lower left quadrant). Finally, transition metals on the nanomaterial surface (e.g. Fe2+ on carbon NT or metal NP) can generate via Fenton reaction (Fig. 3, lower right quadrant). Thus, similar to ambient UFP, ROS generation by nanomaterials could lead to possible adverse biological effects through an oxidant injury mechanism. The magnitude, localization and site of tissue injury will depend on where the exposure to the nanomaterials takes place. ROS generation by the particle can lead to protein, lipids and membrane damage [3]. In addition, once the NPs are taken up into the cell, for their interaction with subcellular organelles and biological systems can lead to further ROS production (Fig. 2) [3, 21, 121]. One example is disruption of one-electron transfers in the mitochondrial inner membrane.
Figure 2.
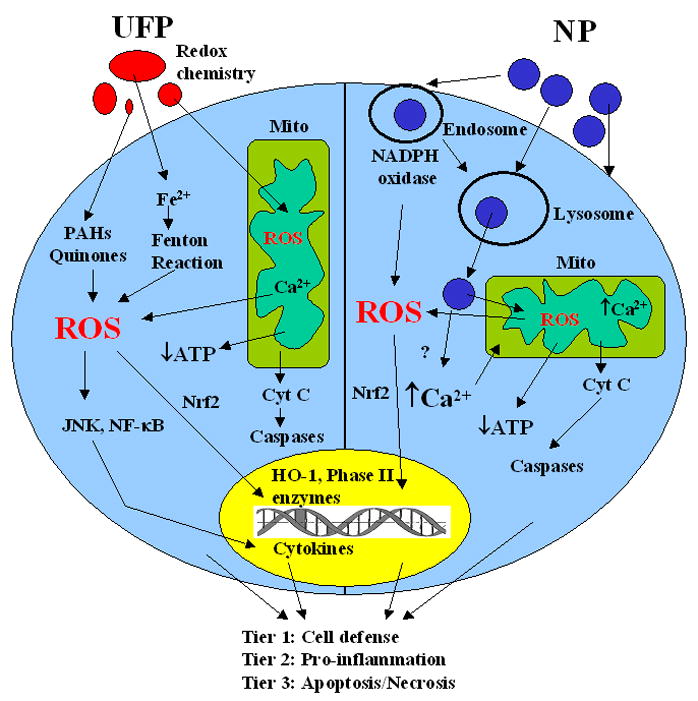
Comparison of the mechanisms of ROS generation induced by UFP and NM out- or inside of cells. Ambient UFP usually contains large amount of organic chemical such as PAHs and quinines and transition metals such as Fe, Cu, which can generate ROS through redox chemistry both out- and inside of cells. UFP have also been found to lodge in mitochondria, causing damage to mitochondrial function and structure, which can also produce more ROS. Cells under oxidative stress will have tiered responses including cell defense (Tier 1), pro-inflammation (Tier 2), and mitochondria-mediated cell death (Tier 3). NM are uniform in size, can also generate ROS via crystal structural defects or under UV conditions. NM are taken up into cells via endocytosis, which includes phagocytosis, clathrin-dependent endocytosis, caveolae-mediated endocytosis, or macropinocytosis depending on specific cell types. After cells take up NM, endosomes are formed, ROS can be produced via the formation of NADPH oxidase. After a series of fusion and fission processes, endosomes will fuse with lysosomes. NM can break loose from lysosomes and interact with other organelles such as mitochondria, which can produce more ROS. The cells under oxidative stress will go through tiered oxidative stress responses as described previously.
Figure 3.
