Abstract
3,3′-Diindolylmethane (DIM), a major condensation product of indole-3-carbinol (I3C), exhibits chemopreventive properties in animal models of cancer. Recent studies have shown that DIM stimulates interferon-gamma (IFN-γ) production and potentiates the IFN-γ signaling pathway in human breast cancer cells via a mechanism that includes increased expression of the IFN-γ receptor. The goal of this study was to test the hypothesis that DIM modulates the murine immune function. Specifically, the effects of DIM were evaluated in a panel of murine immune function tests that included splenocyte proliferation, reactive oxygen species (ROS) generation, cytokine production and resistance to viral infection. DIM was found to induce proliferation of splenocytes as well as augment mitogen- and IL-2-induced splenocyte proliferation. DIM also stimulated the production of ROS by murine peritoneal macrophage cultures. Oral administration of DIM, but not intraperitoneal injection (i.p.), induced elevation of serum cytokines in mice, including interleukin (IL)-6, granulocyte-colony stimulating factor (G-CSF), IL-12 and IFN-γ. Finally, in a model of enteric virus infection, oral DIM administration to mice enhanced both clearance of reovirus from the GI tract and the subsequent mucosal IgA response. Thus, DIM is a potent stimulator of immune function. This property might contribute to the cancer inhibitory effects of this indole.
Keywords: 3,3′-diindolylmethane; Immune stimulation; Cytokine; Lymphocyte proliferation; reactive oxygen species
1. Introduction
3,3′-Diindolylmethane (DIM), a major acid condensation product of indole-3-carbinol (I3C), is a promising antitumor agent derived from Brassica vegetables [1]. The anticarcinogenic effects of DIM have been shown in animal models of spontaneous, carcinogen-induced or transplanted tumors [2,3]. Because of their effectiveness and low level of toxicity, I3C and DIM have become widely used adjunct therapies for recurrent respiratory papillomatosis (RRP), caused by certain types of human papillomaviruses (HPVs) [4,5]. In view of DIM’s pronounced antitumor activities in rodents and humans, there is considerable interest in the modes of action of this compound.
Several studies in our laboratory have shown that DIM evokes a biphasic response on proliferation of cultured breast tumor cells. At higher concentrations (≥ 30 μM), DIM inhibits proliferation and induces apoptosis in a manner that is independent of estrogen receptor (ER) status [6–10]. In contrast, at lower concentrations (< 30 μM), DIM induces proliferation of ER-replete MCF-7 cells and activates ER binding to DNA by a mechanism that is independent of ligand binding to the receptor [11]. Since non-physiological concentrations of DIM are required for its antiproliferative effects but lower concentrations of DIM induce cell proliferation, the antitumor effects of this indole could very well involve modes of action beyond direct cytostasis.
One alternative mechanism that explains the action of DIM might be through modulation of the immune response. Previous studies provide some evidence that exposure to I3C could influence major immune responses, including natural killer cell activity, antibody production, and T-cell-mediated delayed-type hypersensitivity [12,13]. Notably, DIM upregulates the expression of IFN-γ and IFN-γ receptor and potentiates the effects of IFN-γ-induced expression of MHC I in human breast cancer cells [14,15]. IFN-γ is a central regulator of immune and inflammatory responses [16–18], that contribute to inhibition of primary and transplanted tumor development [19–23] as well as antiviral response [18]. Thus, if DIM has generalized immune stimulatory properties, this capacity might indeed contribute to its anticarcinogenic effects.
The purpose of the present study was to test the hypothesis that DIM modulates the murine immune response. Specifically, we assessed the effects of DIM on four different endpoints of immune function that included splenoocyte proliferation, reactive oxygen species (ROS) generation, cytokine production and resistance to viral infection. Addition of DIM to cultured cells enhanced both splenocyte proliferation and ROS production by peritoneal macrophages. Moreover, oral administration of DIM to mice increased serum concentrations of G-CSF, IL-6, IL-12 and IFN-γ, as well as enhanced the host response to enteric reovirus infection. These results strongly suggest that DIM has potent immunomodulating activities that are consistent with the antitumor and antiviral activities of this dietary indole.
