Summary
Background
Malawi, which has about 80 000 deaths from AIDS every year, made free antiretroviral therapy available to more than 80 000 patients between 2004 and 2006. We aimed to investigate mortality in a population before and after the introduction of free antiretroviral therapy, and therefore to assess the effects of such programmes on survival at the population level.
Methods
We used a demographic surveillance system to measure mortality in a population of 32 000 in northern Malawi, from August, 2002, when free antiretroviral therapy was not available in the study district, until February, 2006, 8 months after a clinic opened. Causes of death were established through verbal autopsies (retrospective interviews). Patients who registered for antiretroviral therapy at the clinic were identified and linked to the population under surveillance. Trends in mortality were analysed by age, sex, cause of death, and zone of residence.
Findings
Before antiretroviral therapy became available in June, 2005, mortality in adults (aged 15–59 years) was 9·8 deaths for 1000 person-years of observation (95% CI 8·9–10·9). The probability of dying between the ages of 15 and 60 years was 43% (39–49) for men and 43% (38–47) for women; 229 of 352 deaths (65·1%) were attributed to AIDS. 8 months after the clinic that provided antiretroviral therapy opened, 107 adults from the study population had accessed treatment, out of an estimated 334 in need of treatment. Overall mortality in adults had decreased by 10% from 10·2 to 8·7 deaths for 1000 person-years of observation (adjusted rate ratio 0·90, 95% CI 0·70–1·14). Mortality was reduced by 35% (adjusted rate ratio 0·65, 0·46–0·92) in adults near the main road, where mortality before antiretroviral therapy was highest (from 13·2 to 8·5 deaths per 1000 person-years of observation before and after antiretroviral therapy). Mortality in adults aged 60 years or older did not change.
Interpretation
Our findings of a reduction in mortality in adults aged between 15 and 59 years, with no change in those older than 60 years, suggests that deaths from AIDS were averted by the rapid scale-up of free antiretroviral therapy in rural Malawi, which led to a decline in adult mortality that was detectable at the population level.
Funding
Wellcome Trust and British Leprosy Relief Association.
Introduction
The combination of high rates of HIV infection and the substantial decrease in life expectancy for people with untreated HIV/AIDS has changed mortality rates at the population level in many African countries.1 However, although this can be demonstrated in models, empirical evidence for this effect on populations has been limited to a few community-based demographic surveillance studies2,3 since most African countries do not record vital registration data.4 Studies of changes in mortality rates associated with HIV/AIDS need information about the HIV status of individuals or about the causes of death, which can be ascertained by so-called verbal autopsies (retrospective interviews) or, more indirectly, by assessment of age-specific and sex-specific mortality.5–7
Antiretroviral therapy greatly improves survival in HIV patients.8,9 Cohort studies have shown sustained reductions in mortality rates in treated patients, in both developing and developed countries.8,9 The scale-up of programmes for antiretroviral therapy is proceeding fast, to the extent that the survival benefits might now be detectable at the population level.
In Malawi, adult HIV prevalence has stabilised at about 14% since the late 1990s.10 Antiretroviral therapy was first available in the central and southern regions, from three sites (two free and one with subsidised costs) that treated only 1500 people by 2002.11 With support from the Global Fund for AIDS, Tuberculosis and Malaria, a national programme providing free access to antiretroviral therapy began in Malawi in 2004, and by the end of December, 2006, 81 821 patients had registered for this treatment.12–14
As part of the Karonga Prevention Study in northern Malawi,15–19 we investigated the effect of the introduction of antiretroviral therapy on HIV-related mortality within a population under a demographic surveillance system based on continuous registration.
Methods
In 2002, we set up a demographic surveillance system based on continuous registration in a population of 32 000 in Karonga district. The methods have been described in detail elsewhere.20 An initial house-to-house census recorded personal identifiers and sociodemographic data for all individuals, and economic data and physical locations for all households. Continuous demographic surveillance was launched successively in 230 geographically defined clusters, each with 15–60 households, immediately after completion of the census in the respective cluster. Within each cluster, one village informant was trained to record and report births, deaths, and migrations. Births and deaths were followed up every month by investigators, and migrations were checked every year. A new census of the total population, which was initiated 2 years after the start to assess the accuracy and completeness of continuous registration, showed that the routine system had registered 516 (99%) of 521 deaths, 1540 (97%) of 1588 births, and 12 115 (92%) of 13 168 migrations.20
The national programme of free (at the point of delivery) antiretroviral therapy started in June, 2004. At that time, the nearest clinic was located about 200 km from the study area. The first clinic that provided antiretroviral therapy in Karonga district opened in June, 2005, in Karonga town, which is 80 km from the study area. According to national guidelines, patients who had HIV/AIDS in WHO clinical stage 3 or 4 or in stage 2 with a CD4 cell count of less than 250 cells per mm3 were started on a standard antiretroviral therapy regimen (of stavudine, lamivudine, and nevirapine) at the Karonga clinic.
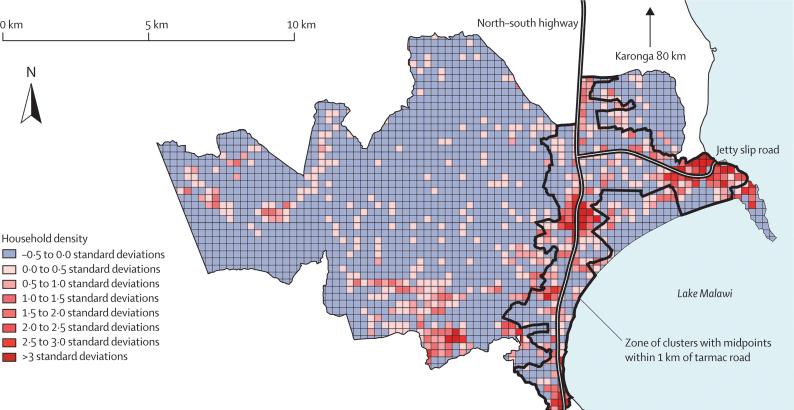
Malawi's north–south highway bisects the study area, and is the only tarmac road with public transport that links the study area to the town of Karonga. A short tarmac slip-road inside the study area leads to a small port and truck stop on the lake. Houses are typically scattered, in the north of Malawi, without obvious village boundaries, but the study area included a zone of higher density settlement, with local bars and trading centres located along the highway and by the truck stop at the lake. Previous fieldwork had shown that the population in this zone was more economically active and mobile than the rest of the population, and that HIV prevalence was much higher than in the more remote areas. A higher density zone around the tarmac roads was defined to stratify the population geographically. We used ArcView 3.1 for spatial analysis to determine the distance from the midpoints of clusters to the nearest road. Figure 1 shows a map of the relative household density in the study area and the delineation of the 1 km zone around the tarmac roads. Clusters with midpoints within 1 km of the roads covered 21% of the study area, and 41 139 person-years (51% of the entire study population) were observed within this zone.
