Abstract
Midazolam is a drug that creates temporary anterograde amnesia. In a within-subjects, double-blind experiment, participants studied a list of stimuli after receiving an injection of midazolam in one session and after receiving saline in another session. The lists consisted of three types of stimuli: words, photographs, and abstract pictures. Recognition memory was tested at the end of each session. Memory was reliably poorer in the midazolam condition than the saline condition, but this amnesic effect was significantly smaller for pictorial stimuli than for words and almost nonexistent for abstract pictures. We argue that the less familiar the stimulus, the less likely it is to be associated with an experimental context. These data bolster our claim that unitization increases the chances of episodic binding and that drug-induced amnesia prevents episodic binding regardless of unitization.
There is evidence that recognition can be based on either the retrieval of episodic information or the backup process of familiarity (Diana, Reder, Arndt, & Park, 2006; Jacoby, 1991; Jacoby & Dallas, 1981; Joordens & Hockley, 2000; Mandler, 1980; Reder, Angstadt, Cary, Erickson, & Ayers, 2002; Reder et al., 2000; Yonelinas, 1994, 1999). Research with amnesic patients (Huppert & Piercy, 1976, 1978; Piercy & Huppert, 1972) and with normal subjects under the influence of a drug that produces temporary amnesia suggests that the recollective process but not the familiarity-based process is specifically vulnerable to anterograde amnesia (Hirshman, Fisher, Henthorn, Arndt, & Passannante, 2002; Mintzer, 2003). When stimuli that have a high degree of preexperimental familiarity are not included among the experimental stimuli, amnesic patients are able to discriminate recently experienced (studied) words and pictures from foils (words and pictures that were not studied in the experiment; Huppert & Piercy, 1976). However, these patients cannot determine whether the words and pictures were just studied or studied a day earlier. This suggests that the temporal (contextual) information was not associated with the stimuli and that the judgments are based only on familiarity (Balota, Burgess, Cortese, & Adams, 2002; Balota & Ferraro, 1996; Reder et al., 2000, 2002). Likewise, Hirshman et al. (2002) found that subjects under the influence of midazolam, a benzodiazepine that causes transient anterograde amnesia, produce more hits and false alarms to high-frequency items than to low-frequency items; this result is consistent with the view that the drug blocks the formation of an episodic trace.
Recently Musen, Szerlip, and Szerlip (1999) found evidence of priming in color naming when words studied earlier with the colors were reinstated. Nonwords and random shapes did not provide an analogous facilitation. The authors concluded that only words could be associated with the colors. We believe that just as people are unable to associate very unfamiliar stimuli with colors, they are unable to bind unfamiliar stimuli to contexts (i.e., to form episodic links). This impairment in the process of binding stimuli to contexts has implications for recollection (see Reder et al., 2002). Therefore, we hypothesize that unfamiliar stimuli are also at a disadvantage when it comes to recollection.
Although Reder et al. (2000, 2002) suggested that recognition judgments can be based on retrieval of episodic traces or on familiarity, they also claimed that stimuli with greater preexperimental familiarity are more vulnerable to spurious “old” judgments arising from the familiarity process. The amnesiacs in Huppert and Piercy's (1976, 1978) experiments were successful in recognition because the stimuli were not high in preexperimental familiarity and therefore not vulnerable to spurious recognition. Although the patients could recognize low-frequency words and pictures on the basis of the familiarity process, they could not discriminate whether those stimuli were seen the day before or 10 min before the test.
We propose that the less familiar a stimulus is, the more difficult it is to encode and, as a consequence, the more difficult it is to bind to an episodic trace or to another stimulus. Conversely, the more familiar a stimulus, the easier it is to encode. For example, lexical decision and word naming are faster for high-frequency than low-frequency words (Balota & Chumbley, 1984). We believe that ease of encoding affects ease of binding. Previous research using midazolam has supported the results found with amnesic patients: Familiarity judgments are unaffected by amnesia, but ability to form new associations is impaired whether or not the amnesia is drug induced (Hirshman et al., 2002; Huppert & Piercy, 1976, 1978). Therefore, we expect that to the degree that stimuli are too difficult to bind to an episodic node and are recognized on the basis of familiarity, recognition performance will be unaffected by a psychopharmacological intervention that produces temporary amnesia.