2. Materials and Methods
2.1. Mice
C57BL/6 mice (6–8 wk old) were purchased from Charles River Laboratory and were housed 4–5 mice per cage at 22–24 °C in rooms with 50% humidity and a twelve-hour light: dark cycle. All animals were given mouse chow and water ad libitum. Mice were housed for at least one week before experimental use, and age-matched animals were employed as described for each functional assay. All animal studies were conducted in conformance with NIH guidelines.
2.2. In vitro effects of DIM on splenic lymphocyte proliferation
Spleens were obtained from euthanized mice, passed through a nylon mesh screen and single cells suspended in RPMI-1640 medium, supplemented with 10% (v/v) fetal bovine serum, 25 mM HEPES buffer, and 50 μM 2-mercaptoethanol. After treating with Red Blood Cell Lysis Buffer (eBioscience), splenocytes were washed twice in cold PBS and resuspended in supplemented RPMI-1640. To determine the effect of DIM on splenocyte proliferation, spleen cells (1 × 105) were cultured in 1 mL of supplemented RPMI-1640 in a 24-well plate at 37 °C, 95% humidity and 5% CO2 in the absence or presence of the stimulators concanavalin A (Con A, Sigma, 1.5 μg/mL) or murine IL-2 (Biosource, 150 ng/mL). Cultures were incubated with and without DIM (1–20 μM) for 2 d and 3 μCi of [3H]thymidine (New England Nuclear) was added to each well. Plates were incubated for an additional 8 h and then cells were harvested by centrifugation and washed three times with 10% (w/v) trichloroacetic acid. Cell pellets were dissolved in 400 μL of 0.3 M NaOH and incubated for 30 min at 25 °C. Aliquots (350 μL) were transferred to scintillation vials containing 4 mL of aqueous scintillation fluid and radioactivity was measured using a liquid scintillation counter.
2.3. In vitro effects of DIM on reactive oxygen species (ROS) production in macrophage cultures
Murine peritoneal macrophages were elicited by injection of 3 mL of sterile Brewer’s thioglycollate medium (4.05 g/100 mL) (Sigma) into the peritoneal cavity of C57BL/6 mice. After 4 d, mice were euthanized. PBS (10 ml) was injected into the peritoneum, and lavage fluid was removed. Peritoneal cells were washed twice by centrifugation, resuspended in supplemented RPMI-1640 medium, and then allowed to adhere to the wells for 2 h in 5% CO2 at 37 °C. Non-adherent cells were removed by three washes with fresh culture medium and the remaining adherent peritoneal macrophages used for ROS assay.
Macrophages (5 × 105 per well) were cultured in 12-well plates with no additive, with DIM (10 to 40 μm) or with the positive control LPS (100 ng/ml) for 2, 6, or 24 h. For detection of ROS, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) (Molecular Probes, 4 μg/mL) was added to each well 2 hours prior to harvest. CM-H2DCFDA is a cell-permeant indicator for reactive oxygen species that is nonfluorescent until removal of the acetate groups by intracellular esterases and oxidation occurs within the cell. Cells were washed three times with PBS and detached with Trypsin-EDTA solution (Sigma). Fluorescence intensity was measured in the suspended cells using a Beckman-Coulter Elite ESP flow cytometer (BD Scientific) equipped with Win MDI 2.8 flow cytometry software (http://facs.scripps.edu).
2.4. In vivo effects of DIM on serum cytokine induction
C57BL/6 male mice (n=3) were administered with 100 μL corn oil control or DIM (30 mg/kg) in corn oil by oral gavage or by intraperitoneal injection. This dose was used based on its effectiveness in tumor xenograft studies [24]. Mice were euthanized and blood collected 1 h, 3 h, 5 h, 8 h or 24 h after DIM administration. Blood was allowed to clot on ice for 60 min and the resultant serum was stored at −70 °C until cytokine measurement.
Serum cytokines were analyzed using a RayBio Mouse Cytokine Array 1 (Ray Biotech) according to manufacturer’s instructions. This array enables detection of GCSF, GM-CSF, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40p70, IL-12p70, IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, SCF, sTNFRI, TNF-alpha, thrombopoietin, and VEGF. Briefly, membranes were incubated with blocking buffer at 25 °C for 30 min and then incubated with 1 ml serum sample (2-fold dilution) overnight at 4 °C. The membranes were washed three times and incubated with biotin-conjugated antibodies for 2 h at 25°C. After washing three times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated streptavidin for an additional 1 h. HRP was detected by using chemilumenescent substrate. Cytokines that were elevated in the focused antibody array were further assessed using commercial ELISAs employing antibody pairs for murine IFN-γ, IL-12 and IL-6 (Biosource), as well as murine G-CSF (R&D Systems) according to manufacturer’s instructions.
