Abstract
Objectives
To explore views on high quality diabetes care based on an analysis of existing diversity in diabetes care programmes and related quality indicators.
Methods
A review of systematic reviews was performed. Four databases (MEDLINE database of the National Library of Medicine, COCHRANE database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Database-CINAHL and Pre-Cinahl) were searched for English review articles published between November 1989 and December 2006. Methodological quality of the articles was assessed. A standardized extraction form was used to assess features of diabetes care programmes and diabetes quality indicators with special reference to those aspects that hinder the conceptualization of high quality diabetes care. Based on these findings the relationship between diversity in diabetes care programmes and the conceptualization of high quality diabetes care was further explored.
Results
Twenty-one systematic reviews met the inclusion criteria representing a total of 185 diabetes care programmes. Six elements were identified to produce a picture of diversity in diabetes care programmes and hinder their standardization: 1) the variety and relative absence of conceptual backgrounds in diabetes care programmes, 2) confusion over what is considered a constituent of a diabetes care program and components of the implementation strategy, 3) large variety in type of diabetes care programmes, settings and related goals, 4) a large number and variety in interventions and quality indicators used, 5) no conclusive evidence on effectiveness, 6) no systematic results on costs.
Conclusions
There is large diversity in diabetes care programmes and related quality indicators. From this review and our analysis on the mutual relationship between diversity in diabetes care programmes and the conceptualization of high quality diabetes care, we conclude that no single conceptual framework used to date provides a comprehensive overview of attributes of high quality diabetes care linked to quality indicators at the structure, process and outcome level. There is a need for a concerted action to develop a standardized framework on high quality diabetes care that is complemented by a practical tool to provide guidance to the design, implementation and evaluation of diabetes care programmes.
Keywords: diabetes, quality of care, systematic review
Background
Diabetes mellitus is a chronic disease for which the worldwide epidemic proportions are described at multiple occasions stressing the significant burden because of its morbidity, mortality and socio-economic cost [1–8]. Improving diabetes care is a topic of concern as evidence suggests there is still a wide variation in care among individuals, with rates of recommended care processes to be unacceptably low [9–15]. In response to deficiencies in diabetes care, and particularly after the St. Vincent Declaration [16], a large number of quality improvement programmes have been launched in primary care, outpatient, hospitals and community settings to improve care for mainly adult type 2 diabetic patients. Most of these programmes are considered ‘complex interventions’ because of their multifaceted approach to improve quality of care [17–19]. Complex interventions are characterized by a variety of interconnecting parts which may act both independently and interdependently [20]. Common labels and concepts that are used to define such programmes are integrated care [21, 22], disease management [23, 24], case management [25, 26], co-ordinated care [27] or managed care [28]. Although these programmes often have common goals, they largely vary in what elements are put forward to be associated with high quality diabetes care. Some aspects of diversity in integrated chronic care programmes have previously been documented by Ouwens and colleagues [29]. The authors highlight the difficulty of comparing the clinical and cost-effectiveness of the different programmes and stress that only 15% of the effects reported are significant, resulting from mainly short-term evaluations. Mechanisms that explain success or failure of the programmes remain unclear and for some interventions even unknown [30]. Some of the aforementioned difficulties have been documented in systematic reviews that highlight diversity in diabetes care programmes [31, 32]. There is, however, to our knowledge no review that provides a comprehensive overview of elements that produce a picture of diversity in diabetes care programmes and quality indicators. This paper is an attempt to provide such overview. In addition, we provide an in-depth analysis how different views on high quality diabetes care programmes and indicators affect the conceptualization of high quality diabetes care.
Methods
We reviewed the scientific literature to identify systematic reviews on diabetes care programmes conducted in primary care, outpatient, community and hospital settings. A review of systematic reviews was deliberately chosen as a method for this review as it allowed both the assessment of systematic reviews on elements that cause diversity in diabetes care programmes, as well as the individual programmes that were included in the reviews.
A diabetes care program was defined according to the definitions of the Disease Management Association of America [33] and applied to diabetes care. A diabetes care program is a system of coordinated health care interventions and communications using a systematic approach to diabetes care and in which patients self-care efforts are significant [33]. A ‘systematic approach to care’ was further operationalized using the classification scheme from Shojania and colleagues [34] who defined eleven distinct categories of quality improvement interventions adapted from the Cochrane Effective Practice and Organization Of Care (EPOC) group [35, 36]. These categories are: patient education, promotion of self-management, clinician education, audit and feed-back, case management, team changes, electronic patient registry, clinician reminders, facilitated relay of clinical information to clinicians, patient reminder systems and continuous quality improvement.
Data sources
Four databases (MEDLINE database of the National Library of Medicine, COCHRANE database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Database-CINAHL and Pre-Cinahl) were searched for English articles published between November 1989 and December 2006. This period was chosen since most diabetes care programmes were developed and implemented after the St. Vincent Declaration of October 1989. The following free text and (combinations of) Medical Subject Headings (MeSH) were used: primary health care, ambulatory care, hospitals, disease management, continuity of patient care, comprehensive health care, case management, patient care team, patient care planning, guidelines, practice guidelines, critical pathways, delivery of health care, delivery of integrated health care, managed care programmes, quality of health care. Furthermore, the terms shared care, coordinated care, disease management, integrated care in combination with diabetes mellitus were searched for as title and/or abstract words. A hand search of bibliographies from relevant reviews was also conducted.
Study selection
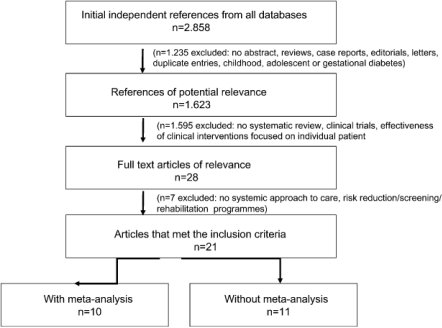
Two review authors (L.B., G.G.) independently assessed articles for eligibility, extracted data and assessed study quality. Disagreements about eligibility, extracted data and quality were resolved by consensus between these authors. Where discrepancies remained, the paper was reviewed by a third author (J.H.). Titles were rejected if they did not deal with adult patients with type 1 or type 2 diabetes or were case reports, editorials, letters and duplicate entries. Abstracts were rejected if they were not systematic reviews. Studies were rejected if they did not report on a systematic approach to care that evaluated structural and/or organizational aspects and/or outcomes of diabetes care. Reviews on risk reduction programmes, screening programmes and rehabilitation programmes were excluded from the review since they do not encompass the whole continuum of care. The selection and inclusion of the studies is presented in Box 1(QUORUM statement flow diagram).
Box 1.
QUORUM statement flow diagram: selection and inclusion of studies in the review.
