Abstract
Background
In individuals with cerebral palsy (CP), adaptation and plasticity in the neuromuscular system can lead to detrimental changes affecting gait. Cycling may be an effective method to improve mobility. The biomechanics of cycling in adolescents with CP have been studied, but further analysis of the frequency and amplitude characteristics of the electromyographic (EMG) signals can assist with interpretation of the cycling kinematics.
Methods
Data were analyzed from ten adolescents with typical development (TD) (mean = 14.9 SD = 1.4 years) and ten adolescents with CP (mean = 15.6 SD = 1.8 years) as they cycled at two different cadences. Analyses of the lower extremity EMG signals involved frequency and amplitude analysis across the cycling revolution.
Findings
Examination of cycling cadence revealed that adolescents with CP had altered EMG characteristics in comparison to adolescents with typical development across the entire crank revolution for all muscles. Analyses of individual muscles indicated both inappropriate muscle activation and weakness.
Interpretation
A more comprehensive analysis of EMG activity has the potential to provide insight into how a task is accomplished. In this study, the control of the several muscles, especially the rectus femoris, was significantly different in adolescents with cerebral palsy. This, combined with muscle weakness, may have contributed to the observed deviations in joint kinematics. Interventions that increase muscle strength with feedback to the nervous system about appropriate activation timing may be beneficial to allow individuals with CP to cycle more efficiently.
Keywords: Electromyography, cycling, cerebral palsy
Introduction
Cerebral palsy (CP) describes, ”a group of disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain” (Bax et al., 2005). While the initial insult to the brain is non-progressive, adaptation and plasticity in the neuromuscular system can often lead to detrimental neuromuscular changes. For example, individuals with CP can experience muscle spasticity and hypertonia (Damiano et al., 2001), altered control over muscle activation (Stackhouse et al., 2005), and muscle co-contraction (Ikeda et al., 1998). As such, the muscle fibers adapt as evidenced by a higher composition of Type-I muscle fibers in CP (Rose et al., 1994; Rose and McGill, 1998), muscle shortening and decreased active range of motion (Crenna, 1998; Woollacott and Shumway-Cook, 2005), and muscle weakness and atrophy (Elder et al., 2003). These alterations in the muscle structure often lead to gait deviations and a potential decline in ambulatory status as the children mature into adults (Bottos and Gericke, 2003).
Numerous clinical interventions exist in order to address decreased mobility in the individual with CP, but one modality that has received little attention is cycling. Cycling has been proposed in several studies (Brown and Kautz, 1998; Brown et al., 2005; Fujiwara et al., 2003) in adults with hemiplegia due to stroke as being ideal for addressing mobility losses because of the potential to strengthen weakened muscles without enhancing abnormal muscle activity. Because cycling involves multisegmental, complex movements that are generated by the same central neural networks as used during gait, cycling may also enhance motor relearning to improve ambulatory status. Finally, it has been suggested that cycling is a functional, safe and widely accessible mode of exercise for patients with a variety of movement disorders, which could have secondary benefits to cardiorespiratory health as has already been demonstrated in adults with SCI (Mutton et al., 1997; Davis et al., 1990). Some of the preliminary reports on a volitional cycling program for children with CP include improvements in standing, walking, running and jumping ability as measured by the Gross Motor Function Measure (Williams and Pountney, 2007)
While reports on the use of cycling as a rehabilitation modality in CP are limited, more effort has been directed towards understanding the biomechanics of cycling. An initial study by Kaplan (Kaplan, 1995) examined pedaling smoothness and electromyographic (EMG) activation patterns in children with and without CP. In this study, it was found that the children with CP could cycle, but had longer periods of muscle activity, higher levels of muscle co-contraction, and irregular cycling patterns. A more recent study by Johnston et al (Johnston et al., 2007), examined the biomechanics of cycling in adolescents with and without CP at two different cadences against a constant resistance as determined by a percentage of body weight. It was reported that while all individuals with CP were able to cycle, significant deviations were found in comparison to adolescents with typical development (TD) in the joint kinematics in the sagittal, transverse and coronal planes. Concurrent with the altered kinematics, differences were also noted in muscle activity, as assessed through onset and onset timing extracted from the surface EMG signal, particularly with lengthened muscle activation times and increased muscle co-contraction.