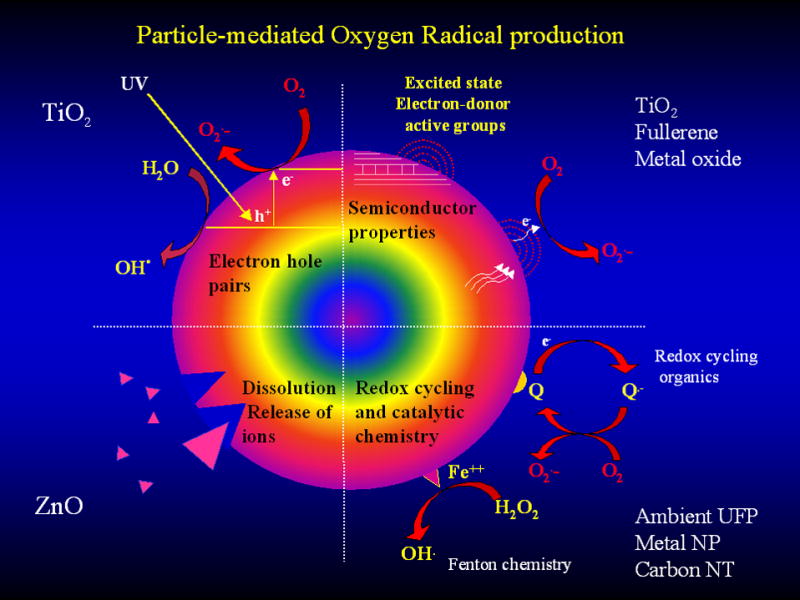
NM surface properties that are responsible for ROS generation. The valence and conductance bands of semiconductor NM can generate electronic states that lead to the formation of , which through dismutation or Fenton chemistry is capable of generating additional ROS. Additionally, photoactivation of TiO2 could generate electron hole pairs that generate and OH. radicals. Transition metals and redox cycling organic chemicals on the particle surface can also participate in ROS generation. Dissolution of the particle surface with the release of metal ions could be particularly relevant to ZnO particle toxicity. These dissolution characteristics could vary with the free surface energy of the particles as well as the pH of the environment or the cell.
Thus, in addition to the intrinsic properties of the material that could generate ROS, additional nanomaterials properties that are responsible for biological interactions could contribute to further ROS production. It is possible, therefore, that engineered NP that are devoid of semiconductor properties, UV activation or transition metals can give rise to ROS generation by perturbing mitochondrial function. For example, cationic polystyrene nanospheres have been shown to induce lysosomal leakage, ROS (H2O2 and ) production, and mitochondrial damage, which can eventually lead to apoptosis of murine macrophages [121]. This is an example of an inert material that does not give rise to spontaneous ROS production, yet is capable of inducing ROS production under biological conditions based on the ability of the nanospheres to target mitochondria.
Due to recent introduction and rapid development of nanotechnology, controlled epidemiological studies on the adverse health effects of NPs are basically non-existing. Animal models for investigating NP toxicity are current being developed and the number of reports demonstrating the pro-inflammatory effects of NPs in the lung is increasing. For example, intratracheal instillation of a low dose of ultrafine colloidal silica particles (UFCS) elicited moderate to severe pulmonary inflammation and tissue injury in ICR mice. While UFCS induced moderate lung inflammation during the acute phase, a significant increase in the apoptotic index could be seen in the lung parenchyma at all times. These lesions correlate with the induction of 8-hydroxyguanosine (8-OHdG) as an oxidative stress marker in lung epithelial cells and activated macrophages [122]. Subacute exposure of C57B1/6 mice to 2–5 nm TiO2 NPs in a whole-body exposure chamber caused a moderate but significant inflammatory response in the lung within the first two weeks of exposure, beyond which the inflammation resolved without permanent damage [123]. Inoue et al have demonstrated that intratracheal administration of 14 nm and 56 nm carbon black NPs induced slight lung inflammation and significant pulmonary edema compared with the vehicle [124].
However, when 14 nm carbon black NPs were co-administered with bacterial endotoxin, these particles intensively aggravated LPS-induced lung inflammation and pulmonary edema [124]. This pathology was accompanied by increased expression of pro-inflammatory cytokines such as interleukin-1β (IL-1β), macrophage inflammatory protein-1α (MIP-1α), macrophage chemoattractant protein-1, MIP-2, and keratinocyte chemoattractant. The level of 8-OHdG in the lung was increased by the nanoparticles independent of the effects of LPS, suggesting that engineered NP could promote the effects of other environmental or inhaled stimuli [124]. The same group also investigated the effects of repeated pulmonary exposure to carbon NPs on the expression of a variety of cytokines in the absence or presence of OVA in ICR mice. These studies have also shown that pulmonary exposure to carbon NP induced the expression of thymus and activation-regulated chemokine (TARC), GM-CSF, and MIP-1α in the lung in the absence of OVA. However, in the presence of OVA, the NP considerably enhanced the expression of TARC, GM-CSF, MIP-1α, IL-2, and IL-10. This enhancing effect was inversely related to the particle size [124]. These authors also went on to show that engineered NP may exert adjuvant effects on OVA-related airway inflammation, similar to what we have shown for ambient PM. This adjuvant effect resulted in exaggerated eosinophil, neutrophil, and mononuclear cell infiltration, as well as an increase in OVA-specific IgG and IgE production. The combination of NP with OVA also increased the formation of 8-OHdG and the production of IL-5, IL-6, IL-13, eotaxin, MCP-1, and RANTES in the lung compared with OVA alone [124].