Data extraction
To assess elements of diversity in diabetes care programmes a standardized extraction form (see Table 3) was created based on three items used in the systematic review on integrated care programmes by Ouwens and colleagues [29]. These authors have documented in a review of systematic reviews important diversity in integrated chronic care programmes using four items. These items are: the use of conceptual backgrounds, goals, (cost) effectiveness and ‘requirements or operational needs for successful implementation’. The latter item was not selected in our review since theoretical components that constitute a diabetes care programme became in this analysis distinguished from components that are part of an implementation strategy. We added four other items to the list that we considered important to assess diversity in diabetes care programmes and quality indicators. These items were: type of diabetes care programmes, setting of care, type and number of distinct interventions and type and number of indicators. For an overview of definitions of these different elements see Table 1. The different care programmes were classified in seven distinct categories according to the type of interventions they represented. These categories were educational programmes, professional and organizational programmes and respective combinations [37].
Table 3.
Elements of diversity evaluated in 21 systematic reviews on diabetes care programmes
| Studies | Conceptual background | Type of program | Goals | Setting | Interventions (Type/number) | Indicators (Type/number) | Effectiveness | Costs/Economic outcomes |
|---|---|---|---|---|---|---|---|---|
| Greenhalgh, 1994 [45] | X | X | ||||||
| Griffin et al., 1998 [46] | X | X | X | X | ||||
| Renders et al., 2001 [32] | X | X | X | X | X | |||
| Norris et al., 2002 [38] | X | X | X | |||||
| Norris et al., 2002 [47] | X | X | X | |||||
| Sarkisian et al., 2003 [48] | X | X | X | X | X | |||
| Gary et al., 2003 [49] | X | X | X | X | X | |||
| Loveman et al., 2003 [50] | X | X | X | X | X | |||
| Warsi et al., 2004 [51] | X | X | X | X | ||||
| Ellis et al., 2004 [52] | X | X | X | |||||
| Ofman et al., 2004 [53] | X | X | X | |||||
| Vermeire et al., 2005 [54] | X | X | X | X | ||||
| Deakin et al., 2005 [55] | X | X | X | X | X | X | ||
| Murray et al., 2005 [56] | X | X | X | X | ||||
| Ouwens et al., 2005 [29] | X | X | X | X | X | X | ||
| Knight et al., 2005 [57] | X | X | X | X | X | |||
| Bowker et al., 2005 [58] | X | X | X | X | ||||
| Shojania et al., 2006 [34] | X | X | X | X | ||||
| Glazier et al., 2006 [59] | X | X | X | X | ||||
| Steuten et al., 2006 [39] | X | X | X | X | X | X | ||
| Lindenmeyer et al., 2006 [60] | X | X | X | X | X |
Table 1.
Definitions of elements used to assess diversity in diabetes care programmes and quality indicators
| Item | Definition |
|---|---|
| 1. Presence of a conceptual background | Conceptual backgrounds refer to existing frameworks and models on high quality care, high quality diabetes care or chronic care. |
| 2. Goals | Goals of diabetes care programmes are defined as improvements in continuity, coordination, short- and long-term health or eonomic outcomes [38] and improvements in efficiency and effectiveness of care [39]. |
| 3. Type of diabetes care program | Diabetes care programmes are described as educational when the interventions are aimed at self-management abilities or disease-specific knowledge of patients; professional, when the interventions focus on changing performance of care providers or improving their adherence to guidelines; or organizational, when the interventions interfere in the structure of the primary care process or in any way aimed to improve the continuity of care [37, 40]. |
| 4. Setting | The setting refers to the type of health care setting (primary care, hospital, community) and country where the diabetes care program is conducted. |
| 5. Interventions | Interventions were identified according to the classification scheme from Shojania and colleagues [34] who defined eleven distinct categories of quality improvement interventions adapted from the Cochrane Effective Practice and Organization of Care (EPOC) group [35, 36]. These categories are: patient education, promotion of self-management, clinician education, audit and feed-back, case management, team changes, electronic patient registry, clinician reminders, facilitated relay of clinical information to clinicians, patient reminder systems and continuous quality improvement. |
| 6. Indicators | Quality or performance indicators are considered in this review as measurable items of care which focus upon some aspects of structure, process (clinical or inter-personal) or outcome and for which there is evidence or consensus that it can be used to assess the quality of care provided, and hence change it [41, 42]. |
| 7. (Cost) effectiveness of diabetes care programmes | (Cost)effectiveness is defined in this review as the degree to which the objectives of a program, care, service or system are achieved [43]. |
Methodological quality of systematic reviews
Methodological quality of the reviews was assessed using the QUORUM (Quality Of Reporting Of Meta-analyses) statement checklist [44]. The checklist describes the preferred way to present the abstract, introduction, methods, results and discussion sections of systematic reviews/meta-analysis. It is organized into 20 headings and subheadings regarding searches, selection, validity assessment, data abstraction, study characteristics, quantitative data synthesis and trial flow. A summary of the extraction is presented in Table 2.
Table 2.
QUORUM statement checklist: summary of items reported in 21 reviews (Yes/No/Not aplicable)
| Systematic reviews | Title | Abstract | Introduction | Methods | Results | Discussion |
|---|---|---|---|---|---|---|
| Greenhalgh, 1994 [45] | Y | N | Y | N | N | N |
| Griffin et al., 1998 [46] | Y | Y | Y | Y | Y | Y |
| Renders et al., 2001 [32] | Y | Y | Y | Y | Y | Y |
| Norris et al., 2002 [38] | Y | Y | Y | Y | Y | Y |
| Norris et al., 2002 [47] | Y | Y | Y | Y | Y | Y |
| Sarkisian et al., 2003 [48] | Y | Y | Y | Y | Y | Y |
| Gary et al., 2003 [49] | Y | Y | Y | Y | Y | Y |
| Loveman et al., 2003 [50] | Y | Y | Y | Y | Y | Y |
| Warsi et al., 2004 [51] | Y | Y | Y | Y | Y | Y |
| Ellis et al., 2004 [52] | Y | Y | Y | Y | Y | Y |
| Ofman et al., 2004 [53] | Y | Y | Y | Y | Y | Y |
| Vermeire et al., 2005 [54] | Y | Y | Y | Y | Y | Y |
| Deakin et al., 2005 [55] | Y | Y | Y | Y | Y | Y |
| Murray et al., 2005 [56] | Y | Y | Y | Y | Y | Y |
| Ouwens et al., 2005 [29] | Y | Y | Y | NA | NA | YY |
| Knight et al., 2005 [57] | Y | Y | Y | Y | Y | Y |
| Bowker et al., 2005 [58] | Y | Y | Y | Y | Y | Y |
| Shojania et al., 2006 [34] | Y | Y | Y | Y | Y | Y |
| Glazier et al., 2006 [59] | Y | Y | Y | Y | Y | Y |
| Steuten et al., 2006 [39] | Y | Y | Y | Y | Y | Y |
| Lindenmeyer et al., 2006 [60] | Y | Y | Y | Y | Y | Y |
Results
Description of systematic reviews
The initial search identified a total of 2,858 citations, published between November 1989 and December 2006 (Medline database 2,664 hits, Cochrane Database 127 hits and Cinahl and Pre-Cinahl basic 67 hits). After scanning titles of the citations and abstracts, 28 were accepted for further screening and full text articles were retrieved. After examination of full text articles, 21 systematic reviews were included in the review. Seven reviews were excluded since they did not report on a systematic approach to care or were risk reduction, screening or rehabilitation programmes. Ten studies were systematic reviews with a meta-analysis [32–57] and ten were systematic reviews without a meta-analysis [32–60]. One review was a review of systematic reviews [29].