Examination of the temporal characteristics, such as onset and offset times, of the EMG signal is a first step towards understanding muscle activity during cycling. Further insight may be gained from the analysis of the amplitude and frequency characteristics. For example, the work of Hakansson and Hull (Hakansson and Hull, 2005) demonstrated the difference between recumbent and upright cycling through the analysis of the temporal and amplitude information extracted from the EMG signals. Examination of amplitude and frequency may provide insight into increases or decreases in muscle activity at specific crank positions, as compared to adolescents with TD. Such information could be useful in designing a therapy program, as long-term training or electrical stimulation may lead to improved timing and amplitude of muscle activation. Thus, the purpose of this study was to expand upon the biomechanical analysis of cycling in adolescents with and without CP conducted by Johnston and colleagues to focus on amplitude and frequency analysis of the lower extremity muscle activity to aid in the understanding of the biomechanics of cycling in CP.
Methods
The following sections provide a summary of the experimental design and data collection. Further details on the methodology can be found in Johnston et al (Johnston et al., 2007). Data were analyzed from ten adolescents (3 male, 7 female) with TD (mean age = 14.9, SD = 1.4 years) and ten adolescents (3 male, 7 female) with CP (mean age = 15.6, SD = 1.8 years). The adolescents with CP were diagnosed with spastic diplegic or quadriplegic CP and were classified as a Level III or Level IV on the Gross Motor Function Classification Scale (Palisano et al., 1997). Subjects with CP were recruited from the patient population at Shriners Hospitals for Children, Philadelphia, USA, while the subjects with TD were recruited through advertisement. Written informed consent, approved by the governing Institutional Review Board (IRB), was obtained from each individual over the age of 18, or from the subject’s parents along with written assent from each subject if under the age of 18.
All subjects used a commercially available stationary cycle (Restorative-Therapies, Inc., Baltimore MD, USA), modified specifically to allow for recumbent cycling. Each subject was positioned on the cycle based upon his or her anthropometrics (Figure 1). The distance of the cycle seat to the pedal during maximal leg extension with the pedal vertical was set to be 85% of the distance between the subject’s greater trochanter and calcaneus. The back support on the seat was placed behind the subject while maintaining 15% of the distance between the subject’s greater trochanter and calcaneus on the bench, and was inclined at a 69° angle. The foot was placed on a footplate with the second metatarsal head aligned with the pedal spindle. The length of the crank arm was set to be 30% of the subject’s tibial length.
Figure 1.
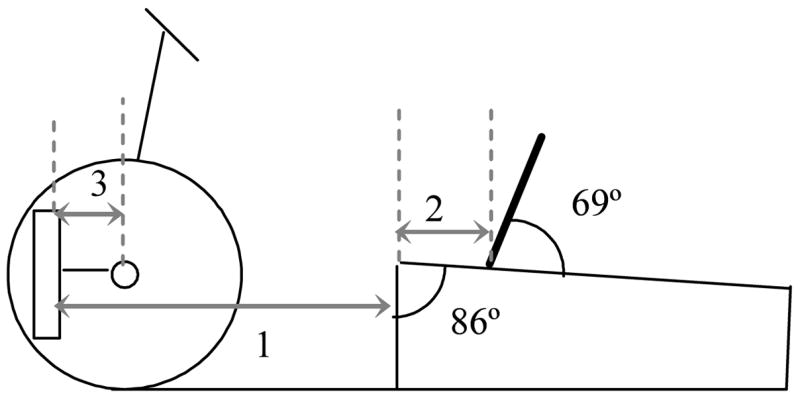
Bicycle set-up as based upon subject anthropometrics. The seat to pedal distance (1) was 85% of the distance from the greater trochanter to the base of the calcaneus. The seat to the greater trochanter distance (2) was 15% of this same measurement, while the crank arm length (3) was 30% of the subject’s tibial length.
