Abstract
Oral cancer is associated with high mortality and morbidity rates, largely as a result of late diagnosis. Although dental practitioners are trained to identify premalignant and malignant lesions, an organized system is needed to offer guidance and to improve access to experts in diagnosis and management of these lesions. In this article, we describe how the British Columbia Oral Cancer Prevention Program (BC OCPP) is addressing this challenge in several ways: by linking community dental practices and referral centres, by creating partnerships between scientists and clinicians that already have resulted in new technologies to enhance early diagnosis, by involving a broad range of stakeholders to ensure population-based screening and by engaging in provincial, national and international outreach.
For citation purposes, the electronic version is the definitive version of this article: www.cda-adc.ca/jcda/vol-74/issue-3/XXX.html
Oral cancer represents a significant challenge worldwide, with close to 300,000 cases identified each year.1 Mortality and morbidity rates associated with oral cancer are high, largely as a result of late diagnosis, and only small improvements in outcome have been achieved over recent decades. Dentists need to play a role in establishing a new paradigm for oral cancer control.
Dental practitioners are trained to diagnose premalignant and malignant lesions, and individual dentists have been making these diagnoses for years. However, there has been no agreement on whom and how to screen for oral cancer or on when and where to refer patients for management. As a result, the problem of late diagnosis has not been addressed. We need an organized system to offer guidance and to improve access to experts in diagnosis and management of these lesions.
The British Columbia Oral Cancer Prevention Program (BC OCPP) is addressing this challenge through a multifaceted program. The program is anchored by a clinical infrastructure that links community dental practices and referral centres to improve detection, risk assessment and management of oral cancers and premalignant lesions.2 Partnerships between scientists and clinicians have produced new technologies aimed at removing barriers to early diagnosis. In this article, we describe these initiatives and the future plans of the BC OCPP for provincial, national and international outreach.
Our History: The Evolution of the BC OCPP
The BC OCPP has evolved through the stepwise addition of a number of critical components leading to a formal program in 1999. The process began in 1992 with a partnership between the BC Oral Biopsy Service (OBS) and scientists in the BC Cancer Agency to identify molecular predictors of cancer risk in patients referred to the OBS by community dentists. The OBS has a long history (since 1980) of providing centralized oral pathology review for community dentists. In 1999, on the basis of these early studies, funding was obtained from the National Institute of Dental and Craniofacial Research, within the National Institutes of Health, United States to launch a longitudinal study aimed at developing biomarkers and improving our ability to assess and manage patients. That year, the first dysplasia clinic was established to coordinate this activity. Now, there are 5 clinics and plans for continued expansion across the province.
New Screening Technologies: A Driving Force for Change
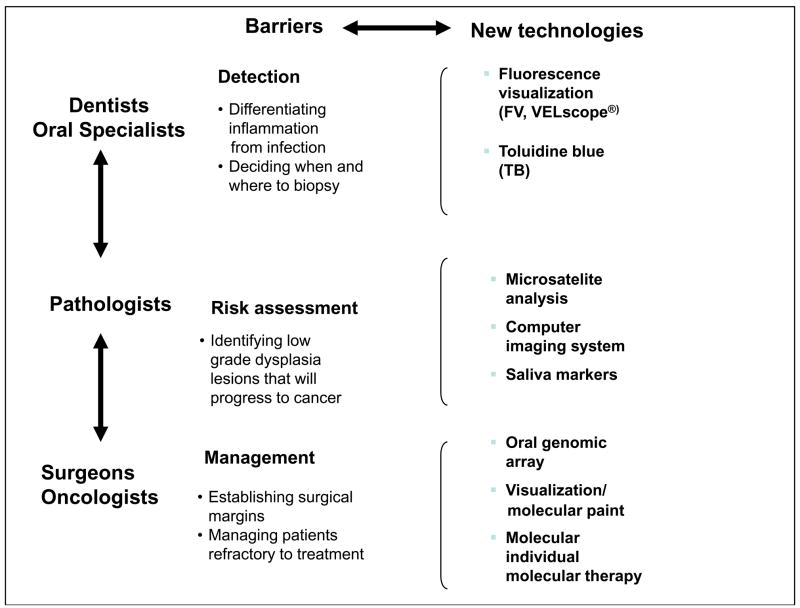
Early in the development of the BC OCPP, several major barriers to the detection, risk assessment and management of early disease were identified (Fig. 1). Oral premalignant lesions and early cancer vary considerably in clinical appearance and often resemble benign conditions, making clinical detection difficult. Currently, the risk of premalignant lesions progressing to cancer is assessed histologically. A high-grade lesion (severe dysplasia and carcinoma-in-situ) indicates a high risk of cancer development. Our longitudinal study has shown that most of these lesions progress to invasive cancers; hence, we recommend treatment. Most low-grade lesions (mild and moderate dysplasia) do not progress to cancer. A major barrier in risk assessment is to identify the small proportion of these low-grade lesions that will progress into cancer. Finally, oral premalignant lesions and cancer frequently extend beyond the boundary of the clinically visible lesion. A major barrier in their management is to identify the extent of the lesions to facilitate complete removal.