Figure 1.
Map of the study area showing the distribution of households
Grid of 250 m × 250 m relative to the mean household density in the whole study area (45 households per km2). The zone of clusters with midpoints within 1 km of the nearest road is marked.
Each death was assigned to a cluster on the basis of the household membership of the deceased at the time of death. This definition excluded short-term moves (eg, for nursing care) that might have occurred before the death. Health assistants with additional training did verbal autopsies for all deaths within the continuous registration area. They used a semistructured questionnaire that was based on the INDEPTH standardised verbal autopsies questionnaire,21 an adaptation of the standard WHO questionnaire. Questionnaire data for each person were supplemented by a review of patient-held health records, hospital case notes, and any previous HIV test results recorded by other related studies in the area. Since 2005, interviewers asked specifically whether the deceased had been on antiretroviral therapy. Three medically or paramedically qualified individuals (medical doctors or clinical officers) independently reviewed each questionnaire to assign the most likely underlying cause of death. Direct causes of death (eg, cryptococcal meningitis in AIDS cases) were not coded. For deaths attributable to tuberculosis, HIV/AIDS was defined as the underlying cause if symptoms of immunosuppression preceded the onset of cough or other symptoms of tuberculosis, or if the individual was known to be HIV positive. If the temporal sequence between signs of immunosuppression and tuberculosis was not clear, the cause of death was classified as “tuberculosis or AIDS”. Discrepantly coded cases were discussed and resolved if possible, or coded as “non-specifiable” if consensus could not be reached.
All patients who attended the Karonga clinic for antiretroviral therapy were also invited to participate in a separate operational study that consisted of active home follow-ups of non-attenders. More than 95% of patients consented to participate in that study, and their identity details were stored in a secured area of the central project database. Ethical approval for these studies was obtained from the National Health Sciences Research Committee of Malawi and the ethics committee of the London School of Hygiene and Tropical Medicine.
Statistical analysis
We assessed overall and cause-specific mortality rates. To define the person-time denominator in the population, we observed every individual from the time they were first seen in the baseline census, from birth, or from the date of migration into the area after the baseline census, until the day of their death (if they were still a member of a household in the area at the time of death) or until migration out of the area. Multiple episodes of observation (and gaps) were allowed if individuals moved out of the surveillance area and later returned.
To allow comparison with other studies, crude and age-standardised death rates (for the whole population) and the age-standardised death rate for adults of 15 years and older are also presented, with direct standardisation to the INDEPTH standard population for sub-Saharan Africa.22 For the analysis of all-cause mortality, the failure event was death. To estimate AIDS-specific mortality, the failure event was death from AIDS or “tuberculosis or AIDS”; deaths from all other known and unknown causes were censored. To estimate mortality from all other causes except AIDS, we censored deaths from AIDS and deaths from tuberculosis or AIDS.
Because of the staggered start of the baseline census for continuous registration, geographical areas contributed different amounts of observation time in different calendar periods. We therefore calculated the probability of dying between certain ages before antiretroviral therapy was available with a Poisson regression model, weighted by the relative population size in the different enumeration areas. The lifetime risk of dying of HIV/AIDS23 was calculated from the Kaplan-Meier failure function as the cumulative probability of dying from AIDS when the curve reached its plateau (at 70 years of age).
To assess the effect of antiretroviral therapy, mortality rates were initially compared between three periods: before the start of the national roll-out, when antiretroviral therapy became available in the northern region, and when antiretroviral therapy became available in Karonga Town. Since mortality rates had not changed before the opening of the Karonga clinic (data not shown), we compared mortality rates in the first two periods combined, with those from the last period. To allow for the staggered start of the baseline census, we adjusted mortality rate ratios for enumeration area. We also adjusted them for age and sex to account for potential changes in age distribution and sex ratio over time. The Poisson regression model was stratified according to age (15–29, 30–44, 45–59, and 60 years and older) and according to the distance of each area of residence to the nearest tarmac road (whether 1 km or less, or more than 1 km).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between August, 2002, and the end of February, 2006, we recorded 916 deaths in 39 321 individuals during 81 278 person-years of observation, which equates to a crude death rate of 11 per 1000 per year (95% CI 10·6–12·0) and an age-standardised death rate of 12 per 1000 person-years of observation. Of these deaths, 574 were in adults older than 14 years (for whom we recorded 42 657 person-years of observation), and 373 deaths were in 18 927 adults aged 15–59 years (with 38 015 person-years of observation). Therefore, the adult mortality rate was 9·8 per 1000 person-years of observation (95% CI 8·9–10·9), with 9·6 (8·3–11·1) in men and 10·0 (8·6–11·5) in women.
269 (46·9%) of the 574 deaths in adults older than 14 years occurred at health facilities, 262 (45·6%) at home, 30 (5·2%) outdoors, and 13 (2·3%) at traditional healer camps. Table 1 shows the probable causes for these 574 deaths. Verbal autopsies were available for 570 (99·3%), and a probable cause was established for 509 deaths (227 [88%] of men and 282 [89%] of women). More female than male deaths were attributed to AIDS: 145 (51%) of 282 women died of AIDS, compared with 98 (43%) of 227 men (p=0·065).