Each subject was instructed to pedal at a cadence of 30 revolutions per minute (rpm) and 60 rpm using the cycle’s tachometer for feedback after training. Three trials of 10 to 15 seconds duration were collected for each subject at each cadence once the target cadence was reached. Six of the ten subjects with CP were able to attain and maintain 60 rpm cycling cadence. The four subjects with CP who could not cycle at 60 rpm could cycle at 30 rpm and were tested only at this lower speed. All individuals cycled against a constant resistance load based upon each subject’s weight using a formula adapted from Dore et al. (Dore et al., 2000):
Surface EMG data (sampling rate = 1200 Hz) were collected using the MA-310 recording system (Motion Lab Systems, Baton Rouge, LA, USA). Activity from the following muscles were recorded bilaterally: the rectus femoris, vastus lateralis, medial hamstrings, biceps femoris, anterior tibialis, lateral gastrocnemius, and soleus. Standard EMG sensors for the MA-310 were employed with placement following standardized procedures. All signals were digitally filtered between 20 Hz and 350 Hz, with the lower bound being set to remove movement artifact from the EMG signal. The individual electrode gains were adjusted to provide the maximum gain within the specifications of the analog-to-digital converters (rails are +/− 2.5VDC) using a real-time display of the raw EMG data.
The EMG data were extracted from five complete cycling revolutions in which the desired cadence was maintained, and processed in MATLAB (The MathWorks Inc., Natick MA, USA). Data from the right and left sides were combined after adjusting for the 180° phase difference between the sides. For the analysis, 0° was defined as the position where the pedal was furthest away the subject, while 180° was the closest position. Frequency analysis was performed with the continuous wavelet transform using a revised methodology to that detailed in previous studies (Lauer et al., 2005; Lauer et al., 2006). The three dimensional scalogram output of the continuous wavelet transform was reduced to a time-frequency curve by calculating the mean frequency for each cycle interval. Amplitude analysis was performed by rectifying the raw EMG data, and then filtering the signal with a second order low-pass Butterworth filter with phase correction and a cut-off of 10 Hz to create a linear envelope.
Statistical analyses of the amplitude and frequency curves for each muscle were performed using a functional analysis of variance (fANVOA) model (Ramsay and Silverman, 1997). A Fourier basis function of 20 expansions terms was fit to each curve, imposing a roughness penalty to ensure smoothness of the function up to the second derivative. The data set was divided into four groupings, representing a designation of cadence (30 vs. 60 rpm) and group (TD vs. CP). Using the amplitude or frequency functions for a given grouping, a linear model was constructed using the following formulas:
| (1) |
| (2) |
where g indicated grouping designation, m is the number of functions representative of that grouping, μ is the grand mean function across all the functions, α are the specific effects on the function of being within a grouping g, and ε is the unexplained variation specific to the mth curve. The terms for each of the four general linear models was determined using a least squares fit. To be able to define a set of a terms unique to each grouping, an additional constraint was placed that Σ αg (°) = 0 for all degree increments. Overall differences between groupings were assessed using the F-ratio function, based on the F-statistic, calculated for each degree interval.
Results
The average amplitude and frequency curves for the lower extremity muscles for all four groupings (TD30, TD60, CP30 and CP60) are represented in Figures 2 through 8. Differences between the curves for all groupings across all muscles were significant at all degree increments, as represented by the F-ratio function being above the 5% significance level for the F-distribution (3,354). Overall, the adolescents with CP exhibited elevated frequency curves across the entire crank revolution in comparison to the adolescents with TD for all muscles. Within each group, both the adolescents with CP and with TD responded to an increase in cycling cadence with a decreasing frequency but increasing amplitude across the entire crank revolution.