All considered, the available data suggest that engineered NP may contribute to pulmonary morbidity by eliciting pro-inflammatory effects in the lung and/or by acting as an adjuvant for allergic inflammation. It is possible, therefore, that through the elicitation of an oxidative stress mechanism, engineered NP may contribute to pro-inflammatory disease processes in the lung.
There is no evidence at this stage, however, that engineered NP is contributing to any known human pulmonary disease. Understanding the link between particle-induced oxidative stress and inflammation provides us with a toxicological paradigm on which to base the toxicity screening of engineered NP.
Conclusions
It has been established that there is a close association between exposure to ambient particulate pollutants and increased cardiorespiratory morbidity and mortality. Among the ambient particles, the pulmonary effects of PM10 and PM2.5 have been more extensively studied than the effects of UFP. However, given the physical chemical properties of UFP, it is very likely that these particles are more dangerous from the perspective of oxidant injury and inflammation than larger sized particles. Moreover, rapid expansion of the field of nanotechnology has introduced the potential that engineered NP may also become airborne and may contribute to pulmonary disease by novel mechanisms of injury that could include oxidant injury. Thus, the potential exists that these materials could contribute to adverse health effects and we need to take that into consideration in developing methods to screen for NP toxicity. The oxidative stress paradigm currently constitutes one of the best toxicological paradigms on which to base the screening for NP toxicity. However, novel paradigms for injury should also be considered.
Acknowledgments
Funding for this study was provided by the US Public Health Service Grants, U19 AI070453, RO1 ES10553, and RO1 ES015498, as well as the U.S. EPA STAR award (RD-83241301) to the Southern California Particle Center. This work is also supported by the UC Lead Campus for Nanotoxicology Training and Research, funded by UC TSR&TP. This work has not been subjected to the EPA for peer and policy review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 2.MacNee W, Donaldson K. Exacerbations of COPD - Environmental mechanisms. Chest. 2000;117:390S–397S. doi: 10.1378/chest.117.5_suppl_2.390s. [DOI] [PubMed] [Google Scholar]
- 3.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 4.Sunyer J, Basagana X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol. 2001;30:1138–1140. doi: 10.1093/ije/30.5.1138. [DOI] [PubMed] [Google Scholar]
- 5.Downs SH, Schindler C, Liu L-JS, Keidel D, Bayer-Oglesby L, Brutsche MH, Gerbase MW, Keller R, Kunzli N, Leuenberger P, Probst-Hensch NM, Tschopp JM, Zellweger JP, Rochat T, Schwartz J, ckermann-Liebrich U the SAPALDIA Team. Reduced Exposure to PM10 and Attenuated Age-Related Decline in Lung Function. The New England Journal of Medicine. 2007 12/6;357:2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 6.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, Chung KF, Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. The New England Journal of Medicine. 2007 12/6;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 7.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. The New England Journal of Medicine. 2007 2/1;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 8.Mittleman MA. Air pollution, exercise, and cardiovascular risk. N Engl J Med. 2007 9/13;357:1147–1149. doi: 10.1056/NEJMe078139. [DOI] [PubMed] [Google Scholar]
- 9.Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007 Oct;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002 1/29;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 11.Nemmar A, Nemery B, Hoet PH, Vermylen J, Hoylaerts MF. Pulmonary inflammation and thrombogenicity caused by diesel particles in hamsters: role of histamine. Am J Respir Crit Care Med. 2003 12/1;168:1366–1372. doi: 10.1164/rccm.200306-801OC. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005 12/21;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 13.Xia T, Kovochich M, Nel A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin Occup Environ Med. 2006;5:817–836. doi: 10.1016/j.coem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Kim S, Wang M, Froines J, Sioutas C, Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal Toxicol. 2002;14:459–486. doi: 10.1080/089583701753678571. [DOI] [PubMed] [Google Scholar]