Table 1.
Probable causes of death in 574 adults aged older than 14 years
|
Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15–44 years | 45–59 years | ≥60 years | Total | 15–44 years | 45–59 years | ≥60 years | Total | ||
| Communicable diseases | 83 (68·6%) | 30 (56·6%) | 22 (26·2%) | 135 (52·3%) | 115 (78·8%) | 37 (69·8%) | 29 (24·8%) | 181 (57·3%) | |
| HIV/AIDS | 61 (50·4%) | 22 (41·5%) | 8 (9·5%) | 91 (35·3%) | 99 (67·8%) | 32 (60·4%) | 5 (4·3%) | 136 (43·0%) | |
| Tuberculosis or HIV/AIDS | 6 (5·0%) | 1 (1·9%) | 0 | 7 (2·7%) | 7 (4·8%) | 1 (1·9%) | 1 (0·9%) | 9 (2·8%) | |
| Tuberculosis | 5 (4·1%) | 0 | 1 (1·2%) | 6 (2·3%) | 0 | 2 (3·8%) | 4 (3·4%) | 6 (1·9%) | |
| Pneumonia | 2 (1·7%) | 3 (5·7%) | 6 (7·1%) | 11 (4·3%) | 2 (1·4%) | 1 (1·9%) | 8 (6·8%) | 11 (3·5%) | |
| Malaria | 0 | 0 | 1 (1·2%) | 1 (0·4%) | 0 | 0 | 0 | 0 | |
| Acute febrile disease, non-specifiable | 3 (2·5%) | 1 (1·9%) | 0 | 4 (1·6%) | 1 (0·7%) | 0 | 7 (6·0%) | 8 (2·5%) | |
| Other communicable disease | 6 (5·0%) | 3 (5·7%) | 6 (7·1%) | 15 (5·8%) | 6 (4·1%) | 1 (1·9%) | 4 (3·4%) | 11 (3·5%) | |
| Non-communicable diseases | 15 (12·4%) | 16 (30·2%) | 44 (52·4%) | 75 (29·1%) | 23 (15·8%) | 14 (26·4%) | 58 (49·6%) | 95 (30·1%) | |
| Cardiovascular | 0 | 6 (11·3%) | 22 (26·2%) | 28 (10·9%) | 2 (1·4%) | 8 (15·1%) | 33 (28·2%) | 43 (13·6%) | |
| Gastrointestinal | 7 (5·8%) | 2 (3·8%) | 6 (7·1%) | 15 (5·8%) | 5 (3·4%) | 3 (5·7%) | 7 (6·0%) | 15 (4·7%) | |
| Direct obstetric | 0 | 0 | 0 | 0 | 11 (7·5%) | 0 | 0 | 11 (3·5%) | |
| Cancer | 2 (1·7%) | 2 (3·8%) | 3 (3·6%) | 7 (2·7%) | 3 (2·1%) | 2 (3·8%) | 8 (6·8%) | 13 (4·1%) | |
| Endocrine | 2 (1·7%) | 1 (1·9%) | 3 (3·6%) | 6 (2·3%) | 0 | 0 | 3 (2·6%) | 3 (0·9%) | |
| Respiratory | 0 | 1 (1·9%) | 0 | 1 (0·4%) | 0 | 0 | 1 (0·9%) | 1 (0·3%) | |
| Other non-communicable disease | 4 (3·3%) | 4 (7·5%) | 10 (11·9%) | 18 (7·0%) | 2 (1·4%) | 1 (1·9%) | 6 (5·1%) | 9 (2·8%) | |
| External causes | 11 (9·1%) | 4 (7·5%) | 2 (2·4%) | 17 (6·6%) | 2 (1·4%) | 1 (1·9%) | 3 (2·6%) | 6 (1·9%) | |
| Accidental | 8 (6·6%) | 4 (7·5%) | 1 (1·2%) | 13 (5·0%) | 2 (1·4%) | 0 | 2 (1·7%) | 4 (1·3%) | |
| Suicide | 1 (0·8%) | 0 | 1 (1·2%) | 2 (0·8%) | 0 | 0 | 1 (0·9%) | 1 (0·3%) | |
| Homicide | 0 | 0 | 0 | 0 | 0 | 1 (1·9%) | 0 | 1 (0·3%) | |
| Unknown intent | 2 (1·7%) | 0 | 0 | 2 (0·8%) | 0 | 0 | 0 | 0 | |
| Cause of death unknown | 12 (9·9%) | 3 (5·7%) | 16 (19·0%) | 31 (12·0%) | 6 (4·1%) | 1 (1·9%) | 27 (23·1%) | 34 (10·8%) | |
| Total | 121 (100·0%) | 53 (100·0%) | 84 (100·0%) | 258 (100·0%) | 146 (100·0%) | 53 (100·0%) | 117 (100·0%) | 316 (100·0%) | |
Of the 373 deaths in adults aged 15–59 years, verbal autopsies were available for 372 (99·4%). In this age-group, probable causes were established for 352 deaths (159 [91·4%] of deaths in men and 192 [96·5%] in women). 229 (65·1%) of these 352 deaths were attributed to AIDS. 139 (72·0%) women and 90 (56·6%) men died of AIDS—ie, 60·7% of all AIDS deaths were in women.