2.7. In vivo effects of DIM on response to reovirus challenge
Reovirus serotype 1, strain Lang (T1/L) obtained from Dr. Chris Cuff (West Virginia University) was grown in L929 fibroblast cells at 34 °C in DMEM medium with 5% (v/v) fetal bovine serum (FBS), 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 ug/ml of amphotericin B (Gibco). A third-passage plaque-purified virion was used for mouse infection and prepared by 1,1,2-trichloro-1,2,2-trifluoroethane extraction and discontinuous CsCl gradient centrifugation [25]. Titers of purified viruses were determined by standard plaque assay [26].
For reovirus studies, female C577BL/6 mice were housed in flow microisolator cages under negative laminar flow in a BSL 2 room at the Michigan State University Research Containment Facility. Mice were orally gavaged with 180 μL corn oil (control) or 30 mg/kg DIM in 180 μL corn oil every other day for 8 days. Two days after the first DIM treatment, mice were infected by oral gavage with 3 × 107 plaque forming units (PFU) of reovirus in a total volume of 100 μL borate-buffered saline (pH, 7.4) containing 0.3% (w/v) of gelatin [27]. Fecal pellets were collected 0, 2, 4, 6, 8, 10, 12 and 14 d after viral infection. Pellets to be used for RNA analysis were immediately frozen at −20 °C. Fecal pellets for Ig study were suspended at 0.1 g/ml in phosphate buffered saline, held on ice for 2 h and then sonicated for 15 sec. Solutions were centrifuged at 16,000 × g for 10 min at 4 °C and stored at −20 °C. At experiment termination, mice were bled from the saphenous vein into heparinized tubes and then euthanized. Plasma was separated from clotted blood samples and stored at 4 °C.
Total RNA was extracted from fecal suspension in phosphate buffered saline using TRIZOL (Invitrogen) and real time PCR for reovirus L2 gene expression was performed as reported previously [28]. PCR primers for λ2 core spike (L2 gene) of reovirus T1/L were forward,5′-ctg acg tcg atc agg tcg ttg- 3′ and reverse, 5′-gat gtg gca tgc atg cat gag- 3′. Purified reoviruses were added into 10% (w/w) of fecal pellet suspension from vehicle mice at concentrations of 0, 101, 102, 103, 104,105,106, 107 PFU/ml to generate a standard curve that was applied to sample quantification.
For measurement of reovirus-specific Ig responses, fecal supernatants and sera were assayed for virus-specific IgA and IgG2A antibody responses by the ELISA method of Major and Cuff [29] as modified by Li et al. [28]. Absorbance at 450 nm was used as endpoint for fecal pellets. For sera, antibody titers represented the geometric mean of the highest serum dilutions to yield absorbance of 0.2 or higher.
2.10. Statistical Analysis
Data were analyzed using Sigma Stat software (Jandel Scientific, San Rafael, CA). For comparisons of two groups of data, Student’s t test was performed. For comparison of multiple groups of data, a Kruskal-Wallis one-way ANOVA on ranks and SNK post hoc test were used. Data sets were considered significantly different when p<0.05.
3. Results
3.1. DIM induces and augments splenocyte proliferation
Proliferation of splenocytes is a widely used marker of the immune activation [30,31]. The effect of DIM concentrations ranging from 1 to 20 μM on proliferation of splenocytes was therefore examined using a [3H]thymidine incorporation assay. A concentration-dependent increase in the proliferation of splenic cells was observed following addition of DIM alone (Fig. 1A). Effects were maximal at 10 μM with cell proliferation being twice that of the control. Con A- and IL-2-induced proliferation in cultures containing 10 μM DIM were 4.2- and 8.2-times higher, respectively, than that for vehicle-treated cultures (Fig. 1B, 1C). The relative decrease in splenocyte proliferation was observed at higher concentrations of DIM (15 μM and 20 μM) although they are still significantly higher compared with vehicle controls. These data thus suggest that DIM alone activated proliferation of splenocytes and, furthermore, strongly augmented stimulation by the agonists Con A and IL-2.