The present experiment was designed to investigate whether stimuli that are especially unfamiliar would show a reduced effect from a drug that creates temporary amnesia. To the degree that a subject cannot create a binding to a stimulus under normal circumstances, the impact of midazolam should be minimized. Musen et al. (1999) used words, nonwords, and random shapes in their priming task. We chose to use abstract drawings, photographs of faces and outdoor scenes, and words.
We predicted that memory for words would be hurt most by the drug because normally they can be easily bound to context. In contrast, unfamiliar abstract pictures are difficult to encode, having no stored memory representation. The attention required to parse such stimuli consumes the resources that otherwise would bind a stimulus to context. The more familiar a stimulus, the more likely that it will be unitized, or transformed into a single chunk (Charness, 1976; Chase & Simon, 1973; Simon, 1974). Once unitized, a chunk can be bound to an episodic trace. Therefore, we expected the drug intervention to have less effect on memory for the abstract pictures, which are not easily perceived as a unit.
We included another category of pictures that we expected to be more easily parsed than the abstract pictures, specifically, photographs of faces and outdoor scenes. Although not unusual in appearance, they were not easily labeled in a way that would enable them to be discriminated from other items on the test list (i.e., generic labels such as “male face” or “mountain scene” would be of little help). We suspect that often features or sets of features from a nonfamiliar face or scene are perceived as lower-level chunks and that these will be bound to the context, rather than the entire face or scene. When a face (e.g., Marilyn Monroe), scene (e.g., the Eiffel Tower), or abstract picture (e.g., painting by Jackson Pollock or Mark Rothko) has been experienced many times, it becomes unitized, and then that entire chunk is bound to an episode in which it is experienced, but we did not use identifiable faces, scenes, or abstract pictures.
In summary, we predicted that memory for abstract pictures would be least vulnerable to the effects of the amnestic drug because there would be no preexisting chunks to bind to context and binding resources would be consumed processing the pictures. Memory for photographs of faces and outdoor scenes would be intermediately affected because sometimes the entire photograph or enough lower-level chunks would be bound to the context to facilitate later recognition. We also expected the photographs to be most vulnerable to foils because the features of these photographs were shared by many of the stimuli. Memory for words was expected to be most affected by the drug because, under saline, words are the most easily bound to context, and they therefore should show the greatest decrement when the recollection process is impaired.
METHOD
Participants
Twenty-five participants with a mean age of 22 received $120 upon completion of the experiment.
Design and Materials
The study employed a 3 (stimulus type: abstract pictures vs. photographs vs. words) × 2 (drug condition: midazolam vs. saline) within-subjects design, with the saline and midazolam sessions 1 week apart. Assignment of drug condition to session was counterbalanced and double-blind.
From the MRC psycholinguistics on-line database, we selected nouns that are four to nine letters long, have a concreteness rating between 300 and 700, and have a mean Kucera and Francis frequency of 15.1 Examples of the pictorial stimuli are shown in Figure 1. The color photographs of unfamiliar landscapes and cityscapes were collected from the Internet. The black-and-white photographs of unfamiliar faces came from the Facial Recognition Technology (FERET) Database (2001). The abstract color pictures included stimuli developed by Koutstaal et al. (2003), created in our lab using Adobe Photoshop, and found on the Internet. We discarded any abstract stimuli that were readily judged as meaningful or given an interpretation.
Fig. 1.

Examples of the photographs and abstract pictures used in the experiment; faces were shown in black and white, and the rest of the stimuli in color.