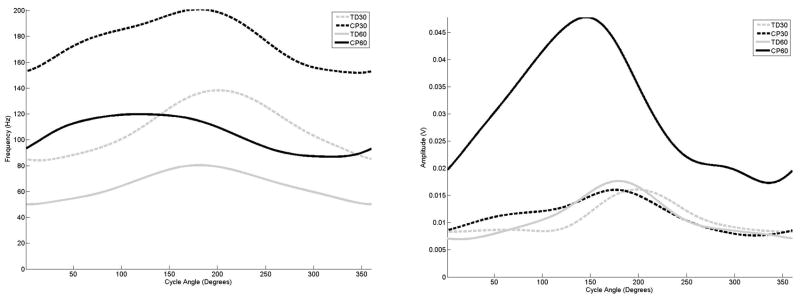
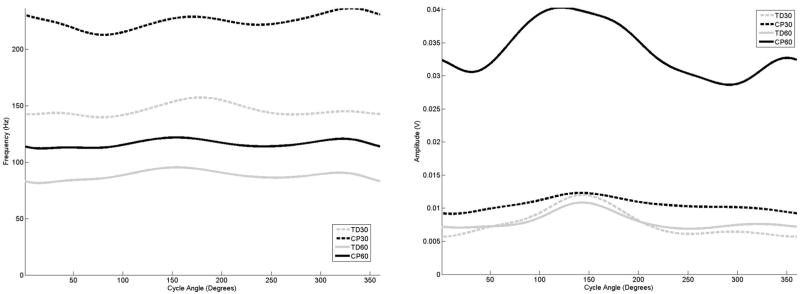
Figure 2.
The average frequency (left) and amplitude (right) curves for the rectus femoris as a function of cycle angle for the four groupings examined in this study,
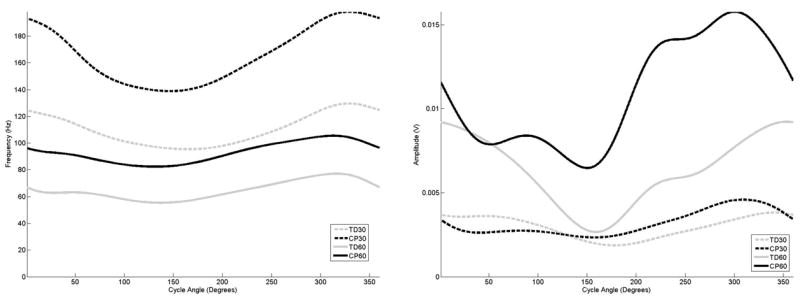
Figure 8.
The average frequency (left) and amplitude (right) curves for the soleus as a function of cycle angle for the four groupings examined in this study,
The examination of the curves for the quadriceps muscles (Figures 2 and 3) for the adolescents with TD indicated that with decreasing mean frequency in response to increasing cadence, the amplitude increased and exhibited earlier onset and offset times. In comparison, when cycling at 30 rpm the rectus femoris in the adolescents with CP activated much earlier, although peaks in both frequency and amplitude did occur at the same range of the crank revolution. At 60 rpm, the maximum frequency and amplitude level of rectus femoris in the adolescents with CP was 90 degrees out of phase in comparison to the adolescent with TD. In the vastus lateralis, at 30 rpm the adolescents with CP displayed an earlier frequency peak, but the amplitude peaked later in comparison to the adolescents with TD. At 60 rpm, the adolescents with CP exhibited almost a constant frequency level, with amplitude indicating an earlier onset and much later offset in comparison.
Figure 3.