Fig. 1.
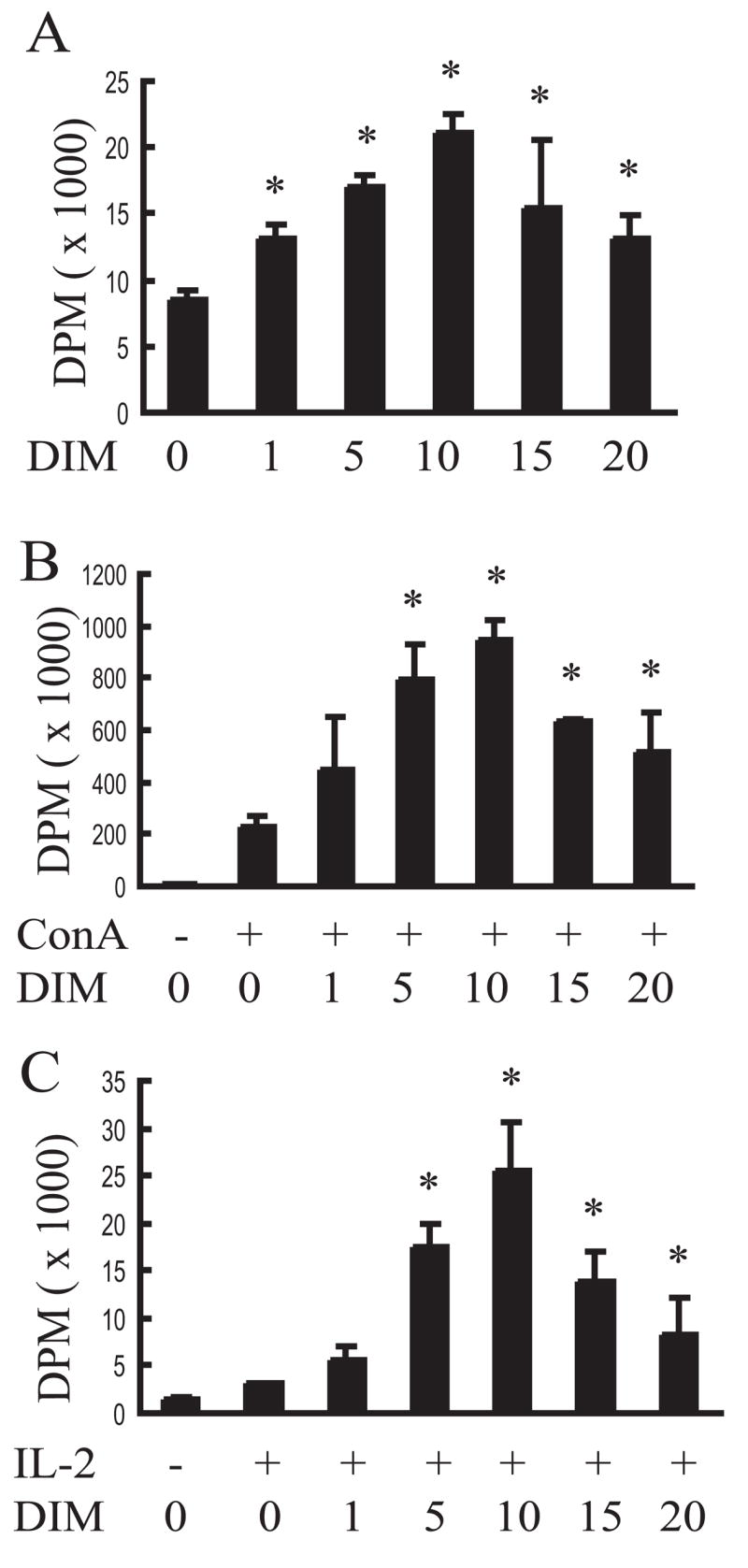
DIM enhances proliferation of splenocyte cultures. Spleen cells (1 × 105/well) were cultured with various concentrations of DIM in the absence (A) or presence of Con A (1.5 μg/mL) (B) or IL-2 (150 ng/mL) (C) for 2 d and [3H]thymidine was added to each well. Plates were incubated for an additional 8 h and [3H]thymidine incorporation determined. Results were expressed as the mean value (X ± SD) and they are representative of at least three individual experiments. In Figure 1A, asterisks indicate significant difference between DMSO-treated and DIM-treated samples (P<0.05); and in Figure 1B and 1C, asterisks indicate significant difference between co-treatment with DMSO and ConA/IL-2 and co-treatment with DIM and ConA/IL-2 (P<0.05).