Procedure
Prospective participants were carefully screened and signed an informed-consent form prior to the medical procedure. At each session, they received a single intravenous injection of either 0.03 mg/kg (to a maximum of 2.5 mg) of midazolam or a matching volume of saline. The effects of midazolam are almost immediate, but dissipate and are typically gone within 60 min.
Prior to the midazolam or saline injection, all participants were told that they would view a series of items on a laptop computer and rate how pleasant each was, using a 4-point Likert scale from very much to not at all. They were also told to expect a recognition test on the stimuli at the end of the session. Approximately 2 min after receiving the injection, participants viewed the three types of stimuli, presented in a random order. Each item remained on the screen for 1 s, after which the participant rated its pleasantness by clicking on the scale, using the built-in mouse pad on the computer. Time to rate each stimulus was self-paced, and the study phase lasted for approximately 12 to 15 min.
Participants were then given the Modified Digit Span task (MODS; Lovett, Reder, & Lebiere, 1997), which lasted approximately 20 min and tested each participant's working memory. Immediately after the working memory task, participants were given a recognition test on the items judged earlier. They responded to each item by pressing one of two keys on the keyboard: “yes” if they had seen the item before and “No” if they had not seen it before. Each test item remained on screen until a response was made. Each session, regardless of drug condition, took approximately 1 hr to complete.
RESULTS
Two participants' data were excluded from the analyses because they fell asleep during the testing. Both participants reported receiving little or no sleep the previous night and thus were particularly vulnerable to the sedation. A multifactor analysis of variance (ANOVA) was conducted on d′ scores, hit rates, and false alarm rates. We report prep values, which represent the probability of replicating an experimental effect (Killeen, 2005).
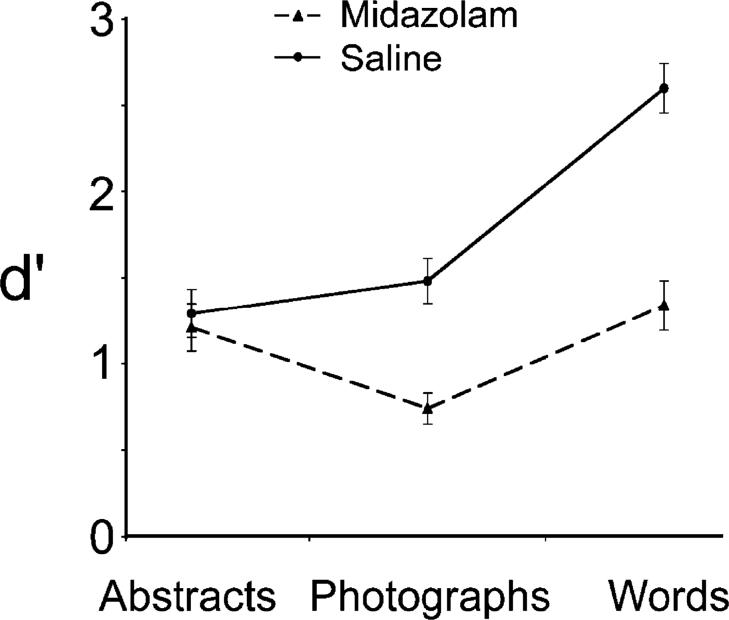
Figure 2 presents the mean d′ scores as a function of stimulus type and drug condition. For d′, there was a main effect of drug condition, F(1, 22) = 66.2, prep > .999, ηp2 = .75, such that memory was better under saline than under midazolam. There was also a main effect of stimulus type, F(2, 44) = 33.3, prep > .999, ηp2 = .60, reflecting the fact that words were recognized better than other stimuli.
Fig. 2.
Mean d′ as a function of stimulus type and drug condition.