The average frequency (left) and amplitude (right) curves for the vastus lateralis as a function of cycle angle for the four groupings examined in this study,
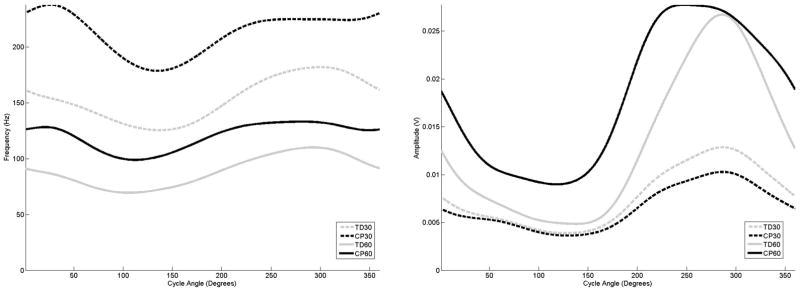
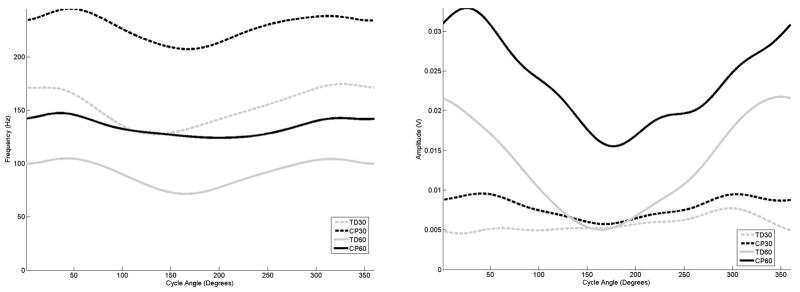
The examination of the curves for the hamstring muscles (Figures 4 and 5) for the adolescents with TD also exhibited a drop in mean frequency with increasing amplitude in response to increasing cadence, and that the biceps femoris and medial hamstring exhibited earlier onset and offset times. The examination of the biceps femoris muscle (4) for the adolescents with CP indicated that regardless of cadence, frequency peaks occurred at the same crank angle position as the adolescents with TD, although amplitude activity was elevated and onset and offset times differed. For the medial hamstring (5), the adolescents with CP also exhibited a frequency peak at the same point of the crank cycle as the adolescents with TD, although the frequency activity in the adolescent with CP was much longer in duration, and almost to the point of a sustained level at 60 rpm.
Figure 4.
The average frequency (left) and amplitude (right) curves for the biceps femoris as a function of cycle angle for the four groupings examined in this study,
Figure 5.
The average frequency (left) and amplitude (right) curves for the medial hamstring as a function of cycle angle for the four groupings examined in this study,
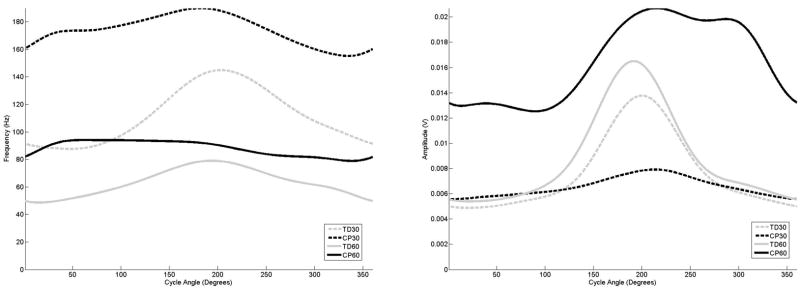
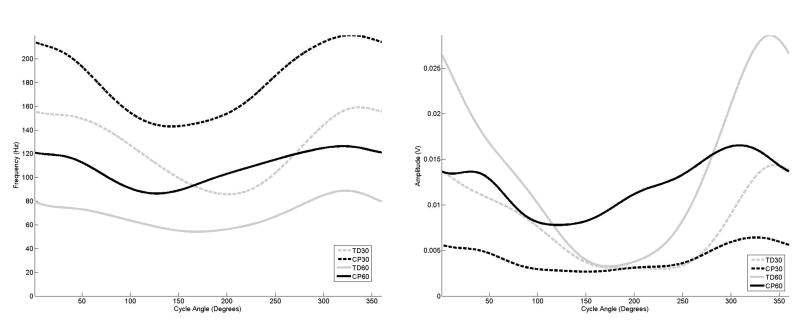
The examination of the curves for the distal muscles (Figures 6–8) demonstrated the same frequency, amplitude and timing shifts as observed in the more proximal muscles. For all groupings, the anterior tibialis muscle (Figure 6) exhibited almost sustained frequency and amplitude levels throughout the entire crank cycle. For the gastrocnemius and soleus (Figures 7 and 8), the adolescents with CP exhibited a frequency peak at the same point of the crank cycle as the adolescents with TD, although the elevated frequency activity in the adolescents with CP was much longer in duration, and almost to the point of sustained activity at 60 rpm for the gastrocnemius muscle.