Figure 1. Tools to overcome barriers to screening for oral disease at detection, risk assessment and management levels.
Source: BC OCPP.
These barriers have stimulated the development and validation of new technologies, some of which are shown in Fig. 1 and described briefly here.
Fluorescence Visualization
The VELscope (LED Dental Inc., White Rock, B.Ca fluorescence visualization device, is an example of a simple hand-held instrument that detects alterations in normal autofluorescence that are associated with morphologic and biochemical changes to tissue during cancer development. In dysplasia clinics, it has proved valuable in the detection of high-risk lesions, in the delineation of surgical margins and in follow-up after treatment.3,4 The device is only now being evaluated for detection in community settings.
Toluidine Blue
Toluidine blue has a long history and established validity in the detection of oral cancers but its value in identifying oral premalignant lesions has been contentious, as not all such lesions stain with the dye. We have shown that toluidine blue staining of oral premalignant correlates with the presence of high-risk molecular patterns and with progression to cancer and that this relationship holds true for both low and high-grade dysplasia.5 This approach is used routinely in our dysplasia clinics for detection and risk assessment.
Microsatellite Analysis
The development of oral cancer requires the accumulation of multiple genetic alterations. Detection of these alterations can be a powerful predictor of risk of premalignant lesions progressing to cancer. One of the more promising approaches is assessment of tissue for loss of heterozygosity. We have found 3 loss of heterozygosity patterns that separate low-grade lesions into different levels of risk of progression to cancer6–9 and cancer recurrence after treatment.10 These patterns are being validated in the ongoing longitudinal study that is being conducted at the dysplasia clinics.
High-Resolution Computer Imaging Systems
Conventional histologic assessment of a biopsy sample relies on detecting specific visible features (such as dysplasia) and the degree of epithelial involvement.11 High-resolution computer imaging systems reveal these features in greater detail, as well as the frequency and distribution of such changes in cells. These systems are being applied to assess both tissue samples and exfoliated cells. Preliminary studies have shown that such imaging can identify nuclear changes in low-grade dysplasia that are associated with the presence of high-risk molecular clones and with risk of cancer formation.12
Saliva Markers
Currently, a large amount of research is focused on the identification of genetic changes in saliva samples to determine whether they can be used to detect cancer and premalignant disease.13,14 This is a promising noninvasive approach, which is still in an early stage of development. We plan to validate these markers in patients seen at the dysplasia clinics.
Genomic Profiling for Patient Management
We are developing a miniaturized gene chip called the OPL (oral premalignant lesion) risk prediction chip to analyze very small biological samples, such as those collected from patients during longitudinal monitoring. The information will improve the prediction of risk of progression.15 It will also be used in patient management to design molecular probes that can be painted in the patient’s mouth to track the spread of genetically altered cells and to guide the selection of individualized drug interventions specific to molecular alterations within the tumour.