Of greater interest was how midazolam differentially affected memory for the stimulus classes.2 Memory for both words and photographs differed by drug condition, F(1, 22) = 79.8, prep > .999, ηp2 = .78, and F(1, 22) = 37.0, prep > .999, ηp2 = .63, respectively, but memory for abstract pictures was not affected, F < 1. There was an interaction between drug condition and stimulus type, F(2, 44) = 22.1, prep > .999, ηp2 = . 50, such that midazolam did not have an equal effect on all stimulus types, F(1, 22) = 28.9, prep > .999, ηp2 = .57. In particular, the difference in d′ between the midazolam and saline conditions was smaller for abstract pictures than for photographs, F(1, 22) = 20.8, prep = .99, ηp2 = .49, and was smaller for photographs than for words, F(1, 22) = 48.6, prep > .999, ηp2 = .69.
Figure 3 presents mean hit and false alarm rates for each stimulus type for the two drug conditions. These data help to illuminate the cause of the different d′ patterns. First, consider the hit rate data, shown in the top portion of the figure. There were significantly more hits in the saline than in the midazolam condition, F(1, 22) = 35.9, prep > .999, ηp2 = .62. There was also a reliable interaction of drug condition and stimulus type, F(2, 44) = 10.3, prep = .99, ηp2 = .32. Inspection of the figure shows that the hit rate did not differ among the three stimulus types under midazolam, F(2, 44) = 1.3, prep = .65, ηp2 = .06. However, in the saline condition, abstract stimuli produced significantly fewer hits than did photographs, F(1, 22) = 6.4, prep = .93, ηp2 = .22, and these two stimulus types produced significantly fewer hits than words, F(1, 22) = 53.9, prep > .999, ηp2 = .71. The effect of the drug was smaller for abstract pictures than for words, F(1, 22) = 20.7, prep = .99, ηp2 = .48, and was smaller for abstract pictures than for photographs, F(1, 22) = 5.3, prep = .91, ηp2 = .49.
Fig. 3.
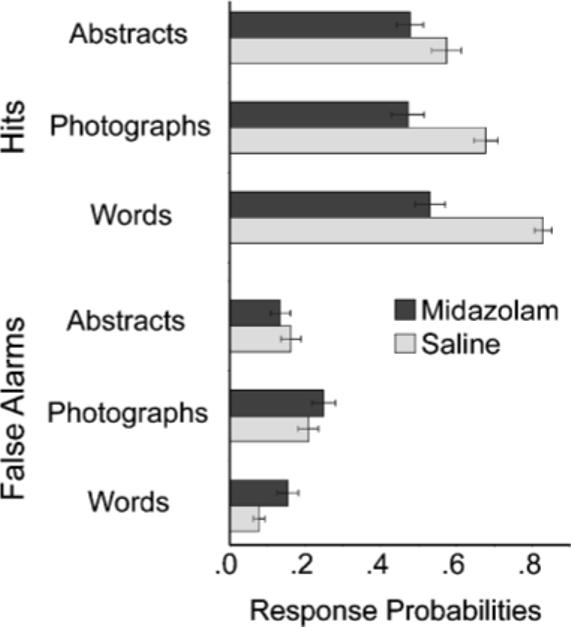
Proportion of hits (top) and false alarms (bottom) as a function of stimulus type and drug condition.
The false alarm data are displayed in the bottom half of Figure 3. False alarms differed by stimulus type, F(2, 44) = 11.3, prep > .99, ηp2 = .34, reflecting the fact that abstract pictures produced significantly fewer false alarms than photographs, F(1, 22) = 8.5, prep = .96, ηp2 = .28. There was also a reliable interaction of drug condition and stimulus type, F(2, 44) = 4.8, prep = .94, ηp2 = .18. Abstract pictures did not produce more false alarms under midazolam than under saline, F(1, 22) = 1.0, prep = .62, ηp2 = .04, and in fact produced slightly fewer false alarms under midazolam than under saline, a pattern opposite that found in the other conditions. When abstract stimuli were excluded from analysis, there were more false alarms under midazolam than under saline, F(1, 22) = 5.2, prep = .90, ηp2 = .18, and drug condition did not interact with stimulus type, F(1, 22) = 1.2, prep = .65, ηp2 = .05.