Elements of diversity in diabetes care programmes
Both the 21 systematic reviews (for an overview see Table 3) as well as the total of 185 individual diabetes care programmes represented in the reviews were assessed on the features they represented. An overview of all 185 diabetes care programmes and a reference list are available upon request or can be downloaded from http://www.diabetesproject.be.
Conceptual backgrounds in diabetes care programmes
Only four of the systematic reviews explicitly analyzed the use of conceptual backgrounds in diabetes care programmes. Some reviews used the conceptual frameworks of disease and/or case management to classify diabetes care programmes under review [38, 39, 57]. Of all individual diabetes care programmes only 5% (n=9) were theory based. Disease and case management are the frameworks most commonly used in diabetes care programmes. The Chronic Care Framework of Wagner and colleagues [61] was used in only three studies. The Innovative Care for Chronic Conditions Framework [62] as well as diabetes specific conceptual frameworks were not reported.
Goals and type of diabetes care programmes
Measurement of effectiveness was the goal most commonly cited in diabetes care programmes with only very few studies specifying other types of goals. Diabetes care programmes that were classified in this review as ‘educational’ were most prevalent (27%; n=50), followed by combined ‘educational and organizational’ care programmes (16%; n=29), ‘organizational’ programmes (14%; n=26), ‘professional’ programmes (14%; n=25), combined ‘educational and professional’ programmes (12%; n=23), ‘educational, professional and organizational’ programmes (12%; n=23) and ‘professional and organizational’ programmes (5%; n=9).
Setting of diabetes care programmes
The settings in which most programmes occurred were primary care and/or community facilities (47%; n=87), with primary care physician offices to be most prevalent. Other settings included hospitals (28%; n=52), either academic or general, and hospital-based diabetes or endocrinology units. Especially U.S.-based programmes were conducted in outpatient facilities (16%; n=29), HMOs (8%; n=15) and clinical pharmacies (1%; n=2). Most of the programmes were conducted in the U.S. (57%; n=105), Europe (28%; n=52), Australia (5%; n=9), Canada (3%; n=5) and other countries including Israel, Korea, Taiwan, Cuba, New Zealand, Argentina, Hawaii and South Africa (7%; n=14), reflecting different health care systems and policies.
Interventions in diabetes care programmes
Interventions varied substantially across studies and no study reported on more than four intervention arms. The different reviews revealed the use of one to eight quality improvement interventions that defined a diabetes care programme. Forty-five percent (n=83) indicated the use of one quality improvement strategy, 18% (n=33) two strategies, 19% (n=35) three strategies, 11% (n=20) four strategies and 7% (n=14) five strategies or more. When only one intervention was described this was often referred to as shared care, team changes, facilitated relay, clinician education and case management which in their turn reflect multiple intervention strategies.
Next a description is given of the interventions found in the reviews according to the eleven categories of the Cochrane Effective Practice and Organization Of Care (EPOC) group.
Patient education/promotion of self-management Patient education and/or promotion of self-management were present in 60% (n=111) of the diabetes care programmes, although many modes of instruction and interventionists were reported. Interventions comprised didactic teaching methods (individual patient counselling and/or group counselling), didactic goal setting, goal setting negotiated teaching method, situational problem solving, cognitive reframing interventions or teaching methods such as telephone outreach and/or the provision of equipment (e.g., home glucometers), printed and electronic educational materials or access to resources (e.g., system for electronically transmitting home glucose measurements and receiving insulin dose changes based on data). Interventionists reported were nurses, dieticians, physicians, community workers, psychologists and health educators with nurse educators to be the most common interventionists to provide patient education. Education provided by clinicians is less prevalent in diabetes care programmes.
Patient reminder systems Patient reminder systems were present in 16% (n=30) of the diabetes care programmes, sometimes in combination with clinician reminder systems. They included any effort (e.g., telephone calls) to remind patients about upcoming appointments or important aspects of self-care.
Clinician education Clinician education was present in 29% (n=54) of the diabetes care programmes. These interventions comprised the promotion of an increased understanding of principles guiding clinical care or awareness of specific recommendations for a target condition or patient population. Subcategories of clinician education included distribution of educational materials, educational outreach visits and conferences or workshops.
Clinician reminders Clinician reminders were present in 16% (n=30) of the diabetes care programmes, sometimes in combination with patient reminder systems. These interventions referred to paper-based or electronic systems intended to prompt a health professional (mostly physicians) to recall patient-specific information (e.g., most recent HbA1c value) or to perform a specific task (e.g., perform a foot examination).
Team changes About one-third of the programmes (33%; n=61) included changes to the structure or organization of the primary health care team. These changes encompassed 1) the expansion or revision of professional roles (e.g., nurse or pharmacists play a more active role in patient monitoring or adjusting medication regimens), 2) adding a team member or ‘shared care’ (e.g., routine visits with personnel other than the primary care physician) or 3) the use of multidisciplinary teams in the primary ongoing management of patients.
Case management Case management was present in 12% (n=22) of the diabetes care programmes. This intervention referred to any system for coordinating diagnosis, treatment or ongoing patient management by a person or a multidisciplinary team in collaboration with or supplementary to the primary care physicians.
Facilitated relay of clinical information to clinicians Facilitated relay of clinical information to clinicians was present in 7% (n=13) of the diabetes care programmes. This type of intervention referred to clinical information collected from patients and transmitted to clinicians by means other than the existing medical record. Examples included electronic or Web-based tools through which patients provided self-care data and which clinicians reviewed, as well as point-of-care testing supplying clinicians with immediate HbA1c values.
Audit and/or feed-back Audit and/or feed-back interventions were present in 7% (n=13) of the diabetes care programmes. These interventions included summaries of clinical performance of health care delivered by an individual clinician or clinic over a specified period, which were transmitted back to the clinician (e.g., the percentage of a clinician's patients who achieved a target glycosylated hemoglobin (HbA1c) level.
Electronic patient registries/changes in electronic medical records The use of electronic patient registries and/or changes in electronic medical records was present in 7% (n=13) of the diabetes care programmes. Electronic patient registries referred to an electronic tracking system for patients with diabetes.
-
Systems for continuous quality improvement Systems for continuous quality improvement were reported in only three studies. These interventions were described as either techniques of continuous quality improvement, total quality management or any iterative process for assessing quality problems, developing solutions to those problems, testing their impacts, and then reassessing the need for further action.
Our review demonstrates that no systematic review on diabetes care program provides conclusive evidence on what kind and how many interventions should be included in a diabetes care program to ensure improvements in quality of care, irrespective of the initiator (HMO, MCO, Diabetes Care Center, community clinics, insurance company) the setting (primary care, outpatient, hospital and community settings) or the co-ordinating caregiver (general practitioner, nurse educator, internist, diabetologist or pharmacist) of the program. Another important observation is that very few systematic reviews reveal information when to implement or how to adapt the quality improvement interventions in accordance to the individual needs and preferences, ethnicity, educational level and the stage and the severity of the disease. Only one systematic review [59] reported on (features of) quality improvement interventions for socially disadvantaged populations and elderly people.