Our Future: Movement to a Province-Wide Screening Program
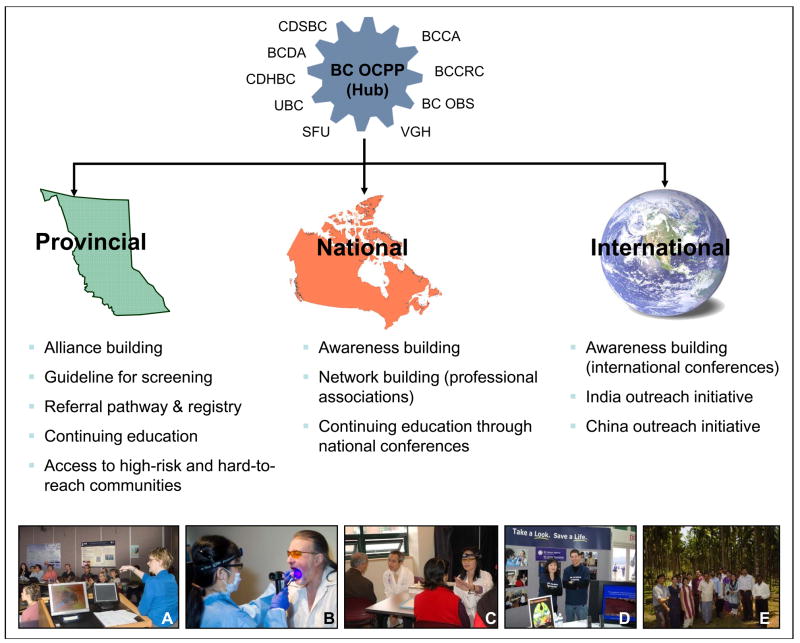
To have an impact on the incidence of oral cancer, a broad range of stakeholders must be involved, including (but not limited to) professional societies, educational institutions, health care facilities, government and the public. A combined effort will guide the evolution of oral cancer screening toward population-based coverage. Creating such a network is becoming a key function of the BC OCPP (Fig. 2). Activities that require these alliances include the standardization of screening activities in the dental community and their integration into day-today practice, the further evolution of referral and management infrastructures and the development of strategies for population-based screening.
Figure 2.
Creating a network to achieve population-based oral cancer screening. The BC OCPP’s organizational hub is linked with a broad range of stakeholders. Its outreach activities, which extend to the provincial national and international levels, include: (a) Partnership with community dentists — continuing education for knowledge transfer and network building. (b) Outreach through community screening in a high-risk, medically underserved area in Vancouver’s downtown east side. (c) Partnership with community representatives to provide oral cancer screening in local health fairs for the elderly Chinese in China Town, Vancouver. (d) Awareness building and continuing education at the Pacific Dental Conference, one of the largest dental conferences in North America. (e) Oral cancer screening camps in rural areas and farms in India to detect cases at early stage.
Note: BCCA = BC Cancer Agency, BCCRC = BC Cancer Research Centre, BC OBS = BC Oral Biopsy Service, VGH = Vancouver General Hospital, SFU = Simon Fraser University, UBC = University of British Columbia, CDHBC = College of Dental Hygienists of British Columbia, BCDA = British Columbia Dental Association, CDSBC = College of Dental Surgeons of British Columbia. Source: BC OCPP
Central to the standardization of screening activity is the provision of guidelines for the early detection of oral cancer with step-by-step procedures for the assessment of clinical lesions, for obtaining oral mucosal tissue samples and interpreting biopsy results. Such guidelines are included in this issue of the JCDA.11,16 Also included is information on strategies for communicating better with the patient about screening17 and on follow-up of abnormalities,11 often requested by dentists. This issue also includes a summary of a dialogue on screening activity by a focus group of community dentists organized in Vancouver late last year.18 The goal of this initiative was to involve dentists directly in identifying barriers and facilitators to screening in community practices and to use this discourse to establish effective strategies for integrating screening into routine clinical practice. These same dentists are also serving as a pilot peer group that is exploring the use of new technology in the community to help set up protocols for effectively evaluating and transferring new technology into such settings.
Infrastructure organization and at least a degree of centralization are needed for any screening program to provide high-quality, cost-effective services. We recognize a need for continued growth and strengthening of the referral pathway.2 Also important is the establishment of a screening registry, which will coordinate screening and diagnostic services and facilitate appropriate management and use of the referral pathway, avoiding unnecessary delays in diagnosis and treatment. Much of this activity is already evolving within the OBS. Creation of a central database within this structure will allow us to monitor program standards regularly to identify areas for improvement. A screening program will be deemed effective only if quality criteria, including target figures and performance indicators, are set and achieved. As the program develops, expected outcomes include improvement in screening participation, changes in rates of detection from biopsies and decreased morbidity and mortality.