DISCUSSION
This study demonstrates that the effect of midazolam, a drug used commonly in medical procedures to produce temporary amnesia, is differentially expressed across various types of stimuli. In particular, the study illustrates the important role of experience in the two processes underlying recognition, recollection and familiarity (Diana et al., 2006; Reder et al., 2000, 2002). Midazolam hurt performance for words and, to a lesser degree, for photographs, and effectively did not hurt performance at all for abstract pictures. Previous research has suggested that midazolam prevents binding in memory (e.g., Ghoneim, 2004; Park, Quinlan, Thornton, & Reder, 2004), creating temporary amnesia by blocking the formation of new episodic traces. Our results with these stimulus types provide more evidence for the notion that recognition can be based on familiarity or recollection and that it is only the latter process that is selectively vulnerable to midazolam. Midazolam prevents the binding of the representation of a stimulus to an experimental context, a key prerequisite for a recollection response.
Our data are consistent with the results of Huppert and Piercy (1976, 1978), who found that patients with anterograde amnesia can still recognize pictures as long as judgments do not require list discrimination (i.e., remembering which list a picture came from). Our results are also consistent with the priming study of Musen et al. (1999), which showed that it is easier to create an association to a word than to an unfamiliar stimulus. Our hypothesis that familiarity affects the probability of encoding as well as the probability of a false alarm can also explain a finding of Koutstaal et al. (2003): Older adults, compared with younger adults, showed poorer recognition for all stimulus classes except abstract pictures. Older adults generally have more trouble recollecting than do young adults and as a result rely more heavily on familiarity (Buchler & Reder, in press). That tendency does not affect recognition of abstract pictures, because even young adults have trouble building episodic traces for abstract pictures. Furthermore, age does not increase vulnerability to commit false alarms to unfamiliar, abstract pictures, because older adults do not have more experience with these pictures than do younger adults. For unfamiliar, abstract pictures, unlike other stimulus classes used by Koutstaal et al., false alarm rates did not differ between the two age groups.
In summary, midazolam introduces a specific dissociation among stimulus types such that when participants cannot capitalize on unitization—a process that results from the lasting effects of experience—performance is relatively unaffected by the drug. Unitization is a prerequisite for building contextual associations that enable recollection, and midazolam affects recollection only when these associations could otherwise be built.
Acknowledgments
This work was supported by Grant 2-R01-MH52808 from the National Institute of Mental Health. We thank John Anderson, Norbou Buchler, and Heekyeong Park for comments on the manuscript and John O'Donnell, program director of the Nurse Anesthesia Program, University of Pittsburgh School of Nursing, for providing nurses.
Footnotes
We selected words of both low frequency (M = 1 per million occurrences of words) and high frequency (M = 29 per million occurrences of words). The word-frequency effects were not of interest because they served only to replicate the findings of Hirshman et al. (2002), but we varied word frequency to ensure that the expected pattern would emerge, and it did. To save space, we do not report the statistics here.
The effect of drug on d′ was approximately the same for both levels of word frequency, F < 1. Faces and scenes produced virtually identical patterns of performance and were affected in the same way by the drug manipulation, F < 1.