Figure 6.
The average frequency (left) and amplitude (right) curves for the anterior tibialis as a function of cycle angle for the four groupings examined in this study,
Figure 7.
The average frequency (left) and amplitude (right) curves for the gastrocnemius as a function of cycle angle for the four groupings examined in this study,
Discussion
The purpose of this study was to expand upon the biomechanical analysis of cycling in adolescents with and without CP conducted by Johnston et al (Johnston et al., 2007) by providing frequency and amplitude analysis of the EMG activity of lower extremity muscle groups. The potential benefits of volitional cycling in non-ambulatory children with CP has been preliminarily investigated (Williams and Pountney, 2007). However, if interventions such as volitional or FES assisted cycling are to be employed to assist with motor relearning and muscle strengthening, then a better understanding of muscle activity during the task will assist with clinical implementation.
The amplitude curves of the lower extremity muscles (Figures 2–8) indicated that in the adolescents with CP, muscles exhibited earlier onset times and later offset times in comparison to the adolescents with TD. Thus, the differences in timing as reported by Johnston et al, and from which this data were taken, were preserved in the analysis. However, further examination of the amplitude curves indicates that at the lower cadence the adolescents with CP had lower amplitude measures, and that with increasing speed, the amplitude measures increased a disproportionate amount as compared to the TD group. This is in combination with an elevated frequency content of the EMG in the direct comparison of cycling cadences (TD30 to CP30 and TD60 to CP60), with differences in the range of 20 to 80 Hz. Thus, the potential for cycling to strengthen weakened muscles without enhancing abnormal muscle activity, as has been demonstrated in adults with hemiplegia, would be a great benefit to the child with CP. Further, based upon the results achieved in children with CP using electrical stimulation to illicit isometric muscle contractions (Stackhouse et al., 2007), introducing FES to the cycling paradigm could provide even further strengthening gains, as well as contribute to better muscle activation.
One muscle that could potentially benefit by enhancement with electrical stimulation during the cycling task is the rectus femoris. This muscle (Figure 2) in the adolescents with CP, especially at 60 rpm, exhibited an activation pattern that was out of phase in comparison to the adolescent with TD. Thus, co-contraction of the hamstring muscles was occurring to compensate for the inappropriate activation of the RF muscle, and the VL muscle was responding to the altered RF pattern by an almost constant level of muscle activity. Thus, altered motor control patterns in the rectus femoris muscle, combined with potential muscle weakness, may contribute to the altered kinematics, especially in the sagittal plane as reported by Johnston (Johnston et al., 2007). Therefore, a possible therapeutic option to alter the cycling pattern may be to use cycling with electrical stimulation to provide for muscle strengthening of all the muscles, as well as for feedback for motor relearning of the rectus femoris muscle.
The study by Johnston et al also reported increased co-contraction of the muscles around the ankle joint for the adolescents with CP, and movement into dorsiflexion and plantar flexion movement for both groups was less than what has been reported in upright cycling studies (Jorge and Hull, 1986). In the Johnston study, it was speculated that the pedal design, which was weighted to favor movement in dorsiflexion, might have affected muscle activity by requiring an increased plantarflexion moment to counteract this. For both groups and cadences, the anterior tibialis muscle (Figure 6) demonstrated almost constant activity with only a slight amplitude elevation between the designated onset/offset times. The lateral gastrocnemius (Figure 7) and soleus muscles (Figure 8), in contrast, exhibited greater amplitude and frequency changes between the active and inactive states. It may be inferred that the control strategy adapted for the ankle was to provide stability at the ankle with constant anterior tibialis activity, thus requiring greater plantar flexor activity than would normally be used.