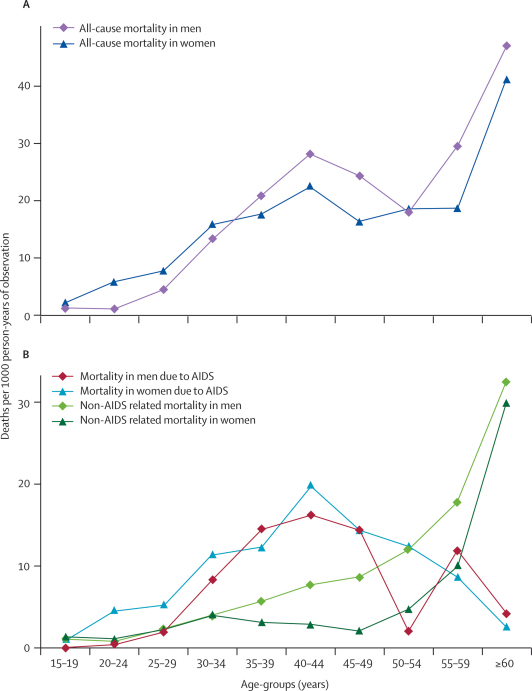
Mortality rates from all causes by sex and age in the period before antiretroviral therapy was introduced in June 2005 are shown in Figure 2. Death rates initially peaked between the ages of 40 and 44 years for both men and women, and then rose again at later ages. Death rates increased at an earlier age in women than in men; and were 3·4 times higher (95% CI 1·3–8·9) at age 20–24 years (adjusted for area). This differential was lost by the ages of 30–34 years, and at older ages rates of death in women were slightly lower than in men. Figure 2 also shows age-specific and sex-specific mortality from AIDS and from all other causes combined for the same period; the high mortality in young women and the peak between the ages of 40 and 44 years is clearly attributable to AIDS. Overall adult mortality from all causes was almost identical in women and men (rate ratio [RR] adjusted for age and area 0·97, 95% CI 0·81–1·17), but AIDS-specific mortality was higher in women (RR adjusted for age and area 1·36, 1·01–1·82). The overall age-standardised AIDS mortality rate in adults older than 14 years was 6·3 deaths per 1000 person-years of observation, with 4·9 in men and 6·5 in women.
Figure 2.
Mortality rates by sex and age-group before availability of antiretroviral therapy
Data are from August, 2002 to June, 2005, with 438 deaths in 31 665 person-years of observation.
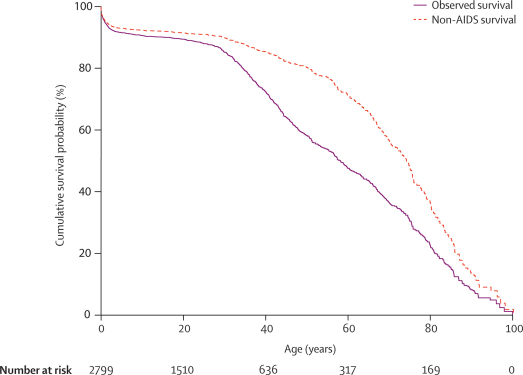
Figure 3 and table 2 show survival probability by age and adult mortality risks in the period before antiretroviral therapy. Before the opening of the Karonga clinic, the probability of dying from any cause between the exact ages of 15 and 60 years (45q15, adjusted for area) was 43% (95% CI 39–49) for men and 43% (38–47) for women. Of the 289 deaths in adults aged 15–59 years in this period, 181 (63%) were attributed to AIDS. The probability of dying of any cause other than AIDS between ages 15 and 60 years (45q15, adjusted for area) was 19% (95% CI 15–25) for men and 15% (12–20) for women. Two other measures for the probability of dying are shown in table 2 to allow comparison with other studies. Assuming unchanged HIV-incidence and mortality, a child born into the surveillance population before the introduction of antiretroviral therapy would have had a 37% lifetime risk of dying from AIDS (35% for boys and 38% for girls).
Figure 3.
Kaplan–Meier survival function for the entire study population before introduction of antiretroviral therapy
Data are from August, 2002, to June, 2005, with 438 deaths in 31 665 person-years of observation.
Table 2.
Probability of dying between the ages of 15 and 60, 20 and 50, and 30 and 65 years in the surveillance population before opening of the antiretroviral therapy clinic in June, 2005, adjusted for area
|
All-cause mortality |
Mortality from all causes except AIDS |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Deaths |
Person-years of observation |
Probability |
Deaths |
Person-years of observation |
Probability |
|||||||||||||
| All | Men | Women | All | Men | Women | All | Men | Women | All | Men | Women | All | Men | Women | All | Men | Women | |
| Ages 15–60 years | 289 | 131 | 158 | 28 244 | 13 459 | 14 786 | 43% | 43% | 43% | 89 | 51 | 38 | 28 244 | 13 459 | 14 786 | 17% | 19% | 15% |
| Ages 20–50 years | 229 | 103 | 126 | 19 696 | 9372 | 10 323 | 30% | 31% | 30% | 57 | 33 | 24 | 19 696 | 9 372 | 10 323 | 10% | 12% | 9% |
| Ages 30–65 years | 244 | 124 | 120 | 12 685 | 5772 | 6913 | 45% | 45% | 44% | 76 | 46 | 30 | 12 685 | 5 772 | 6913 | 20% | 22% | 18% |
Overall mortality rates before and after opening of the antiretroviral therapy clinic in Karonga district are shown in table 3. After the opening of the clinic, all-cause mortality in adults aged 15–59 years decreased by 10% (the rate ratio [RR] adjusted for age, sex, and area was 0·90, 95% CI 0·70–1·15), which is equivalent to nine deaths averted in the 8-month observation period after introduction of antiretroviral therapy. All-cause mortality in individuals aged 60 years and older did not change. AIDS mortality decreased by 19% in the 15–59-year age-group (adjusted RR 0·81, 0·58–1·12), with no change in mortality from causes other than AIDS.
Table 3.