3.2 DIM induces ROS production in macrophage cultures
Activated macrophages synthesize and release ROS, which are important in immunologic clearance of tumor targets and infectious agents [32–34]. The effect of DIM on ROS production by peritoneal macrophages was assessed by flow cytometry (Fig. 2). DIM induced ROS production in a concentration-dependent fashion to as much as three times that of background. This induction level was similar to that of the positive control, LPS. Thus, DIM also appeared to activate macrophages.
Fig. 2.
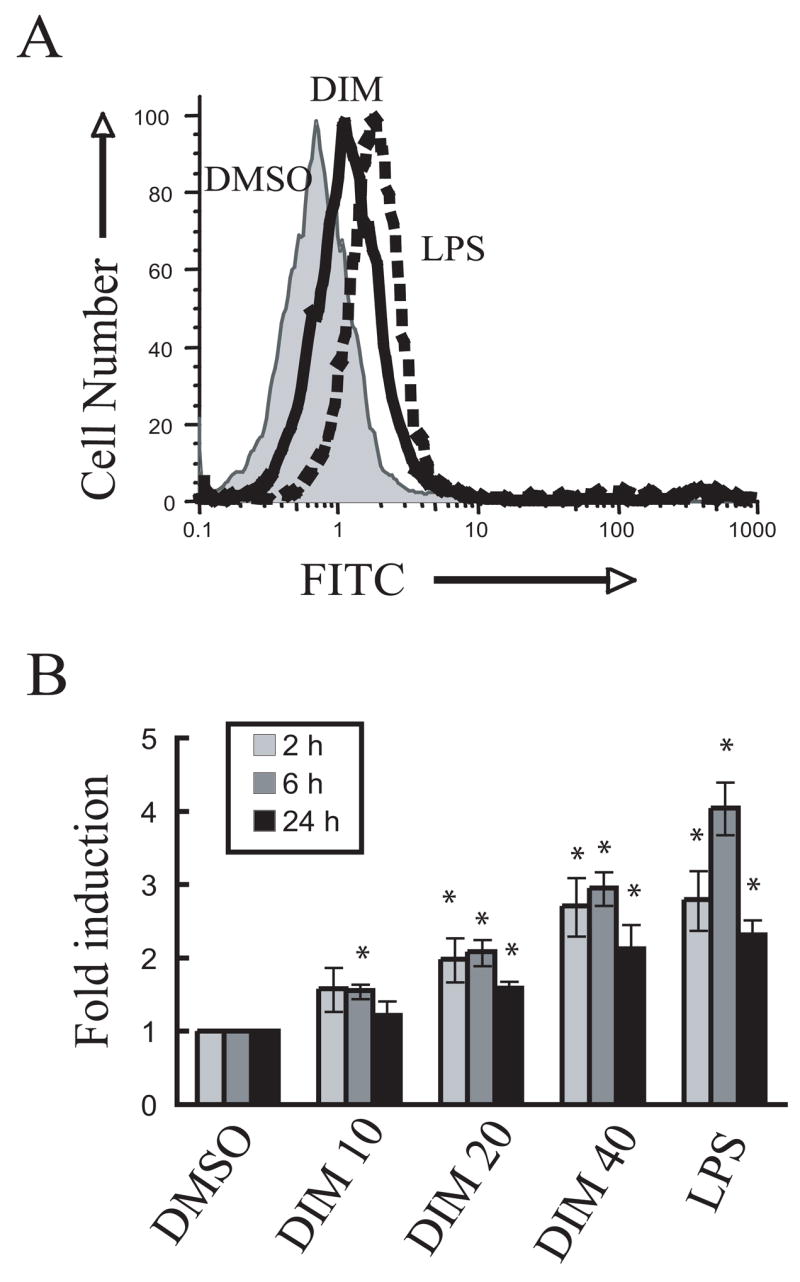
DIM induces ROS production in peritoneal macrophage cultures. (A) Peritoneal macrophages (5 × 105 per well) were cultured with CM-H2DCFDA with vehicle DMSO vehicle, DIM (20 μM) or 100 ng/mL of LPS for 2 h at 37 °C. Cells were detached by subjected to FACS analysis (B). Cultures were incubated with vehicle, DIM (10–40 μM) or LPS for 2 h, 6 h or 24 h. Cells were detached by trypsin/EDTA and analyzed by FACS. ROS production is presented as fold induction over the DMSO treatment after subtraction the fluorescence intensity of background. Results are presented as the mean ± SD (n=3). Asterisks indicate significant difference between DMSO-treated and DIM-treated/LPS-treated samples (P<0.05).