Indicators in diabetes care programmes
In diabetes care programmes, a large variety of indicators, definitions, classification schemes and measurements are used. The different reviews revealed the use from one to twelve quality indicators. Two types of broad classification schemes are reported. A first type of ‘patient indicators’ includes glycemic control, microvascular and macrovascular outcomes, resource utilization, provider-reported counselling and patient-reported self-care. Other classification schemes are based on the Donabedian triangle of structure (e.g., healthcare settings and resources), process (e.g., annual eye examinations) outcome (e.g., hospitalization rate) [63]. In this, structural indicators are defined as the physical and organizational properties of the settings in which the care is provided. Process indicators refer to all that is done for a population of patients including preventive services and measures, diagnosis and treatment. Outcome indicators are the end results of care, that is, the changes in patient or population health status, life expectancy, quality of life and health care costs [39]. Only one study reported on structural indicators referring to access and continuity of care. A combination of outcome and process indicators was measured in only 14% (n=26) of the studies. Most diabetes care programmes adopt a glucocentric view of type 2 diabetes, favoring glycemic control over indicators of micro- and macro-vascular management or cardiovascular outcomes. The most common glycemic indicators cited in the diabetes care programmes were HbA1c levels and fasting plasma glucose (FPG). Glycemic control as a process measure and outcome measure was measured in 19% (n=35) and 80% (n=148) of the studies, respectively. Indicators of micro- and macro-vascular management were measured in only 19% (n=35) of the studies. Provider-reported counselling indicators as those pertaining to foot care, self-monitoring of blood glucose and smoking cessation get very little attention in diabetes care programmes compared to indicators of glycemic control. Other provider related indicators such as satisfaction were reported in only two studies. Patient-reported or humanistic outcome indicators such as self-care behaviors, generic and diabetes-specific health related quality of life, satisfaction and psycho-social status (e.g., self-efficacy, anxiety, onset distress and depression) were identified in, respectively, 11% (n=20), 7% (n=13), 4% (n=7) and 6% (n=11) of the diabetes care programmes.
It must be noted that diabetes self-management is often an imprecisely defined term that captured activities including patient education for diabetes complications and management (e.g., foot care and self monitoring of blood glucose), alcohol, food intake and exercise advice and lifestyle visits [34]. Patient knowledge with regard to, e.g., footcare and other treatment modalities was measured in 13% (n=24) of the diabetes care programmes.
Preference-based generic health related quality of life indices such as the Health Utilities Index or Quality of Well-Being Scale are not reported. Health economic or resource utilization outcome indicators including diabetes-related and health-care practitioner visits, hospitalizations and emergency department visits and were measured in, respectively, 5% (n=9), 3% (n=5) and 1% (n=2) of the diabetes care programmes. No single care program reported on absenteeism from work. Indicators referring to costs were measured in only three of the programmes. Least measured are the extent of effective communication and patient-centered reasoning and attitudes [58]. An overall finding is that the indicators used in diabetes care programmes are operationalized with different and often not validated measurement instruments [64]. A last element found is that there is often no link between aims of the diabetes care programmes and evaluated structure, process and outcome indicators in a substantial part of studies on diabetes care programmes, especially when efficiency of care is concerned [39].
(Cost) effectiveness of diabetes care programmes
Systematic reviews that measure the effectiveness of mainly disease and case management programmes reveal modest but clinically and statistically significant improvements in HbA1c and provider compliance (annual monitoring of HbA1c, retinopathy screening, screening for foot lesions and peripheral neuropathy, lipid concentrations and proteinuria), but no improvements in hospitalization rates and duration of stay, patient satisfaction, patient knowledge, weight, body mass index, blood pressure, lipid concentrations nor compliance [34, 38, 57]. Improvements in HbA1c show on average a net decrease of –0.5% (interquartile range, –1.35% to 0.1%) [38], with only a few studies specifying what is considered an important clinical improvement over time [34]. No systematic review reported on the effectiveness of diabetes care programmes on long-term health and quality of life outcomes, including cardiovascular disease events, renal failure, amputations, visual impairment and mortality. Only three individual diabetes care programmes reported on mortality rates and one on the number of amputations after intervention.
The potential benefit of either nursing [34, 50] or pharmacist-led [60] interventions in diabetes care programmes was evaluated in only three systematic reviews. Not the professional background as such of the nurse or pharmacist case managers seemed to be important, but the possibility to make at least some independent medication changes was associated with positive effects on HbA1c [34]. In programmes that focused on the effectiveness of pharmacist-led integrated management and education programmes, important improvements in HbA1c and hypoglycemic episodes were noted. Positive effects on HbA1c were also found if algorithm-based diabetes management was applied by pharmacists. The potential benefit of other professionals including primary care physicians, diabetologists, internists and dieticians are not evaluated in a systematic way.
The costs of diabetes care programmes are not reviewed in a systematic way [29] and studies yield conflicting and limited evidence around the cost-effectiveness of diabetes care programmes. Other beneficial effects of diabetes care programmes on how the approach and infrastructure (e.g., practice guidelines, information systems and resources) could be used for the care of people with other chronic diseases has not yet been evaluated in systematic reviews either.
Discussion
This review of systematic reviews is to our knowledge the first to provide a comprehensive overview of elements that create a picture of diversity in diabetes care programmes and quality indicators. We recognize, however, the methodological limitations to our review. First, we have not searched for systematic reviews on diabetes care programmes in every available database. Second, as we solely focused on systematic reviews and the articles they represented, we recognize there is a risk for publication bias. In addition, our review has demonstrated that critical appraisal of complex interventions remains difficult with limited conclusive evidence to build on. The latter is in line with findings from Lenz and colleagues [31], who published a methodological review of systematic reviews and found that reviews do not meet the challenge to sufficiently disclose the ‘active ingredients’ [20] of complex interventions in diabetes care.
A major finding from our review is the extent of diversity in diabetes care programmes that consequently highlight different views on high quality diabetes care. For this reason we argue the need to develop a standardized framework on high quality diabetes care. In this context, ‘standardization’ does not imply the use of a rigid definition in what is considered high quality diabetes care. We must at any time recognize the import differences of the health care settings in which diabetes care programmes are conducted, as well as the cultural and socio-economic characteristics and preferences of the different target populations and providers. Before any attempt on the standardization of the concept of high quality diabetes care can take place, a profound insight is required on how diversity in diabetes care programmes, quality indicators and the conceptualization of high quality diabetes care are mutually related. We consider three elements to clarify this mutual relationship including; 1) the absence of a universally accepted definition on high quality care, and diabetes care in particular, 2) limitations that characterize existing models and frameworks on high quality (diabetes) care, and 3) poor understanding of how quality indicators at the structure, process and outcome level in diabetes care are mutually related. These elements will now be discussed in more detail.