Trends in adult mortality before and after opening of the antiretroviral clinic in Karonga district
|
All causes |
Adults 15–59 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15–29 years | 30–44 years | 45–59 years | ≥ 60 years | 15–59 years | All ≥15 years | AIDS death | Non-AIDS death | Unknown cause of death | ||
| Entire study population | ||||||||||
| Before antiretroviral therapy | ||||||||||
| Deaths | 59 | 148 | 82 | 149 | 289 | 438 | 181 | 89 | 19 | |
| Person-years of observation | 16 347 | 7866 | 4031 | 3421 | 28 244 | 31 665 | 28 244 | 28 244 | 28 244 | |
| Deaths per 1000 person-years of observation | 3·6 | 18·8 | 20·3 | 43·6 | 10·2 | 13·8 | 6·4 | 3·2 | 0·7 | |
| After antiretroviral therapy | ||||||||||
| Deaths | 16 | 44 | 25 | 51 | 85 | 136 | 48 | 34 | 3 | |
| Person-years of observation | 5587 | 2840 | 1344 | 1220 | 9771 | 10 991 | 9771 | 9771 | 9771 | |
| Deaths per 1000 person-years of observation | 2·9 | 15·5 | 18·6 | 41·8 | 8·7 | 12·4 | 4·9 | 3·5 | 0·3 | |
| Adjusted rate ratio | 0·83* (0·47–1·47) | 0·86* (0·61–1·21) | 0·98* (0·62–1·56) | 1·00* (0·73–1·38) | 0·90† (0·70–1·15) | 0·94† (0·77–1·14) | 0·81† (0·58–1·12) | 1·16† (0·77–1·75) | 0·52† (0·16–1·70) | |
| Area closer than 1 km to the road | ||||||||||
| Before antiretroviral therapy | ||||||||||
| Deaths | 35 | 105 | 56 | 85 | 196 | 281 | 124 | 61 | 11 | |
| Person-years of observation | 8695 | 4119 | 1985 | 1814 | 14 799 | 16 613 | 14 799 | 14 799 | 14 799 | |
| Deaths per 1000 person-years of observation | 4·0 | 25·5 | 28·2 | 46·9 | 13·2 | 16·9 | 8·4 | 4·1 | 0·7 | |
| After antiretroviral therapy | ||||||||||
| Deaths | 9 | 20 | 11 | 27 | 40 | 67 | 26 | 11 | 3 | |
| Person-years of observation | 2 713 | 1368 | 638 | 582 | 4720 | 5302 | 4720 | 4720 | 4720 | |
| Deaths per 1000 person-years of observation | 3·3 | 14·6 | 17·2 | 46·4 | 8·5 | 12·6 | 5·5 | 2·3 | 0·6 | |
| Adjusted rate ratio | 0·84* (0·40–1·80) | 0·58* (0·36–0·94) | 0·64* (0·33–1·24) | 0·99* (0·64–1·52) | 0·65† (0·46–0·92) | 0·76† (0·57–0·99) | 0·67† (0·44–1·03) | 0·57† (0·30–1·10) | 0·88† (0·25–3·04) | |
| Area further than 1 km from the road | ||||||||||
| Before antiretroviral therapy | ||||||||||
| Deaths | 24 | 43 | 26 | 64 | 93 | 157 | 57 | 28 | 8 | |
| Person-years of observation | 7651 | 3747 | 2047 | 1607 | 13 445 | 15 052 | 13 445 | 13 445 | 13 445 | |
| Deaths per 1000 person-years of observation | 3·1 | 11·5 | 12·7 | 39·8 | 6·9 | 10·4 | 4·2 | 2·1 | 0·6 | |
| After antiretroviral therapy | ||||||||||
| Deaths | 7 | 24 | 14 | 24 | 45 | 69 | 22 | 23 | 0 | |
| Person-years of observation | 2873 | 1472 | 706 | 638 | 5052 | 5689 | 5052 | 5052 | 5052 | |
| Deaths per 1000 person-years of observation | 2·4 | 16·3 | 19·8 | 37·6 | 8·9 | 12·1 | 4·4 | 4·6 | 0 | |
| Adjusted rate ratio | 0·82* (0·35–1·94) | 1·48* (0·88–2·49) | 1·72* (0·87–3·31) | 1·03* (0·64–1·66) | 1·37† (0·94–1·98) | 1·24† (0·92–1·65) | 1·07† (0·64–1·79) | 2·36† (1·32–4·20) | .. | |
Rate ratio, adjusted for area and sex, with 95% CI.
Rate ratio, adjusted for area, sex, and age, with 95% CI.
Trends in adult mortality differed in the two zones that were defined according to distance from the tarmac road (p=0·003, test for interaction in adults aged 15–59 years). Mortality in adults aged 15–59 years before the introduction of antiretroviral therapy was much higher in the 1 km zone close to the tarmac roads than in the rest of the study area (rate ratio [RR] 1·91, 95% CI 1·49–2·48). Table 3 shows that the mortality rate was reduced by 35% in adults aged 15–59 years who lived within 1 km of the tarmac roads (RR adjusted for age and area 0·65, 0·46–0·92) after the opening of the antiretroviral therapy clinic. Reductions were seen in each of the age-groups, 15–29, 30–44, and 45–59 years. In the zone further away from the road, mortality increased slightly in those aged 15–59 years. Mortality rates in adults aged 60 years or older did not change in either zone (area-adjusted RR: 1 km-zone 0·99, remote zone 1·03).
In the zone within 1 km of the tarmac roads, AIDS mortality in adults aged 15–59 years was reduced by 33% after the opening of the Karonga antiretroviral therapy clinic (rate ratio [RR], adjusted for age, sex, and area 0·67, 95% CI 0·44–1·03) and little change was seen in the more remote zone (RR, adjusted for age, sex, and area 1·07, 0·64–1·79). Deaths that were classified as not related to AIDS also decreased close to the road (RR adjusted for age, sex, and area 0·57, 0·30–1·10), but increased further from the road (RR adjusted for age, sex, and area 2·36, 1·32–4·20). 11 men and 12 women died of causes other than AIDS in the remote zone after the introduction of antiretroviral therapy (five from gastrointestinal causes, four cardiovascular, four cancer, three meningitis, three accidents, two from tuberculosis, and one maternal death). Of adults aged 15–59 years who died, three had moved from the near zone to the more remote zone and two in the other direction before the introduction of antiretroviral therapy. After the clinic opened, three moved from the near to the more remote zone.