- 16.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 18.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 19.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiura TS, Li N, Kaplan R, Horwitz M, Seagrave JC, Nel AE. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165:2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- 21.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai Y, Arimoto T, Shinyashiki M, Shimojo N, Nakai Y, Yoshikawa T, Sagai M. Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radic Biol Med. 1997;22:479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- 24.Monks TJ, Hanzlik RP, Cohen GM, Ross D, Graham DG. Quinone chemistry and toxicity. Toxicol Appl Pharmacol. 1992;112:2–16. doi: 10.1016/0041-008x(92)90273-u. [DOI] [PubMed] [Google Scholar]
- 25.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 26.Penning TM, Burczynski ME, Hung CF, McCoull KD, Palackal NT, Tsuruda LS. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem Res Toxicol. 1999;12:1–18. doi: 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- 27.Baulig A, Sourdeval M, Meyer M, Marano F, Baeza-Squiban A. Biological effects of atmospheric particles on human bronchial epithelial cells. Comparison with diesel exhaust particles. Toxicol In Vitro. 2003;17:567–573. doi: 10.1016/s0887-2333(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 28.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 29.Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol. 1997 Oct;146:180–188. doi: 10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- 30.Kasprzak KS. In: The role of metals in oxidative damage and redox cell signaling derangement. Koropatnick J, Zalups R, editors. New York: Taylor & Francis; 2000. pp. 477–527. [Google Scholar]
- 31.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000 8/14;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 32.Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect. 2005 Aug;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baeza-Squiban A, Bonvallot V, Boland S, Marano F. Airborne particles evoke an inflammatory response in human airway epithelium. Activation of transcription factors. Cell Biol Toxicol. 1999;15:375–380. doi: 10.1023/a:1007653900063. [DOI] [PubMed] [Google Scholar]
- 34.Rhoden CR, Lawrence J, Godleski JJ, Gonzalez-Flecha B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci. 2004 Jun;79:296–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- 35.Antonini JM, Taylor MD, Leonard SS, Lawryk NJ, Shi X, Clarke RW, Roberts JR. Metal composition and solubility determine lung toxicity induced by residual oil fly ash collected from different sites within a power plant. Mol Cell Biochem. 2004 Jan;255:257–265. doi: 10.1023/b:mcbi.0000007281.32126.2c. [DOI] [PubMed] [Google Scholar]
- 36.Schaumann F, Borm PJ, Herbrich A, Knoch J, Pitz M, Schins RP, Luettig B, Hohlfeld JM, Heinrich J, Krug N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med. 2004 10/15;170:898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- 37.Ghio AJ. Biological effects of Utah Valley ambient air particles in humans: a review. J Aerosol Med. 2004;17:157–164. doi: 10.1089/0894268041457200. [DOI] [PubMed] [Google Scholar]
- 38.Saldiva PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, Murthy GG, Diaz E, Koutrakis P, Suh H, Tsuda A, Godleski JJ. Lung inflammation induced by concentrated ambient air particles is related to particle composition. Am J Respir Crit Care Med. 2002 6/15;165:1610–1617. doi: 10.1164/rccm.2106102. [DOI] [PubMed] [Google Scholar]
- 39.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 40.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 41.von Klot S, Wolke G, Tuch T, Heinrich J, Dockery DW, Schwartz J, Kreyling WG, Wichmann HE, Peters A. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur Respir J. 2002;20:691–702. doi: 10.1183/09031936.02.01402001. [DOI] [PubMed] [Google Scholar]
- 42.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999;104:1183–1188. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–2413. [PubMed] [Google Scholar]
- 45.Kleinman MT, Sioutas C, Froines JR, Fanning E, Hamade A, Mendez L, Meacher D, Oldham M. Inhalation of concentrated ambient particulate matter near a heavily trafficked road stimulates antigen-induced airway responses in mice. Inhal Toxicol. 2007;19(Suppl 1):117–126. doi: 10.1080/08958370701495345. [DOI] [PubMed] [Google Scholar]