A first element to clarify the relationship is the absence of a universally accepted definition on high quality care, and diabetes care in particular. The large number of definitions used, either generic [65–67] or disaggregated [68–72] demonstrate the complexity and multidimensionality of the concept of quality. Our review demonstrates that most studies consider effectiveness and efficiency as key attributes to define the concept of high quality diabetes care. Other attributes of high quality diabetes care including acceptability, accessibility, equity, solidarity, relevance, appropriateness, respect, choice, safety and accessibility to information [72–76] are less frequently used in diabetes care programmes. Our review also demonstrates that it is not clear from literature which interventions and indicators in diabetes care programmes should be used in relation to the aforementioned attributes. As a consequence, depending on the definition used, diabetes care programmes largely vary in the type and number of interventions and quality indicators used. In this context some authors argue the use of at least three types of quality improvement interventions to define ‘integrated’ care programmes, including a patient-related intervention to support self-management, a professional-directed intervention, and an organizational intervention [29]. Although multifaceted intervention strategies in diabetes care are considered effective on outcomes of care [77–84] they largely vary in the type and number of attributes of quality care they represent, including effectiveness, efficiency, acceptability, accessibility, equity, solidarity, relevance and appropriateness of care.
A second element to clarify the mutual relationship between diversity in diabetes care programmes and the conceptualization of high quality diabetes care is related to a number of limitations that characterize existing models and frameworks on high quality, chronic and diabetes care that provide a conceptual basis to diabetes care programmes. As these frameworks and models still present some important limitations, diabetes care programmes that build on these models present an important risk to reflect the same limitations. The Chronic Care Model (CCM) [85] for example, is a well known and highly valued conceptual model that defines the concept of high quality chronic care, and for which a related set of quality indicators exists [77–85]. The author of the CCM states that improvements in six interrelated components (community resources, self-management support, delivery system redesign, decision support, clinical information systems and organizational support) can produce system reform that enhance patient–provider interactions [77, 78, 80]. There is however, limited evidence to support the validity of this model [26, 79, 86] and additional studies seem to be necessary to determine the association of CCM components with improved outcomes [87]. Another limitation of the CCM is that it has paid little attention to requirements for successful implementation, although the CCM recognizes that improvement in the care of patients with chronic illness will only occur if system leaders make it a priority and provide the leadership, incentives and resources necessary to make improvements happen [88]. At this stage, the type and number of attributes of quality care used in the CCM provide an incomplete answer to the conceptualization of high quality diabetes care.
Only recently, improvements to the CCM have been provided by the WHO through the development of the Innovative Care for Chronic Conditions (ICCC) framework [62]. Although this generic chronic disease model is certainly of use to define the constituents of high quality diabetes care, it does not describe specific changes that must be tailored to unique needs and resources. Neither does it provide an overview of quality indicators linked to structural, processes and outcomes of care.
When we look at limitations of other, more recent frameworks that focus on high quality chronic care [39, 74], we refer to frameworks that have explicitly built on the Donabedian triangle of structure, process and outcome [63]. These frameworks have used a comparable number of attributes of quality care as used in the CCM and the ICCC, but offer limited practical guidance to the design and implementation of diabetes care programmes.
In contrast to the CCM, ICCC and frameworks based on the quality theory of Donabedian, the components of disease management as defined by the Disease Management Association of America (DMAA) [33] have been used on a larger scale in diabetes care programmes. There is, however, a diffuse picture of what disease management really is, as it reflects different forms of healthcare organization and delivery [89]. Six components are considered in a ‘full service’ disease management program, including 1) population identification processes, 2) evidence based practice guidelines or performance standards of care, 3) collaborative practice models to include physicians and support service providers, 4) patient self-management education, 5) process and outcome measurement, and 6) routine reporting and/or feed-back. Less comprehensive programmes include fewer components and are considered ‘disease management support services’.
An important limitation to the disease management framework of the DMAA is that it lacks descriptive detail on organizational, professional and patient-related attributes of quality care, neither does it provide guidance to determine what system changes or actions that organizational teams must make in order to improve outcomes of care [85]. This might be explained as existing ‘full service’ disease management programmes and research into their cost-effectiveness are still in their infancy [39].
Another concept that is often used to provide a conceptual basis to diabetes care programmes is case management. Case management is considered the assignment of authority to a professional, most commonly a nurse case manager, who oversees and is responsible for coordinating and implementing care. Case management has five essential features including 1) identification of eligible patients, 2) assessment, 3) development of an individual care plan, 4) implementation of the individual care plan, and 5) monitoring of outcomes [38]. Case management can be implemented along with disease management, as a single intervention or with other interventions.
Although substantial efforts have been made to refine the concept of disease and case management [24], these concepts do not explicitly include important attributes of high quality diabetes care that refer to equity, accessibility or acceptability of care.
Finally, diabetes specific measures that exist for the design and evaluation of quality care such as those described in the Diabetes Quality Improvement Project (DQIP 1.0 measure set) [90] focus on important components of quality care as well, but the indicators may not be detailed enough to fully evaluate specific quality improvement interventions in the context of controlled studies [64, 91]. Moreover, the latter indicators do not provide guidance in health care systems that strongly differ from US-based incentives for patient and provider behaviors [58].
A third and last element to clarify the mutual relationship between diversity in diabetes care programmes and the conceptualization of high quality diabetes is the poor understanding of how quality indicators at the structure, process and outcome level in diabetes care are mutually related. This leads to a large variability in the use of indicators in diabetes care programmes and consequently has an important impact on how high quality diabetes care is conceptualized. To illustrate this finding we refer to the large number of diabetes care programmes that favor process and outcome indicators over structural and organizational indicators of quality of care such as vision, leadership, quality culture and (financial) incentives. The latter indicators only recently get more attention [77, 92, 93] as sparse but growing evidence suggests an important relation between these indicators of quality care and health and non health(economic) related outcomes in patients and health care providers [93–99]. The same issue is noted for patient-reported or humanistic outcomes that are less frequently used compared to clinical outcomes, despite literature suggesting these variables to be valid and reliable in diabetes care [100, 101].
Conclusion
From our analysis on diversity in diabetes care programmes, quality indicators and the mutual relationship with the conceptualization of high quality diabetes care, we conclude that no single conceptual framework used to date provides a comprehensive overview of attributes of high quality diabetes care linked to quality/performance indicators at the structure, process and outcome level. No framework at this stage at the same time provides practical guidance to the design, implementation and evaluation of diabetes care programmes. We therefore argue the need for a concerted action across healthcare systems to refine the definition and concept of high quality diabetes care and to develop a standardized framework on high quality diabetes care. This framework must be flexible and allow for context specific evaluations of diabetes care programmes. The evidence offered by existing conceptual models on high quality diabetes and chronic care should serve as a theoretical basis to develop the framework. The framework must allow broadening of existing definitions and concepts of high quality diabetes care beyond the attributes of effectiveness and efficiency of care. The framework must also highlight the mutual interdependencies of all relevant diabetes quality indicators and allow for the development of a new generation of diabetes care programmes that include a broad range of quality indicators at the structure, process and outcome level of care. It is of equal importance to complement the framework by a practical tool that provides guidance to researchers, change agents and organizational teams to the implementation and evaluation of diabetes care programmes. A last prerequisite of a framework on high quality diabetes care is that it must improve international comparability of the studies and consequently provide an impetus to innovative research in quality diabetes care.
Contributor Information
Liesbeth A.D. Borgermans, Catholic University of Leuven, Faculty of Medicine, Department of General Practice, Kapucijnenvoer 33, 3000 Leuven, Belgium.