By the end of the observation period, in February, 2006, 99 adults from the study population had accessed antiretroviral therapy at the Karonga clinic and 12 of these (seven women and five men) died during the observation period. The verbal autopsies revealed that an additional eight adults (five women and three men) had received antiretroviral therapy before their death, but had not been identified at the Karonga clinic and therefore apparently received treatment from another source, some probably before the opening of the Karonga clinic in June, 2005. Overall 12 women and eight men who had antiretroviral therapy died during the observation period (accounting for 8% of AIDS deaths).
Of all 107 adults (74 women and 33 men) known to have accessed antiretroviral therapy, 78 (73%) lived within 1 km of the tarmac roads. The rate of access per person-years of observation in people near the road was 2·6 times that in the more distant zone (p<0·0001).
Discussion
Malawi's population of 12·5 million has been severely affected by a generalised HIV epidemic since the early 1990s. In the absence of a vital registration system or reliable mortality statistics from the health services, AIDS mortality has been estimated with mathematical models (EPP and Spectrum)24 based on HIV-prevalence data from antenatal sentinel surveillance. More than half of all adults who died in the study population did so outside of health facilities, which is typical of other rural African settings,25,26 and explains why facility-based analyses of mortality would not be representative in these settings.
Analysis of age-specific mortality and survival in the population showed that premature deaths of adults aged 15–59 years were associated with the effect of the HIV epidemic. Before the antiretroviral therapy clinic opened, the probablility of dying between the ages of 15 and 60 years for men was 43%, and 63% of deaths in this age-group were attributed to AIDS. This is in line with other studies when the prevalence of HIV is taken into account. Adult HIV-prevalence in the study population in 2005 was about 11·4%,27 which was lower than national estimates of 14·4% in 2003 from antenatal surveillance24 and 12% in 2004 from a general population sample.28 Based on the assumed national prevalence of 14·4%, WHO models predict a mortality risk in men aged between 15 and 60 years of 66% for Malawi. In the era before the HIV epidemic, male adult mortality in sub-Saharan Africa was assumed to range between 20% and 50%, and values below 40% have been projected for countries with low HIV prevalence in West and Central Africa.29 A review of WHO and UNAIDS projections showed estimates of greater than 60% for adult mortality in nine of 12 countries in sub-Saharan Africa that had HIV prevalences of more than 10%.29 Our result for the mortality rate from AIDS in adults, standardised for age (6·3 deaths per 1000 person-years of observation), was much higher than empirical estimates, based on verbal autopsies from three other demographic surveillance sites. In Tanzania, which had a lower prevalence of HIV, the rates were 1·3 deaths per 1000 person-years of observation in Rufiji and 1·8 in Ifakara.30 In Manhiça, Mozambique, the rate was 2·4 deaths per 1000 person-years of observation.30 In KwaZulu Natal, South Africa, where the HIV prevalence was estimated at 21·5% in 2003–04,31 the rate was 11·4 per 1000 person-years of observation.30
Our results show not only the effect of HIV on mortality at the population level, but also the beginning of a reversal of this effect by antiretroviral therapy. After the opening of the first clinic in the district, mortality rates fell by 10% in 15–59-year-olds. In the area close to the roads, where baseline mortality rates were much higher, associated with higher HIV prevalences,32 all-cause mortality fell by 35% (rate ratio 0·65, 0·46–0·92).
Detection of effects at the population level such a short time after the introduction of antiretroviral therapy in the district might seem surprising, but could be expected from the high proportion of deaths attributable to HIV before the clinic opened (65%), the high HIV prevalence (11% overall and higher near the road), good uptake of antiretroviral therapy, and the ability of such treatment to prevent deaths which would otherwise be imminent.
Using mathematical models fitted to repeated surveys of the seroprevalence of HIV, we have previously estimated that about 2% of adults in Karonga district needed antiretroviral therapy in 2005 (ie, would have died within 2 years).33 If we assume that the demographic surveillance area is typical of the district as a whole, 334 of the 16 720 adults aged 15 years and older in the surveillance population needed treatment. By the end of February, 2006, 8 months after the opening of the Karonga clinic, 107 adults in the study population—almost a third of those who were in need—had accessed antiretroviral therapy. 60·1% of all patients at the clinic were women. That a similar proportion (60·7%) of AIDS deaths in our study population were in women suggests that access to treatment was balanced between the sexes. A community-based study from the era before antiretroviral therapy in Malawi reported high rates of mortality in HIV patients eligible for antiretroviral therapy (62 and 88 deaths per 100 person-years of observation for patients in clinical stages 3 and 4, respectively).34 Data from the programme in Malawi have shown that 81% of patients survived after 6 months of antiretroviral therapy, and 74% after 12 months.35 Data from the Ministry of Health's routine monitoring of the Karonga clinic show that of 1091 patients who registered from June, 2005, to September, 2006, 452 (41·4%) were in clinical stage 3 and 615 (56·4%) were in stage 4. The mortality risk was 25·0% within the first 6 months after initiation of antiretroviral therapy and declined rapidly with time on antiretroviral therapy: only 41 (19·4%) of the 211 deaths occurred after the third month (Prof AD Harries, personal communication). Of the 99 patients of the Karonga clinic who were in the study population, 66 (66·7%) had initiated antiretroviral therapy before December, 2005—ie, more than 3 months before the end of the observation period. With respect to the substantial effect that antiretroviral therapy has on survival and the large number of adults in the surveillance population who had accessed antiretroviral therapy, our findings about reductions in mortality in the study population can plausibly be attributed to antiretroviral therapy.