- 46.Muranaka M, Suzuki S, Koizumi K, Takafuji S, Miyamoto T, Ikemori R, Tokiwa H. Adjuvant activity of diesel-exhaust particulates for the production of IgE antibody in mice. J Allergy Clin Immunol. 1986;77:616–623. doi: 10.1016/0091-6749(86)90355-6. [DOI] [PubMed] [Google Scholar]
- 47.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 48.Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 49.Arimoto T, Yoshikawa T, Takano H, Kohno M. Generation of reactive oxygen species and 8-hydroxy-2′-deoxyguanosine formation from diesel exhaust particle components in L1210 cells. Jpn J Pharmacol. 1999;80:49–54. doi: 10.1254/jjp.80.49. [DOI] [PubMed] [Google Scholar]
- 50.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- 51.Li N, Wang M, Oberley TD, Sempf JM, Nel AE. Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J Immunol. 2002;169:4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- 52.Jung EJ, Avliyakulov NK, Boontheung P, Loo JA, Nel AE. Pro-oxidative DEP chemicals induce heat shock proteins and an unfolding protein response in a bronchial epithelial cell line as determined by DIGE analysis. Proteomics. 2007;7:3906–3918. doi: 10.1002/pmic.200700377. [DOI] [PubMed] [Google Scholar]
- 53.Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 2002;18:315–320. doi: 10.1023/a:1019548517877. [DOI] [PubMed] [Google Scholar]
- 54.Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol Appl Pharmacol. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 55.Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, Scheepers PT, Van Loveren H. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- 56.Hao M, Comier S, Wang M, Lee JJ, Nel A. Diesel exhaust particles exert acute effects on airway inflammation and function in murine allergen provocation models. J Allergy Clin Immunol. 2003;112:905–914. doi: 10.1016/j.jaci.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Ichinose T, Takano H, Sadakane K, Yanagisawa R, Yoshikawa T, Sagai M, Shibamoto T. Mouse strain differences in eosinophilic airway inflammation caused by intratracheal instillation of mite allergen and diesel exhaust particles. J Appl Toxicol. 2004;24:69–76. doi: 10.1002/jat.949. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto A, Hiramatsu K, Li Y, Azuma A, Kudoh S, Takizawa H, Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol. 2006;121:227–235. doi: 10.1016/j.clim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Ichinose T, Furuyama A, Sagai M. Biological effects of diesel exhaust particles (DEP). II. Acute toxicity of DEP introduced into lung by intratracheal instillation. Toxicology. 1995;99:153–167. doi: 10.1016/0300-483x(94)03013-r. [DOI] [PubMed] [Google Scholar]
- 60.Lim HB, Ichinose T, Miyabara Y, Takano H, Kumagai Y, Shimojyo N, Devalia JL, Sagai M. Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice. Free Radic Biol Med. 1998;25:635–644. doi: 10.1016/s0891-5849(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 61.Ohta K, Yamashita N, Tajima M, Miyasaka T, Nakano J, Nakajima M, Ishii A, Horiuchi T, Mano K, Miyamoto T. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104:1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 62.Sagai M, Furuyama A, Ichinose T. Biological effects of diesel exhaust particles (DEP). III. Pathogenesis of asthma like symptoms in mice. Free Radic Biol Med. 1996;21:199–209. doi: 10.1016/0891-5849(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 63.Sagai M, Saito H, Ichinose T, Kodama M, Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic Biol Med. 1993;14:37–47. doi: 10.1016/0891-5849(93)90507-q. [DOI] [PubMed] [Google Scholar]
- 64.Takano H, Lim HB, Miyabara Y, Ichinose T, Yoshikawa T, Sagai M. Manipulation of the L-arginine-nitric oxide pathway in airway inflammation induced by diesel exhaust particles in mice. Toxicology. 1999;139:19–26. doi: 10.1016/s0300-483x(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 65.Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med. 1997;156:36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 66.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, Horwitz LD, Brechun N, Diaz-Sanchez D, Nel AE. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 67.Izzotti A, Camoirano A, D’Agostini F, Sciacca S, De Naro Papa F, Cesarone CF, De Flora S. Biomarker alterations produced in rat lung by intratracheal instillations of air particulate extracts and chemoprevention with oral N-acetylcysteine. Cancer Res. 1996;56:1533–1538. [PubMed] [Google Scholar]