All adolescents in the study, regardless of group assignment, exhibited a decrease in the mean frequency and an increase in amplitude with an increase in cycling cadence against the same resistance. In response to increased power output, the neuromuscular system has two options available to it in order to respond. The first is to recruit additional motor units, including type-2 muscle fibers to generate increased force and the second option is to synchronize the firing rate of the motor units that are currently in use (Ricard et al., 2005). If additional motor units were recruited, this should result in an increase in the mean frequency with increasing amplitude (ramp contraction), whereas motor unit synchronization would result in a decrease in the mean frequency with increasing amplitude (ballistic contraction) (Ricard et al., 2005). Given that all groups demonstrated a drop in frequency and an increase in amplitude when cycling cadence increased, motor unit synchronization is more likely the strategy employed during cycling to increase power output required by the muscles in this study. A more detailed analysis of how the overall EMG spectrum was altered in response to cadence, and an examination of a wider range of cycling cadences, would be needed to verify that motor unit synchronization was occurring.
The results of this study are limited in scope as the analyzed EMG signals for the adolescents with CP were for those subjects and trials where the cycling cadence was achieved. As stated in the methodology section of this paper, 4 of the 10 subjects with CP were unable to cycle at 60 rpm, thus muscle responses for those individuals were not recorded at that speed. A comparison of the frequency and amplitude characteristics of the lower extremity muscles at 30 rpm did not show any statistically significant differences in comparison to those adolescents who could cycle at 60 rpm and those who could not, thus more detailed analyses of muscle architecture and motor control are needed to assess the fundamental difference between these two groups. The inability of 4 out of the 10 children with CP to cycle at the higher cadence also furthers the argument that FES cycling may be a better therapeutic option than volitional cycling due to the potential difficulties in achieving higher cadences for some children.
Conclusion
The introduction of amplitude and frequency EMG analyses to enhance the understanding of muscle activity has the potential to provide additional insight into how a task is accomplished, and differences between the normal and pathological condition. In this study, the analysis of the EMG signals during cycling indicated that during the cycling task, the control of the rectus femoris muscle was significantly different in adolescents with CP as compared to the adolescents with TD. This combined with muscle weakness and altered motor control, as exhibited through the frequency and amplitude interaction with a muscle, likely lead to deviations in joint kinematics. An intervention, which increases muscle strength or results in the conversion of the muscle to a more normal phenotype, with feedback to the nervous system about appropriate activation timing, may be beneficial to allow individuals with CP to cycle more efficiently.
Acknowledgments
Funding for this study was provided by Shriners Hospitals for Children, Grants #8530 and #8540. Additional support was provided by NIH/NICHD Grant # 1 R01 HD043859-01A1, NIH/NINDS Grant # 1 R03 NS044875-01A2 and the Pediatric Section of the American Physical Therapy Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–790. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- Brown DA, Kautz SA. Increased workload enhances force output during pedaling exercise in persons with poststroke hemiplegia. Stroke. 1998;29:598–606. doi: 10.1161/01.str.29.3.598. [DOI] [PubMed] [Google Scholar]
- Brown DA, Nagpal S, Chi S. Limb-loaded cycling program for locomotor intervention following stroke. Phys Ther. 2005;85:159–168. [PubMed] [Google Scholar]