This high uptake of antiretroviral therapy in the study area is encouraging, especially since, although treatment was free, visits to the clinic cost about US$3 by public transport, whereas the average monthly household income in this population was US$23 in 2004.36
Our finding that deaths not related to AIDS decreased in the zone near the main roads but not in the more remote zone might be attributable to some misclassification in causes of death. Some of the 23 deaths attributed to other causes (eg, the three deaths from meningitis and two from tuberculosis) could in fact have been due to HIV. Previous studies have shown that analyses of verbal autopsies alone, without knowledge of HIV status, can underestimate deaths caused by AIDS.37 Therefore, assessments of mortality due to AIDS by use of verbal autopsies alone might not be valid.38 Systematic assessments of the ascertainment of AIDS deaths by verbal autopsies have shown that their sensitivity ranges from 76%39 to 92%,40 and that they have a specificity of 66% compared with diagnoses based on HIV serostatus.39 HIV status was only known for a few of those who died. The proportion of individuals with known HIV status rose later in the study. 19% of those who died in the near zone before treatment was available were known to be HIV positive, compared with 30% of those who died after the clinic was set up; further from the road the proportions were 13% and 16%, respectively. The increase in the availability of positive results after the clinic opened could have increased the proportion of deaths attributed to AIDS in the zone close to the road, and contributes to the apparent decline in other deaths in this area.
Since few people moved between the two zones, the observed changes in mortality cannot be explained by migration. The rate of deaths from causes other than AIDS was relatively low in the more remote zone before the clinic opened, which could have contributed to the apparent rise in such deaths in this area.
The fact that mortality fell in the age-group known to be affected most by AIDS mortality (15–59 years), but remained unchanged in older adults, adds credence to the interpretation that the observed trend was due to a reduction in AIDS deaths. Other external changes (eg, in the food supply) would be expected to increase mortality more in older than in younger adults.
The surveillance area contained about 12% of the adult population of the district but 16% of those who attended the Karonga clinic. This suggests that access to antiretroviral treatment in the study area was slightly higher than average in the population. Implementation of antiretroviral therapy across Malawi continues to accelerate, including a new public clinic in the surveillance area since September, 2006. With continuing decentralisation and increased access, population mortality rates should continue to fall.
Acknowledgments
We dedicate this article to Dr Frank Mwaungulu who died in a tragic accident on August 10, 2005. We thank Dr Anne Ben-Smith for helpful comments and editorial support. The study was funded by The Wellcome Trust UK and the British Leprosy Relief Association.
Contributors
This paper was written by AJ, JRG, SF, ACC, HM, FiM, NM, PEMF, and BZ. AJ, SF, ACC, FrM, NM, PEMF, JRG, and BZ contributed to the conception and design of the study. AJ, FrM, HM, FiM, JM, VM, and BM acquired data or actively participated in data management activities. AJ and SF did statistical analysis. AJ, JRG, and SF drafted the report, and all coauthors (except for the late FrM) revised the manuscript. PEMF and BZ, supported by JRG and ACC, obtained funding for the study. All authors have seen and approved the manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.UNAIDS/WHO . 2006 report on the global AIDS epidemic. WHO; Geneva, Switzerland: 2006. The impact of AIDS on people and societies. [Google Scholar]
- 2.Boerma JT, Nunn AJ, Whitworth JA. Mortality impact of the AIDS epidemic: evidence from community studies in less developed countries. AIDS. 1998;12(suppl 1):3–14. [PubMed] [Google Scholar]
- 3.Zaba B, Whiteside A, Boerma JT. Demographic and socioeconomic impact of AIDS: taking stock of the empirical evidence. AIDS. 2004;18(suppl 2):1–7. doi: 10.1097/00002030-200406002-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 5.Blacker J. The impact of AIDS on adult mortality: evidence from national and regional statistics. AIDS. 2004;18(suppl 2):19–26. doi: 10.1097/00002030-200406002-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hosegood V, Vanneste AM, Timaeus IM. Levels and causes of adult mortality in rural South Africa: the impact of AIDS. AIDS. 2004;18:663–671. doi: 10.1097/00002030-200403050-00011. [DOI] [PubMed] [Google Scholar]
- 7.Whiting DR, Setel PW, Chandramohan D, Wolfson LJ, Hemed Y, Lopez AD. Estimating cause-specific mortality from community- and facility-based data sources in the United Republic of Tanzania: options and implications for mortality burden estimates. Bull World Health Organ. 2006;84:940–948. doi: 10.2471/blt.05.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KC, Wong KH, Lee SS. Universal decline in mortality in patients with advanced HIV-1 disease in various demographic subpopulations after the introduction of HAART in Hong Kong, from 1993 to 2002. HIV Med. 2006;7:186–192. doi: 10.1111/j.1468-1293.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Braitstein P, Brinkhof MW, Dabis F. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS/WHO . Annex 1: country profiles. 2006 report on the Global AIDS epidemic. UNAIDS/WHO; Geneva, Switzerland: 2006. p. 412. [Google Scholar]
- 11.Chimzizi R, Harries AD, Gausi F, Mwansambo A, Salaniponi FM, Mpazanje R. Report of a country-wide survey of HIV/AIDS and joint HIV-TB services in Malawi (2002) National Tuberculosis Control Programme; National AIDS Commission; HIV/AIDS Unit, Department of Clinical Services, Ministry of Health and Population; Lilongwe, Malawi: 2003. [Google Scholar]
- 12.Ministry of Health and Population . ART in the public sector in Malawi, results up to 31st December 2006. HIV/AIDS Unit, Department of Clinical Services; Lilongwe, Malawi: 2007. [Google Scholar]