3.3 DIM induces cytokine production in mice
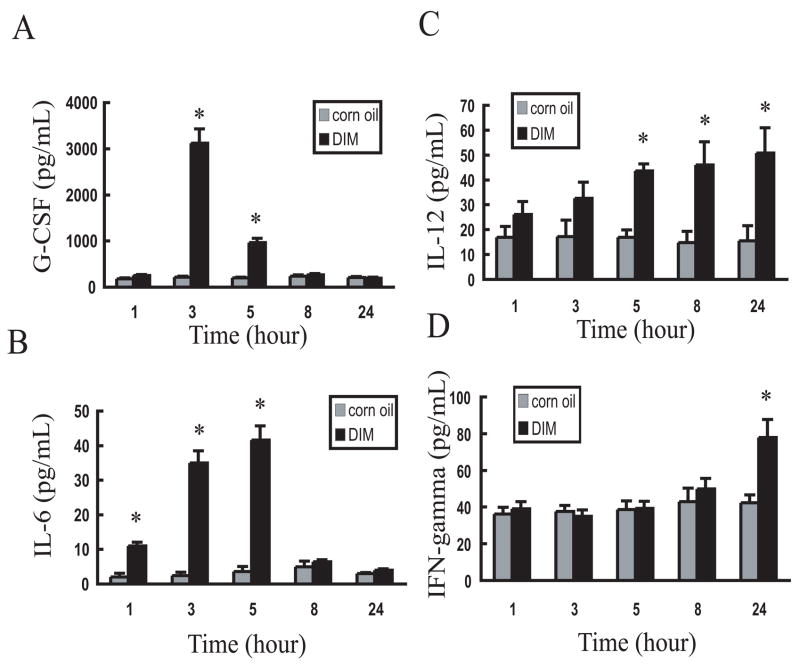
Cytokines play integral regulatory and effector roles in the immune response [35,36]. The effects of oral and i.p. DIM exposure on serum cytokine levels were thus assessed in the mouse over a 24 h period (1 h, 3 h, 5 h, 8h and 24 h) cytokine protein array. Oral administration of DIM markedly increased the concentration of 4 out of 22 serum cytokines tested over this time period, which included IL-6, G-CSF, IL-12 and IFN-γ, whereas no effect was observed when DIM was given by the i.p. route (data not shown). Confirmation by ELISA revealed that DIM stimulated time-dependent increases in serum levels of these cytokines that were maximal at 3 h for G-CSF and at 5 h for IL-6 (Figure 3A and 3B). In contrast, DIM stimulation of IL-12 persisted for at least 24 h (Figure 3C) while induction of IFN-γ was only observed at 24 h (Figure 3D).
Fig. 3.
Oral administration of DIM induces cytokine production in mice. Mice were challenged with corn oil (grey bars) or 30 mg/kg body wt. of DIM in corn oil (black bars)) by oral administration and blood collected at 1, 3, 5, 8, and 24 h after treatment. Serum was analyzed for (A) G-CSF, (B) IL-6, (C) IL-12, (D) IFN-γ by ELISA. Results were expressed as the mean value (X ± SD) and they are representative of at least three individual experiments. Asterisks indicate significant difference between corn oil-treated group and DIM-treated group (P<0.05).