A further objective is to improve access to services by those not receiving regular dental care. This requires outreach involving new community partnerships and alternative strategies to reach high-risk, underserved populations, including the poor, immigrants, aboriginal people and the elderly. In British Columbia, for example, an outreach program at a dental clinic in the Downtown Eastside of Vancouver (Canada’s poorest neighbourhood, with high levels of alcohol consumption and smoking) involves a partnership among the University of British Columbia, the Portland Hotel Society (a private society), the provincial government and the BC OCPP.19 The program has identified the urgent need for dental and oral medicine care in such communities and has shown that the community is responsive to such initiatives and recognizes their value.
Looking Toward the Future: National and International Partnerships
The vision of the BC OCPP is to spread, share and disseminate technology and knowledge to the world to provide better care in oral cancer detection and management. Because two-thirds of oral cancers occur in developing countries, this outreach will involve partnerships across cultures and between nations.1
Our initiatives in British Columbia are “going global.” For example, we have begun outreach in India, where oral cancer is one of the most prevalent cancers. Most cases of the disease occur in rural settings, where many people are poor and illiterate, many children and young adults are continuing to take up betel quid and tobacco chewing habits, and an epidemic of tobacco-induced cancers is predicted.20,21 The challenge of controlling oral cancer in India is huge, but the impact will be great. To achieve this task, joint efforts and collaborations are being developed. The BC OCPP is committed to taking on this challenge and assuming a leadership role through partnership and transfer of technology and its modification to fit the needs of the intended setting. There are plans to extend this outreach to China.
In summary, this is a global disease. We need a global solution. So let us join hands and build partnerships. Together we can make a difference.
Acknowledgments
The authors acknowledge core support for this program, funding of the ongoing longitudinal study and ensuing technology development through grants R01 DE13124 and R01 DE17013 from the National Institute of Dental and Craniofacial Research/National Institutes of Health. The British Columbia Cancer Foundation has provided seed funding for community outreach. Dr. Poh is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research and a Scholar Award from the Michael Smith Foundation for Health Research. We specifically thank the following people for their long-term support of our vision: Dr. Yasaman Shirazi, Dr. Simon Sutcliffe, Dr. Andy Coldman, Dr. Robert Priddy, Dr. Chris Zed, Dr. Kenneth Berean, Dr. Nhu Le, Dr. John Hay, Dr. Barry Sheehan, Dr. Janessa Laskin, Dr. Scott Durham, Dr. Don Anderson, Ms. Heather MacKay, Ms. Jocelyn Johnston and Dr. John O’Keefe. We are grateful to the many other members of the BC OCPP team and especially to the patients enrolled in the ongoing longitudinal study.
Footnotes
Contact Author
Dr. Rosin, Email: Miriam_Rosin@shaw.ca
The other authors have no declared financial interests.
Dr. Rosin is director, BC Oral Cancer Prevention Program, professor, faculty of applied sciences, Simon Fraser University, and senior scientist, cancer control research, BC Cancer Agency and Research Centre.
Dr. Poh is assistant professor, faculty of dentistry, University of British Columbia and staff, British Columbia Cancer Agency.
Dr. Elwood is clinical professor, health care and epidemiology, University of British Columbia and senior scientist, cancer control research, BC Cancer Agency and Research Centre.
Dr. Williams is clinical professor, faculty of dentistry, University of British Columbia and staff, British Columbia Cancer Agency.
Prof. Gallagher is leader, cancer control research, BC Cancer Agency and Research Centre and clinical professor, health care and epidemiology, University of British Columbia.…,.
Dr. MacAulay is leader, cancer imaging, BC Cancer Agency and Research Centre.
Dr. Lam is associate professor, pathology and laboratory medicine, University of British Columbia and senior scientist, cancer genomics and developmental biology, BC Cancer Agency and Research Centre.
Dr. Auluck is a clinician/scientist in a PhD training program in the faculty of dentistry at UBC and at the BC Cancer Agency and Research Centre.
Dr. Zhang is professor and head, division oral medicine and pathology, faculty of dentistry, University of British Columbia.
Dr. Hislop is clinical professor health care and epidemiology, University of British Columbia and senior scientist, cancer control research, BC Cancer Agency and Research Centre Vancouver, British Columbia.