- 13.Libamba E, Makombe S, Mhango E. Supervision, monitoring and evaluation of nationwide scale-up of antiretroviral therapy in Malawi. Bull World Health Organ. 2006;84:320–326. doi: 10.2471/blt.05.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harries AD, Schouten EJ, Libamba E. Scaling up antiretroviral treatment in resource-poor settings. Lancet. 2006;367:1870–1872. doi: 10.1016/S0140-6736(06)68809-0. [DOI] [PubMed] [Google Scholar]
- 15.Fine PE, Ponnighaus JM, Maine N, Clarkson JA, Bliss L. Protective efficacy of BCG against leprosy in Northern Malawi. Lancet. 1986;2:499–502. doi: 10.1016/s0140-6736(86)90367-3. [DOI] [PubMed] [Google Scholar]
- 16.Ponnighaus JM, Fine PE, Maine N, Bliss L, Kalambo M, Ponnighaus I. The Lepra Evaluation Project (LEP), an epidemiological study of leprosy in northern Malawi. II: Prevalence rates. Lepr Rev. 1988;59:97–112. doi: 10.5935/0305-7518.19880014. [DOI] [PubMed] [Google Scholar]
- 17.Fine PE, Ponnighaus JM. Leprosy in Malawi. 2. Background, design and prospects of the Karonga Prevention Trial, a leprosy vaccine trial in northern Malawi. Trans R Soc Trop Med Hyg. 1988;82:810–817. doi: 10.1016/0035-9203(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 18.Glynn JR, Warndorff DK, Fine PE, Munthali MM, Sichone W, Ponnighaus JM. Measurement and determinants of tuberculosis outcome in Karonga District, Malawi. Bull World Health Organ. 1998;76:295–305. [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn JR, Ponnighaus J, Crampin AC. The development of the HIV epidemic in Karonga District, Malawi. AIDS. 2001;15:2025–2029. doi: 10.1097/00002030-200110190-00016. [DOI] [PubMed] [Google Scholar]
- 20.Jahn A, Crampin AC, Glynn JR. Evaluation of a village-informant driven demographic surveillance system in Karonga, Northern Malawi. Demogr Res. 2007;16:219–248. [Google Scholar]
- 21.INDEPTH Network INDEPTH standardized verbal autopsy questionnaire (Revised August 2003), 2003. http://www.indepth-network.org/core_documents/indepthtools.htm (accessed Oct 1, 2005)
- 22.INDEPTH Network . Part II. Mortality at INDEPTH sites. In: Sankoh O, Kahn K, Mwangeni E, Ngom P, Nyarko P, editors. Population and health in developing countries. Population, health and survival at INDEPTH sites. International Development Research Centre; Ottawa, Canada: 2002. p. 356. [Google Scholar]
- 23.Blacker J, Zaba B. HIV Prevalence and life-time risk of dying of AIDS. Health Trans Rev. 1997;7:45–62. [Google Scholar]
- 24.Malawi National AIDS Commission. National Estimate of HIV/AIDS in Malawi in 2003. Lilongwe, Malawi: 2003.
- 25.Kahn K, Tollman SM, Garenne M, Gear JS. Validation and application of verbal autopsies in a rural area of South Africa. Trop Med Int Health. 2000;5:824–831. doi: 10.1046/j.1365-3156.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 26.Todd J, Balira R, Grosskurth H. HIV-associated adult mortality in a rural Tanzanian population. AIDS. 1997;11:801–807. doi: 10.1097/00002030-199706000-00013. [DOI] [PubMed] [Google Scholar]
- 27.McGrath N, Kranzer K, Saul J. Estimating the need for antiretroviral treatment and an assessment of a simplified HIV/AIDS case definition in rural Malawi. AIDS. 2007;21(suppl 6):105–113. doi: 10.1097/01.aids.0000299417.69432.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chipeta J, Schouten EJ, Aberle–Grasse J. HIV prevalence and associated factors. In: National Statistical Office and ORC Macro, MD, USA, eds. Malawi Demographic and Health Survey 2004. Zomba, Malawi and Calverton, MD, USA, 2005: 225–41.
- 29.Ngom P, Clark S. Adult mortality in the era of HIV/AIDS: Sub-Saharan Africa. Workshop on HIV/AIDS and adult mortality in developing countries. Population Division, Department of Economic and Social Affairs, United Nations Secretariat; New York, USA: 2003. [Google Scholar]
- 30.Adjuik M, Smith T, Clark S. Cause-specific mortality rates in sub-Saharan Africa and Bangladesh. Bull World Health Organ. 2006;84:181–188. doi: 10.2471/blt.05.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welz T, Hosegood V, Jaffar S, Batzing–Feigenbaum J, Herbst K, Newell ML. Continued very high prevalence of HIV infection in rural KwaZulu–Natal, South Africa: a population-based longitudinal study. AIDS. 2007;21:1467–1472. doi: 10.1097/QAD.0b013e3280ef6af2. [DOI] [PubMed] [Google Scholar]
- 32.Crampin AC, Glynn JR, Ngwira BM. Trends and measurement of HIV prevalence in northern Malawi. AIDS. 2003;17:1817–1825. doi: 10.1097/00002030-200308150-00011. [DOI] [PubMed] [Google Scholar]
- 33.White RG, Vynnycky E, Glynn JR. HIV epidemic trend and antiretroviral treatment need in Karonga District, Malawi. Epidemiol Infect. 2007;135:922–932. doi: 10.1017/S0950268806007680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oosterhout JJ, Laufer MK, Graham SM. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39:626–631. [PubMed] [Google Scholar]
- 35.Ministry of Health and Population . ART in the public sector in Malawi, results up to 31st March 2007. HIV/AIDS Unit, Department of Clinical Services; Lilongwe, Malawi: 2007. [Google Scholar]
- 36.National Statistics Office . Integrated household survey 2004–2005. Volume I, Household socio-economic characteristics. National Statistics Office; Zomba, Malawi: 2005. pp. 73–75. [Google Scholar]
- 37.Urassa M, Boerma JT, Isingo R. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;15:2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- 38.Porter K, Zaba B. The empirical evidence for the impact of HIV on adult mortality in the developing world: data from serological studies. AIDS. 2004;18(suppl 2):9–17. doi: 10.1097/00002030-200406002-00002. [DOI] [PubMed] [Google Scholar]
- 39.Lopman BA, Barnabas RV, Boerma JT. Creating and Validating an Algorithm to measure AIDS mortality in the adult population using verbal autopsy. PLoS Med. 2006;3:312. doi: 10.1371/journal.pmed.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamali A, Wagner HU, Nakiyingi J, Sabiiti I, Kengeya–Kayondo JF, Mulder DW. Verbal autopsy as a tool for diagnosing HIV-related adult deaths in rural Uganda. Int J Epidemiol. 1996;25:679–684. doi: 10.1093/ije/25.3.679. [DOI] [PubMed] [Google Scholar]