- 68.Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–672. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. Faseb J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 71.Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, Chung KF, Barnes PJ, Ashmore M, Newman-Taylor A. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162:161–166. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- 72.Yamaya M, Hosoda M, Ishizuka S, Monma M, Matsui T, Suzuki T, Sekizawa K, Sasaki H. Relation between exhaled carbon monoxide levels and clinical severity of asthma. Clin Exp Allergy. 2001;31:417–422. doi: 10.1046/j.1365-2222.2001.01013.x. [DOI] [PubMed] [Google Scholar]
- 73.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174:1335–1341. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol. 2007;292:L33–39. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- 75.Wan J, Diaz-Sanchez D. Phase II enzymes induction blocks the enhanced IgE production in B cells by diesel exhaust particles. J Immunol. 2006;177:3477–3483. doi: 10.4049/jimmunol.177.5.3477. [DOI] [PubMed] [Google Scholar]
- 76.Wan J, Diaz-Sanchez D. Antioxidant enzyme induction: a new protective approach against the adverse effects of diesel exhaust particles. Inhal Toxicol. 2007;19(Suppl 1):177–182. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- 77.Yanagisawa R, Takano H, Inoue KI, Ichinose T, Sadakane K, Yoshino S, Yamaki K, Yoshikawa T, Hayakawa K. Components of diesel exhaust particles differentially affect Th1/Th2 response in a murine model of allergic airway inflammation. Clin Exp Allergy. 2006;36:386–395. doi: 10.1111/j.1365-2222.2006.02452.x. [DOI] [PubMed] [Google Scholar]
- 78.Ichinose T, Takano H, Sadakane K, Yanagisawa R, Kawazato H, Sagai M, Shibamoto T. Differences in airway-inflammation development by house dust mite and diesel exhaust inhalation among mouse strains. Toxicol Appl Pharmacol. 2003;187:29–37. doi: 10.1016/s0041-008x(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 79.Sadakane K, Ichinose T, Takano H, Yanagisawa R, Sagai M, Yoshikawa T, Shibamoto T. Murine strain differences in airway inflammation induced by diesel exhaust particles and house dust mite allergen. Int Arch Allergy Immunol. 2002;128:220–228. doi: 10.1159/000064255. [DOI] [PubMed] [Google Scholar]
- 80.Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A. 2007;70:688–695. doi: 10.1080/15287390600974692. [DOI] [PubMed] [Google Scholar]
- 81.Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- 82.Boland S, Baeza-Squiban A, Fournier T, Houcine O, Gendron MC, Chevrier M, Jouvenot G, Coste A, Aubier M, Marano F. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am J Physiol. 1999;276:L604–613. doi: 10.1152/ajplung.1999.276.4.L604. [DOI] [PubMed] [Google Scholar]
- 83.Goldsmith CA, Frevert C, Imrich A, Sioutas C, Kobzik L. Alveolar macrophage interaction with air pollution particulates. Environ Health Perspect. 1997;105(Suppl 5):1191–1195. doi: 10.1289/ehp.97105s51191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin LD, Krunkosky TM, Dye JA, Fischer BM, Jiang NF, Rochelle LG, Akley NJ, Dreher KL, Adler KB. The role of reactive oxygen and nitrogen species in the response of airway epithelium to particulates. Environ Health Perspect. 1997;105(Suppl 5):1301–1307. doi: 10.1289/ehp.97105s51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohtoshi T, Takizawa H, Okazaki H, Kawasaki S, Takeuchi N, Ohta K, Ito K. Diesel exhaust particles stimulate human airway epithelial cells to produce cytokines relevant to airway inflammation in vitro. J Allergy Clin Immunol. 1998;101:778–785. doi: 10.1016/S0091-6749(98)70307-0. [DOI] [PubMed] [Google Scholar]
- 87.Yang HM, Ma JY, Castranova V, Ma JK. Effects of diesel exhaust particles on the release of interleukin-1 and tumor necrosis factor-alpha from rat alveolar macrophages. Exp Lung Res. 1997;23:269–284. doi: 10.3109/01902149709087372. [DOI] [PubMed] [Google Scholar]
- 88.Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P, Plebanski M. Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol. 2004;82:506–516. doi: 10.1111/j.0818-9641.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 89.Schijns VE. Induction and direction of immune responses by vaccine adjuvants. Crit Rev Immunol. 2001;21:75–85. [PubMed] [Google Scholar]