- Crenna P. Spasticity and ‘spastic’ gait in children with cerebral palsy. Neurosci Biobehav Rev. 1998;22:571–578. doi: 10.1016/s0149-7634(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Damiano DL, Quinlivan J, Owen BF, Shaffrey M, Abel MF. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. 2001;8(Suppl 5):40–49. doi: 10.1046/j.1468-1331.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- Davis GM, Servedio FJ, Glaser RM, Gupta SC, Suryaprasad AG. Cardiovascular responses to arm cranking and FNS-induced leg exercise in paraplegics. J Appl Physiol. 1990;69:671–677. doi: 10.1152/jappl.1990.69.2.671. [DOI] [PubMed] [Google Scholar]
- Dore E, Bedu M, Franca NM, Diallo O, Duche P, Van Praagh E. Testing peak cycling performance: effects of braking force during growth. Med Sci Sports Exerc. 2000;32:493–498. doi: 10.1097/00005768-200002000-00035. [DOI] [PubMed] [Google Scholar]
- Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–550. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Liu M, Chino N. Effect of pedaling exercise on the hemiplegic lower limb. Am J Phys Med Rehabil. 2003;82:357–363. doi: 10.1097/01.PHM.0000064722.01940.E4. [DOI] [PubMed] [Google Scholar]
- Hakansson NA, Hull ML. Functional roles of the leg muscles when pedaling in the recumbent versus the upright position. J Biomech Eng. 2005;127:301–310. doi: 10.1115/1.1865192. [DOI] [PubMed] [Google Scholar]
- Ikeda AJ, Abel MF, Granata KP, Damiano DL. Quantification of cocontraction in spastic cerebral palsy. Electromyogr Clin Neurophysiol. 1998;38:497–504. [PubMed] [Google Scholar]
- Johnston TE, Barr AE, Lee SC. Biomechanics of Submaximal Recumbent Cycling in Adolescents With and Without Cerebral Palsy. Phys Ther. 2007 doi: 10.2522/ptj.20060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge M, Hull ML. Analysis of EMG measurements during bicycle pedalling. J Biomech. 1986;19:683–694. doi: 10.1016/0021-9290(86)90192-2. [DOI] [PubMed] [Google Scholar]
- Kaplan SL. Cycling patterns in children with and without cerebral palsy. Dev Med Child Neurol. 1995;37:620–630. doi: 10.1111/j.1469-8749.1995.tb12050.x. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Stackhouse C, Shewokis PA, Smith BT, Orlin M, McCarthy JJ. Assessment of wavelet analysis of gait in children with typical development and cerebral palsy. J Biomech. 2005;38:1351–1357. doi: 10.1016/j.jbiomech.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Stackhouse CA, Shewokis PA, Smith BT, Tucker CA, McCarthy J. A time-frequency based electromyographic analysis technique for use in cerebral palsy. Gait Posture. 2006 doi: 10.1016/j.gaitpost.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Mutton DL, Scremin AM, Barstow TJ, Scott MD, Kunkel CF, Cagle TG. Physiologic responses during functional electrical stimulation leg cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil. 1997;78:712–718. doi: 10.1016/s0003-9993(97)90078-2. [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Ramsay JO, Silverman BW. Functional Data Analysis. Springer-Verlag; New York, NY: 1997. [Google Scholar]
- Ricard MD, Ugrinowitsch C, Parcell AC, Hilton S, Rubley MD, Sawyer R, Poole CR. Effects of rate of force development on EMG amplitude and frequency. Int J Sports Med. 2005;26:66–70. doi: 10.1055/s-2004-817856. [DOI] [PubMed] [Google Scholar]
- Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12:758–768. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- Rose J, McGill KC. The motor unit in cerebral palsy. Dev Med Child Neurol. 1998;40:270–277. doi: 10.1111/j.1469-8749.1998.tb15461.x. [DOI] [PubMed] [Google Scholar]
- Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31:594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse SK, Binder-Macleod SA, Stackhouse CA, McCarthy JJ, Prosser LA, Lee SC. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabil Neural Repair. 2007;21:475–485. doi: 10.1177/1545968306298932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Pountney T. Effects of a static bicycling programme on the functional ability of young people with cerebral palsy who are non-ambulant. Dev Med Child Neurol. 2007;49:522–527. doi: 10.1111/j.1469-8749.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A. Postural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12:211–219. doi: 10.1155/NP.2005.211. [DOI] [PMC free article] [PubMed] [Google Scholar]