Geert Goderis, Catholic University of Leuven, Faculty of Medicine, Department of General Practice, Kapucijnenvoer 33, 3000 Leuven, Belgium.
Marielle Ouwens, Radboud University of Nijmegen, Faculty of Medicine, Centre for Quality of Care, PO Box 9101, KWAZO 114, 6500 HB Nijmegen, The Netherlands.
Johan Wens, University of Antwerp Faculty of Medicine, Department of General Practice, Integrated Health Care and Geriatrics, Universiteitsplein 1, 2610 Wilrijk, Belgium.
Jan Heyrman, Catholic University of Leuven, Faculty of Medicine, Department of General Practice, Kapucijnenvoer 33, 3000 Leuven, Belgium.
Richard P.T. M. Grol, Radboud University of Nijmegen, Faculty of Medicine, Centre for Quality of Care, PO Box 9101, KWAZO 114, 6500 HB Nijmegen, The Netherlands.
Reviewers
Lynn M. Robertson, Research Assistant, Department of Public Health, University of Aberdeen, Aberdeen, UK
Xuanping Zhang, PhD, Health scientist and team leader of the Systematic Review Work Group, Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
One anonymous reviewer
References
- 1.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. British Medical Journal. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010 . Diabetic Medicine. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 3.de Grauw WJ, van de Lisdonk EH, van den Hoogen HJ, van Weel C. Cardiovascular morbidity and mortality in type 2 diabetic patients: a 22-year historical cohort study in Dutch general practice. Diabetic Medicine. 1995;12(2):117–22. doi: 10.1111/j.1464-5491.1995.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 4.Ettaro L, Songer TJ, Zhang P, Engelgau MM. Cost-of-illness studies in diabetes mellitus. Pharmacoeconomics. 2004;22(3):149–64. doi: 10.2165/00019053-200422030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26(3):917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson B. Revealing the cost of Type II diabetes in Europe. Diabetologia. 2002;45(7):S5–12. doi: 10.1007/s00125-002-0858-x. [DOI] [PubMed] [Google Scholar]
- 7.Ostgren CJ, Engstrom S, Heurgren M, Borgquist L. Healthcare utilization is substantial for patients with type 2 diabetes in primary care: a patient-level study in a Swedish municipality. The European Journal of General Practice. 2006;12(2):83–4. doi: 10.1080/13814780600780734. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Engelgau MM, Norris SL, Gregg EW, Narayan KM. Application of economic analysis to diabetes and diabetes care. Annals of Internal Medicine. 2004;140(11):972–7. doi: 10.7326/0003-4819-140-11-200406010-00039. [DOI] [PubMed] [Google Scholar]
- 9.McBean AM, Jung K, Virnig BA. Improved care and outcomes among elderly medicare managed care beneficiaries with diabetes. The American Journal of Managed Care. 2005;11(4):213–22. [PubMed] [Google Scholar]
- 10.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, De Cristofaro A, et al. The quality of health care delivered to adults in the United States. The New England Journal of Medicine. 2003;348(26):2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 11.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA: the Journal of the American Medical Association. 2004;291(3):335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. British Medical Journal. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth EL, Majumdar SR, Guirguis LM, Lewanczuk RZ, Lee TK, Johnson JA. Compliance with clinical practice guidelines for type 2 diabetes in rural patients: treatment gaps and opportunities for improvement. Pharmacotherapy. 2003;23(5):659–65. doi: 10.1592/phco.23.5.659.32203. [DOI] [PubMed] [Google Scholar]
- 14.Harris SB, Stewart M, Brown JB, Wetmore S, Faulds C, Webster-Bogaert S, et al. Type 2 diabetes in family practice. Room for improvement. Canadian Family Physician. 2003;49:778–85. [PMC free article] [PubMed] [Google Scholar]
- 15.McFarlane SI, Jacober SJ, Winer N, Kaur J, Castro JP, Wui MA, et al. Control of cardiovascular risk factors in patients with diabetes and hypertension at urban academic medical centers. Diabetes Care. 2002;25(4):718–23. doi: 10.2337/diacare.25.4.718. [DOI] [PubMed] [Google Scholar]
- 16.Saint Vincent Declaration. 1989 Oct [cited May 2007]. Available from: http://www.crag.scot.nhs.uk/topics/diabetes/vincent.htm.
- 17.Muhlhauser I, Berger M. Patient education-evaluation of a complex intervention. Diabetologia. 2002;45(12):1723–33. doi: 10.1007/s00125-002-0987-2. [DOI] [PubMed] [Google Scholar]
- 18.Muhlhauser I. Evidenzbasierte Therapie- und Schulungsprogramme-Evaluation komplexer Interventionen. [Evidence-based treatment and education programs-evaluation of complex interventions]. Zeitschrift für äuarztliche Fortbildung und Qualitäuatssicherung. 2003;97(4–5):251–6. [PubMed] [Google Scholar]
- 19.Steuten LM, Vrijhoef HJ, van Merode GG, Severens JL, Spreeuwenberg C. The health technology assessment-disease management instrument reliably measured methodologic quality of health technology assessments of disease management. Journal of Clinical Epidemiology. 2004;57(9):881–8. doi: 10.1016/j.jclinepi.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 20.MRC Medical Research Council . A framework for the development and evaluation of RCTs for complex interventions to improve health. London: MRC Medical Research Council; 2000. [Google Scholar]
- 21.Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. British Medical Journal. 1998;316(7125):133–7. doi: 10.1136/bmj.316.7125.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodner L, Spreeuwenberg C. Integrated care: meaning , logic, applications, and implications — a discussion paper. International Journal of Integrated Care [serial online] 2002 Nov 14;2 doi: 10.5334/ijic.67. Available from: http://www.ijic.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellrodt G, Cook DJ, Lee J, Cho M, Hunt D, Weingarten S. Evidence-based disease management. The Journal of the American Medical Association. 1997;278(20):1687–92. [PubMed] [Google Scholar]
- 24.Norris SL, Glasgow RE, Engelgau MM, O'Connor PJ, McCulloch D. Chronic disease management: a definition and systematic approach to component interventions. Current opinion. Disease Management & Health Outcomes. 2003;11(8):477–88. [Google Scholar]
- 25.DeBusk RF, West JA, Miller NH, Taylor CB. Chronic disease management: treating the patient with disease(s) vs. treating disease(s) in the patient. Archives of Internal Medicine. 1999;159(22):2739–42. doi: 10.1001/archinte.159.22.2739. [DOI] [PubMed] [Google Scholar]
- 26.Krein SL, Klamerus ML, Vijan S, Lee JL, Fitzgerald JT, Pawlow A, et al. Case management for patients with poorly controlled diabetes: a randomized trial. The American Journal of Medicine. 2004;116(11):732–9. doi: 10.1016/j.amjmed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Mills PD, Harvey PW. Beyond community-based diabetes management and the COAG coordinated care trial. The Australian Journal of Rural Health. 2003;11(3):131–7. [PubMed] [Google Scholar]
- 28.Miller RH, Luft HS. Managed care plan performance since 1980. A literature analysis. The Journal of the American Medical Association. 1994;271(19):1512–9. [PubMed] [Google Scholar]