REFERENCES
- Balota DA, Burgess GC, Cortese MJ, Adams DR. The word-frequency mirror effect in young, old, and early-stage Alzheimer's Disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46:199–226. [Google Scholar]
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Balota DA, Ferraro FR. Lexical, sublexical, and implicit memory processes in healthy young and healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology. 1996;10:82–95. [Google Scholar]
- Buchler NE, Reder LM. Modeling age-related memory deficits: A two-parameter solution. Psychology and Aging. doi: 10.1037/0882-7974.22.1.104. (in press) [DOI] [PubMed] [Google Scholar]
- Charness N. Memory for chess positions: Resistance to interference. Journal of Experimental Psychology: Human Learning and Memory. 1976;2:641–653. [Google Scholar]
- Chase WG, Simon HA. Perception in chess. Cognitive Psychology. 1973;4:55–81. [Google Scholar]
- Diana R, Reder LM, Arndt J, Park H. Models of recognition: A review of arguments in favor of a dual-process account. Psychonomic Bulletin & Review. 2006;13:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Facial Recognition Technology (FERET) database. National Institute of Standards and Technology, Information Technology Laboratory, Information Access Division, Image Group; Gaithersburg, MD: 2001. (Release 2) [Google Scholar]
- Ghoneim MM. Drugs and human memory (Part 2): Clinical, theoretical, and methodologic issues. Anesthesiology. 2004;100:1277–1297. doi: 10.1097/00000542-200405000-00033. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Fisher J, Henthorn T, Arndt J, Passannante A. Midazolam amnesia and the dual-process models of the word-frequency mirror effect. Journal of Memory and Language. 2002;47:499–516. [Google Scholar]
- Huppert FA, Piercy M. Recognition memory in amnesic patients: Effect of temporal context and familiarity of material. Cortex. 1976;12:3–20. doi: 10.1016/s0010-9452(76)80024-x. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Piercy M. Normal and abnormal forgetting in organic amnesia: Effect of locus of lesion. Cortex. 1978;15:385–390. doi: 10.1016/s0010-9452(79)80065-9. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Joordens S, Hockley WE. Recollection and familiarity through the looking glass: When old does not mirror new. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1534–1555. doi: 10.1037//0278-7393.26.6.1534. [DOI] [PubMed] [Google Scholar]
- Killeen PR. An alternative to null-hypothesis significance tests. Psychological Science. 2005;16:345–353. doi: 10.1111/j.0956-7976.2005.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Reddy C, Jackson EM, Prince S, Cendan DL, Schacter DL. False recognition of abstract versus common objects in older and younger adults: Testing the semantic categorization account. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:499–510. doi: 10.1037/0278-7393.29.4.499. [DOI] [PubMed] [Google Scholar]
- Lovett MC, Reder LM, Lebiere C. Proceedings of the Nineteenth Annual Cognitive Science Conference. Erlbaum; Mahwah, NJ: 1997. Modeling individual differences in a digit working memory task. pp. 460–465. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;89:609–626. [Google Scholar]
- Mintzer MZ. Triazolam-induced amnesia and the word-frequency effect in recognition memory: Support for a dual process account. Journal of Memory and Language. 2003;48:596–602. [Google Scholar]
- Musen G, Szerlip JS, Szerlip NJ. Role of familiarity and unitization on new-association priming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:275–283. [Google Scholar]
- Park H, Quinlan JJ, Thornton ER, Reder LM. The effect of midazolam on visual search: Implications for understanding amnesia. Proceedings of the National Academy of Sciences, USA. 2004;101:17879–17883. doi: 10.1073/pnas.0408075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy M, Huppert FA. Efficient recognition of pictures in organic amnesia. Nature. 1972;5383:564. doi: 10.1038/240564a0. [DOI] [PubMed] [Google Scholar]
- Reder LM, Angstadt P, Cary M, Erickson MA, Ayers MA. A reexamination of stimulus-frequency effects in recognition: Two mirrors for low- and high-frequency pseudowords. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:138–152. doi: 10.1037//0278-7393.28.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder LM, Nhouyvanisvong A, Schunn CD, Ayers MS, Angstadt P, Hiraki K. A mechanistic account of the mirror effect for word frequency: A computational model of remember-know judgments in a continuous recognition paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:294–320. doi: 10.1037//0278-7393.26.2.294. [DOI] [PubMed] [Google Scholar]
- Simon HA. How big is a chunk? Science. 1974;183:482–488. doi: 10.1126/science.183.4124.482. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: A formal dual-process model and an analysis of receiver operating characteristics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]