Sidebar/pull-quote: “Screening is more than an exam: it is a process. Clinically it involves patient communication, diagnostic work-up and management and quality control. It also involves development of creative strategies for removing barriers... technology development can drive such activity. Teamwork is critical in making this happen.” — The BC OCPP
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM GLOBOCAN. cancer incidence. Mortality and prevalence worldwide. IARC cancer base no. 5, version 2.0. Lyon (France): IARC Press; 2002. 2004. [Google Scholar]
- 2.BC Oral Cancer Detection Working Group. Guidelines for the early detection of oral cancer in British Columbia. J Can Dent Assoc. 2008;74(3):XXX. [PubMed] [Google Scholar]
- 3.Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11(2):024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 4.Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12(22):6716–22. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, Durham S, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65(17):8017–21. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 6.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2(6):682–5. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 7.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488–92. [PubMed] [Google Scholar]
- 8.Partridge M, Emilion G, Pateromichelakis S, A’Hern R, Phillips E, Langdon J. Allelic imbalance at chromosomal loci implicated in the pathogenesis of oral precancer, cumulative loss and its relationship with progression to cancer. Oral Oncol. 1998;34(2):77–83. doi: 10.1016/s1368-8375(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 9.Rosin MP, Lam WL, Poh C, Le ND, Li RJ, Zeng T, et al. 3p14 and 9p21 loss is a simple tool for predicting second oral malignancy at previously treated oral cancer sites. Cancer Res. 2002;62(22):6447–50. [PubMed] [Google Scholar]
- 10.Zhang L, Cheng X, Li Y, Poh C, Zeng T, Priddy R, et al. High frequency of allelic loss in dysplastic lichenoid lesions. Lab Invest. 2000;80(2):233–7. doi: 10.1038/labinvest.3780026. [DOI] [PubMed] [Google Scholar]
- 11.Poh CF, Ng SP, Berean KW, Williams PM, Rosin MP, Zhang L. Biopsy and histopathological diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2008;74(3):XXX. [PubMed] [Google Scholar]
- 12.Guillaud M, Zhang L, Poh CF, Rosin MP, MacAulay C. Potential use of quantitative tissue phenotype to predict malignant risk for oral premalignant lesions. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-07-2113. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Loo JA, Wong DT. Human saliva proteome analysis. Ann N Y Acad Sci. 2007;1098:323–9. doi: 10.1196/annals.1384.015. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann BG, Park NJ, Wong DT. Genomic targets in saliva. Ann N Y Acad Sci. 2007;1098:184–91. doi: 10.1196/annals.1384.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsui FL, Watson S, Rosin MP, Zhang L, Lam WL. Development of a diagnostic tool for the evaluation of progression risk of early oral premalignant lesions (abstract A51). Sixth Annual International Conference on Frontiers in Cancer Prevention Research. American Association for Cancer Research; 2007 Dec 5–8; Philadelphia: Pennsylvania. [accessed 2008 Feb 16]. p. 88. Available: www.aacr.org/Uploads/DocumentRepository/2007conf/prev/FinalProgram/cpr07_poster_a.pdf. [Google Scholar]
- 16.Williams PM, Hovan A, Poh CF. The clinical lesion (temporary title) J Can Dent Assoc. 2008;74(3):XXX. [Google Scholar]
- 17.Currie BL, Williams PM, Poh CF. Is the message clear? Talking with your patient about oral cancer screening. J Can Dent Assoc. 2008;74(3):XXX. [PubMed] [Google Scholar]
- 18.Laronde DM, Bottorff JL, Hislop TG, et al. Experiences from the dental office: Initiating oral cancer screening. J Can Dent Assoc. 2008;74(3):XXX. [PubMed] [Google Scholar]
- 19.Poh CF, Hislop G, Currie B, Lee R, Sikorski S, Zed C, et al. Oral cancer screening in a high-risk underserved community —Vancouver Downtown Eastside. J Health Care Poor Underserved. 2007;18(4):767–78. doi: 10.1353/hpu.2007.0106. [DOI] [PubMed] [Google Scholar]
- 20.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19(4):251–62. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 21.Gupta PC. Mouth cancer in India: a new epidemic? J Indian Med Assoc. 1999;97(9):370–3. [PubMed] [Google Scholar]