- 29.Ouwens M, Wollersheim H, Hermens R, Hulscher M, Grol R. Integrated care programmes for chronically ill patients: a review of systematic reviews. International Journal for Quality in Health Care. 2005;17(2):141–6. doi: 10.1093/intqhc/mzi016. [DOI] [PubMed] [Google Scholar]
- 30.Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. British Medical Journal. 2007;334(7591):455–9. doi: 10.1136/bmj.39108.379965.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenz M, Steckelberg A, Richter B, Muhlhauser I. Meta-analysis does not allow appraisal of complex interventions in diabetes and hypertension self-management: a methodological review. Diabetologia. 2007;50(7):1375–83. doi: 10.1007/s00125-007-0679-z. [DOI] [PubMed] [Google Scholar]
- 32.Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care , outpatient and community settings. Cochrane Database of Systematic Reviews. 2001;(1):CD001481. doi: 10.1002/14651858.CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Disease Management Association of America [webpage on the internet]. c2007 [cited 2007 Jun]. Available from: http://www.dmaa.org/
- 34.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. The Journal of the American Medical Association. 2006;296(4):427–40. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 35.Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Medical Care. 2001;39(8 Suppl 2):II2–45. [PubMed] [Google Scholar]
- 36.Shojania KG, McDonald KM, Wachter RM, Owens DK. Closing the quality gap: a critical analysis of quality improvement strategies: Volume 1-Series Overview and Methodology, Agency for Healthcare Research and Quality Publication No. 04-0051-1. Rockville MD: AHRQ; 2004. [cited 2007 May]. Available from: http://www.ahrq.gov/downloads/pub/evidence/pdf/qualgap1/qualgap1.pdf. [PubMed] [Google Scholar]
- 37.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Jr, et al. Interventions used in disease management programmes for patients with chronic illness — which ones work? Meta-analysis of published reports. British Medical Journal. 2002;325(7370):925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. American Journal of Preventive Medicine. 2002;22(4 Suppl):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 39.Steuten L, Vrijhoef B, Severens H, van Merode F, Spreeuwenberg C. Are we measuring what matters in health technology assessment of disease management? Systematic literature review. International Journal of Technology Assessment in Health Care. 2006;22(1):47–57. doi: 10.1017/s0266462306050835. [DOI] [PubMed] [Google Scholar]
- 40.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. British Medical Journal. 2003;327(7425):1219–21. doi: 10.1136/bmj.327.7425.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell SM, Braspenning J, Hutchinson A, Marshall M. Research methods used in developing and applying quality indicators in primary care. Quality & Safety in Health Care. 2002;11(4):358–64. doi: 10.1136/qhc.11.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donabedian A. Evaluating the quality of medical care. The Milbank Memorial Fund Quarterly. 1966;44(3) Suppl-206. [PubMed] [Google Scholar]
- 43.Long AF, Harrison S. Effectiveness: definitions and approaches. In: Long AF, Harrison S, editors. Health services performance: Effectiveness and efficiency. London: Croom Helm; 1985. pp. 1–9. [Google Scholar]
- 44.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 45.Greenhalgh PM. Shared care for diabetes. A systematic review. Occasional Paper (Royal College of General Practitioners) 1994;(67):1–35. [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin S, Kinmonth AL. Diabetes care: the effectiveness of systems for routine surveillance for people with diabetes. [Review] Cochrane Database of Systematic Reviews. 2000;(2):CD000541. doi: 10.1002/14651858.CD000541. [DOI] [PubMed] [Google Scholar]
- 47.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 48.Sarkisian CA, Brown AF, Norris KC, Wintz RL, Mangione CM. A systematic review of diabetes self-care interventions for older, African American, or Latino adults. The Diabetes Educator. 2003;29(3):467–79. doi: 10.1177/014572170302900311. [DOI] [PubMed] [Google Scholar]
- 49.Gary TL, Genkinger JM, Guallar E, Peyrot M, Brancati FL. Meta-analysis of randomized educational and behavioral interventions in type 2 diabetes. The Diabetes Educator. 2003;29(3):488–501. doi: 10.1177/014572170302900313. [DOI] [PubMed] [Google Scholar]
- 50.Loveman E, Royle P, Waugh N. Specialist nurses in diabetes mellitus. [Review] [79 refs] Cochrane Database of Systematic Reviews. 2003;(2):CD003286. doi: 10.1002/14651858.CD003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Archives of Internal Medicine. 2004;164(15):1641–9. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 52.Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: a meta-analysis and meta-regression. Patient Education and Counseling. 2004;52(1):97–105. doi: 10.1016/s0738-3991(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 53.Ofman JJ, Badamgarav E, Henning JM, Knight K, Gano AD, Jr, Levan RK, et al. Does disease management improve clinical and economic outcomes in patients with chronic diseases? A systematic review. The American Journal of Medicine. 2004;117(3):182–92. doi: 10.1016/j.amjmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. [Review] [125 refs] Cochrane Database of Systematic Reviews. 2005;(2):CD003638. doi: 10.1002/14651858.CD003638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2005;(2):CD003417. doi: 10.1002/14651858.CD003417.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive health communication applications for people with chronic disease.[update of Cochrane Database Syst Rev 2004;(4):CD004274] [Review]. Cochrane Database of Systematic Reviews. 2005;(4):CD004274. [Google Scholar]
- 57.Knight K, Badamgarav E, Henning JM, Hasselblad V, Gano AD, Jr, Ofman JJ, et al. A systematic review of diabetes disease management programs. The American Journal of Managed Care. 2005;11(4):242–50. [PubMed] [Google Scholar]
- 58.Bowker SL, Majumdar SR, Johnson JA. Systematic review of indicators and measurements used in controlled studies of quality improvement for type 2 diabetes. Canadian Journal of Diabetes. 2005;29(3):230–8. [Google Scholar]
- 59.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29(7):1675–88. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 60.Lindenmeyer A, Hearnshaw H, Vermeire E, Van Royen P, Wens J, Biot Y. Interventions to improve adherence to medication in people with type 2 diabetes mellitus: a review of the literature on the role of pharmacists. Journal of Clinical Pharmacy and Therapeutics. 2006;31(5):409–19. doi: 10.1111/j.1365-2710.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 61.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Effective Clinical Practice. 1998;1(1):2–4. [PubMed] [Google Scholar]
- 62.World Health Organization Innovative care for chronic conditions: building blocks for action. Global report. Geneva: 2002. Available from: http://www.who.int/diabetesactiononline/about/icccglobalreport.pdf.