- 90.Lambrecht BN. Allergen uptake and presentation by dendritic cells. Curr Opin Allergy Clin Immunol. 2001;1:51–59. doi: 10.1097/01.all.0000010985.57414.74. [DOI] [PubMed] [Google Scholar]
- 91.Lambrecht BN. Dendritic cells and the regulation of the allergic immune response. Allergy. 2005;60:271–282. doi: 10.1111/j.1398-9995.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 92.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 93.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Moser M, Murphy KM. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 95.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 96.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol. 2004;172:5016–5023. doi: 10.4049/jimmunol.172.8.5016. [DOI] [PubMed] [Google Scholar]
- 97.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O’Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 98.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 100.Thepen T, McMenamin C, Girn B, Kraal G, Holt PG. Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin Exp Allergy. 1992;22:1107–1114. doi: 10.1111/j.1365-2222.1992.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 101.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Rijt LS, Prins JB, Leenen PJ, Thielemans K, de Vries VC, Hoogsteden HC, Lambrecht BN. Allergen-induced accumulation of airway dendritic cells is supported by an increase in CD31(hi)Ly-6C(neg) bone marrow precursors in a mouse model of asthma. Blood. 2002;100:3663–3671. doi: 10.1182/blood-2002-03-0673. [DOI] [PubMed] [Google Scholar]
- 103.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-Enriched Particulate Matter Functionally Activates Human Dendritic Cells. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 105.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol. 2006;118:455–465. doi: 10.1016/j.jaci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Finkelman FD, Yang M, Orekhova T, Clyne E, Bernstein J, Whitekus M, Diaz-Sanchez D, Morris SC. Diesel exhaust particles suppress in vivo INF-y production by inhibiting cytokine effects on NK and NKT cells. J Immunol. 2004;172:3808–3813. doi: 10.4049/jimmunol.172.6.3808. [DOI] [PubMed] [Google Scholar]
- 107.Peden DB. Pollutants and asthma: role of air toxics. Environ Health Perspect. 2002;110(Suppl 4):565–568. doi: 10.1289/ehp.110-1241207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J Allergy Clin Immunol. 1995;95:103–115. doi: 10.1016/s0091-6749(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 109.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001;17:909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 110.Takano H, Ichinose T, Miyabara Y, Yoshikawa T, Sagai M. Diesel exhaust particles enhance airway responsiveness following allergen exposure in mice. Immunopharmacol Immunotoxicol. 1998;20:329–336. doi: 10.3109/08923979809038548. [DOI] [PubMed] [Google Scholar]
- 111.Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164:1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- 112.Wichmann HE. Diesel exhaust particles. Inhal Toxicol. 2007;19(Suppl 1):241–244. doi: 10.1080/08958370701498075. [DOI] [PubMed] [Google Scholar]
- 113.Doornaert B, Leblond V, Galiacy S, Gras G, Planus E, Laurent V, Isabey D, Lafuma C. Negative impact of DEP exposure on human airway epithelial cell adhesion, stiffness, and repair. Am J Physiol Lung Cell Mol Physiol. 2003;284:L119–132. doi: 10.1152/ajplung.00039.2002. [DOI] [PubMed] [Google Scholar]
- 114.Ichinose T, Takano H, Miyabara Y, Sagai M. Long-term exposure to diesel exhaust enhances antigen-induced eosinophilic inflammation and epithelial damage in the murine airway. Toxicol Sci. 1998;44:70–79. doi: 10.1006/toxs.1998.2459. [DOI] [PubMed] [Google Scholar]
- 115.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med. 2006;19:74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 117.Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 119.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 122.Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K, Takano H. Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol. 2006;34:958–965. doi: 10.1080/01926230601094552. [DOI] [PubMed] [Google Scholar]
- 123.Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, Shimada A, Yoshikawa T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect. 2006;114:1325–1330. doi: 10.1289/ehp.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]