- 63.Donabedian A. The quality of care. How can it be assessed? The Journal of the American Medical Association. 1988;260(12):1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 64.Majumdar SR, Johnson JA, Bowker SL, Booth GL, Dolovich L, Ghali W, et al. A Canadian consensus for the standardized evaluation of quality improvement interventions in type 2 diabetes. Canadian Journal of Diabetes. 2005;29(3):220–9. [Google Scholar]
- 65.Ellis R, Whittington D. Quality assurance in health care: a handbook. London [etc.]: Arnold, a division of Hodder & Stoughton; 1993. [Google Scholar]
- 66.Institute of Medicine . Crossing the quality chasm: the IOM Health Care Quality Initiative. Washington: Institute of Medicine; 2007. [cited 2007 Mar]; Available from: http://www.iom.edu/CMS/8089.aspx. [Google Scholar]
- 67.Juran JM. Juran on planning for quality. New York: Free Press; 1988. [Google Scholar]
- 68.Donabedian A. Explorations in quality assessment and monitoring. Volume I: The definition of quality and approaches to its assessment. Ann Arbor, MI: Health Administration Press; 1980. [Google Scholar]
- 69.Lohr KN. A strategy for quality assurance. Volume I. Washington DC: The National Academies Press; 1990. [PubMed] [Google Scholar]
- 70.Lohr KN, Donaldson MS, Harris-Wehling J. Medicare: a strategy for quality assurance , V: Quality of care in a changing health care environment. Quality Review Bulletin. 1992;18(4):120–6. doi: 10.1016/s0097-5990(16)30518-8. [DOI] [PubMed] [Google Scholar]
- 71.Peters T. Thriving on chaos: handbook for a management revolution. New York: Harper-Perennial; 1987. [Google Scholar]
- 72.Campbell SM, Roland MO, Buetow SA. Defining quality of care. Social Science & Medicine. 2000;51(11):1611–25. doi: 10.1016/s0277-9536(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 73.Boelen C. Toward unity for health: challenges and opportunities for partnership in health development. A Working paper. Geneva: WHO, Policy Department of Organization of Health delivery; 2000. [WHO/EIP/OSD/2000.9.]. Available from: http://www.who.int/hrh/documents/en/TUFH_challenges.pdf. [Google Scholar]
- 74.De Maeseneer JM, van Driel ML, Green LA, van Weel C. The need for research in primary care. Lancet. 2003;362(9392):1314–9. doi: 10.1016/S0140-6736(03)14576-X. [DOI] [PubMed] [Google Scholar]
- 75.Maxwell RJ. Dimensions of quality revisited: from thought to action. Quality in Health Care. 1992;1(3):171–7. doi: 10.1136/qshc.1.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moss F. Quality in health care: getting to the heart of the matter. In: Best R, editor. The quest for excellence: essays in honour of Robert J Maxwell. London: King's Fund; 1998. [Google Scholar]
- 77.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model , Part 2. The Journal of the American Medical Association. 2002;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 78.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. The Journal of the American Medical Association. 2002;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 79.Piatt GA, Orchard TJ, Emerson S, Simmons D, Songer TJ, Brooks MM, et al. Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care. 2006;29(4):811–7. doi: 10.2337/diacare.29.04.06.dc05-1785. [DOI] [PubMed] [Google Scholar]
- 80.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank Quarterly. 1996;74(4):511–44. [PubMed] [Google Scholar]
- 81.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Managed Care Quarterly. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 82.Wagner EH. The role of patient care teams in chronic disease management. British Medical Journal. 2000;320(7234):569–72. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24(4):695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- 84.Wagner EH, Groves T. Care for chronic diseases. British Medical Journal. 2002;325(7370):913–4. doi: 10.1136/bmj.325.7370.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonomi AE, Wagner EH, Glasgow RE, VonKorff M. Assessment of chronic illness care (ACIC): a practical tool to measure quality improvement. Health Services Research. 2002;37(3):791–820. doi: 10.1111/1475-6773.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olivarius NF, Beck-Nielsen H, Andreasen AH, Horder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. British Medical Journal. 2001;323(7319):970–5. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sperl-Hillen JM, Solberg LI, Hroscikoski MC, Crain AL, Engebretson KI, O'Connor PJ. Do all components of the chronic care model contribute equally to quality improvement? Joint Commission Journal on Quality and Safety. 2004;30(6):303–9. doi: 10.1016/s1549-3741(04)30034-1. [DOI] [PubMed] [Google Scholar]
- 88.Epping-Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of health care for chronic conditions. Quality & Safety in Health Care. 2004;13(4):299–305. doi: 10.1136/qshc.2004.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kesteloot K. Disease management. A new technology in need of critical assessment. International Journal of Technology Assessment in Health Care. 1999;15(3):506–19. [PubMed] [Google Scholar]
- 90.Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The diabetes quality improvement project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24(10):1815–20. doi: 10.2337/diacare.24.10.1815. [DOI] [PubMed] [Google Scholar]
- 91.Booth GL, Zinman B, Redelmeier DA. Diabetes care in the U.S. and Canada. Diabetes Care. 2002;25(7):1149–53. doi: 10.2337/diacare.25.7.1149. [DOI] [PubMed] [Google Scholar]
- 92.Committee on Quality of Health Care in America IoM . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: the IOM Quality of Care Initiative; 2001. [Google Scholar]
- 93.Heisler M, Wagner EH. Improving diabetes treatment quality in managed care organizations: some progress, many challenges. The American Journal of Managed Care. 2004;10(2 Pt 2):115–7. [PubMed] [Google Scholar]
- 94.Rundall TG, Shortell SM, Wang MC, Casalino L, Bodenheimer T, Gillies RR, et al. As good as it gets? Chronic care management in nine leading US physician organisations. British Medical Journal. 2002;325(7370):958–61. doi: 10.1136/bmj.325.7370.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaix-Couturier C, Durand-Zaleski I, Jolly D, Durieux P. Effects of financial incentives on medical practice: results from a systematic review of the literature and methodological issues. International Journal for Quality in Health Care. 2000;12(2):133–42. doi: 10.1093/intqhc/12.2.133. [DOI] [PubMed] [Google Scholar]
- 96.Fleming B, Silver A, Ocepek-Welikson K, Keller D. The relationship between organizational systems and clinical quality in diabetes care. The American Journal of Managed Care. 2004;10(12):934–44. [PubMed] [Google Scholar]
- 97.Khunti K, Ganguli S, Baker R, Lowy A. Features of primary care associated with variations in process and outcome of care of people with diabetes. The British Journal of General Practice. 2001;51(466):356–60. [PMC free article] [PubMed] [Google Scholar]
- 98.Klein R. Can policy drive quality? Quality in Health Care. 1998;7(Suppl):S51–3. [PubMed] [Google Scholar]
- 99.Li R, Simon J, Bodenheimer T, Gillies RR, Casalino L, Schmittdiel J, et al. Organizational factors affecting the adoption of diabetes care management processes in physician organizations. Diabetes Care. 2004;27(10):2312–6. doi: 10.2337/diacare.27.10.2312. [DOI] [PubMed] [Google Scholar]
- 100.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–43. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 101.Maddigan SL, Majumdar SR, Toth EL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with varying degrees of disease severity in type 2 diabetes. Health and Quality of Life Outcomes. 2003;1(1):78. doi: 10.1186/1477-7525-1-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maddigan SL, Majumdar SR, Guirguis LM, Lewanczuk RZ, Lee TK, Toth EL, et al. Improvements in patient-reported outcomes associated with an intervention to enhance quality of care for rural patients with type 2 diabetes: results of a controlled trial. Diabetes Care. 2004;27(6):1306–12. doi: 10.2337/diacare.27.6.1306. [DOI] [PubMed] [Google Scholar]