3.4 DIM augments reovirus clearance and reovirus-induced gut IgA responses
Reoviruses are double-stranded RNA viruses that have been extremely useful models for studying viral pathogenesis and host immunity [37]. Reovirus is shed in feces during intestinal infection thus enabling it to be continuously monitored in feces by real-time PCR of the L2 gene [28]. To further confirm the immunomodulatory effects of DIM, an in vivo reovirus infection model was used (Fig. 4A). In vehicle-treated mice, approximately 1×105 and 3.5×105 L2 RNA copies per gram feces were detected at 2 d and 4 d post-infection (PI), respectively, and fecal L2 RNA was undetectable at 6 d PI and thereafter (Fig. 4B). However, in DIM-treated mice, L2 RNA was undetectable at 2 d PI and only approximately 1.3×105 copies per gram feces were observed at 4 d PI, which were significantly less than that for corn oil-treated mice (P<0.05). These results suggest that oral gavage of DIM strongly suppressed both reovirus infection and shedding.
Fig. 4.
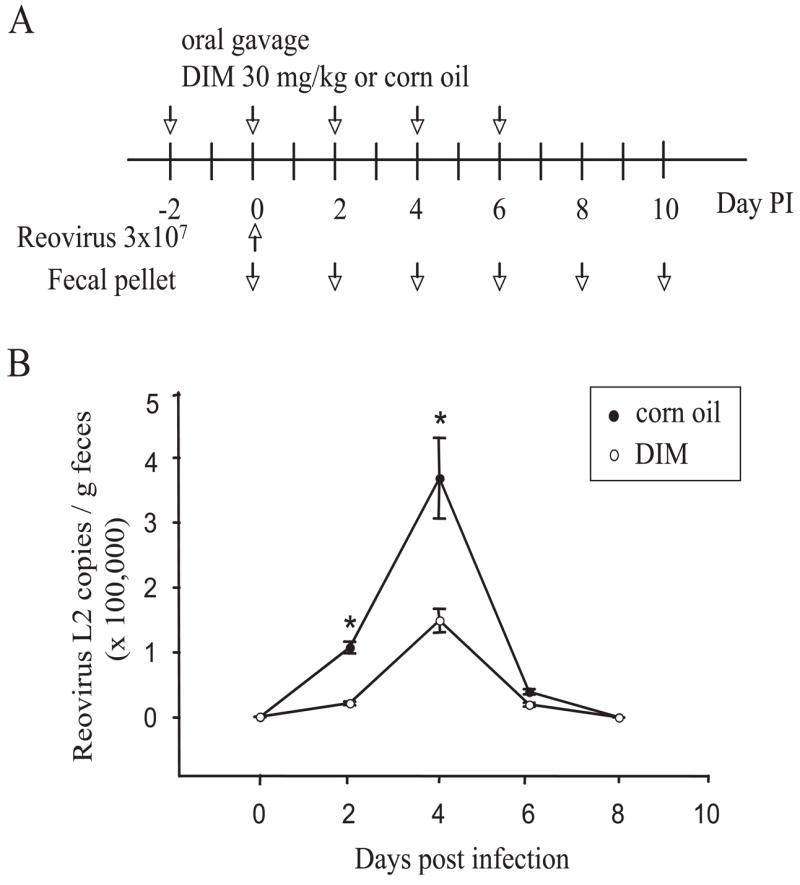
DIM suppresses intestinal reovirus infection. (A) Mice were orally gavaged with 30 mg/kg DIM or corn oil every other day for 8 days. Two days after the first DIM treatment, mice were orally gavaged with 3×107 plaque forming units of reovirus. Fecal pellets were collected at 2 d intervals. (B) Total RNAs were isolated and L2 gene detected by real-time PCR. Data are means ± SEM (n=6) of viral L2 gene copies per gram fecal pellet. Asterisks indicate significant difference between corn oil-treated group and DIM-treated group (P<0.05). Results are representative of two separate experiments.
Reovirus induces a specific mucosal IgA response which likely enhances clearance of the virus during initial and secondary exposures to the agent [25,27]. To assess mucosal IgA response, reovirus-specific IgA in feces was compared over the 14 d course of infection in vehicle- and DIM-treated mice. Increased reovirus-specific IgA was detectable at 8 d PI and thereafter in control mice (Fig. 5A). Induction of reovirus-specific IgA was significantly enhanced at 8 and 10 d PI in DIM-treated mice (P<0.05). In contrast, DIM did not affect reovirus-specific serum IgA or IgG2a titers at 10 d (Fig. 5B). Overall, DIM strongly accelerated both intestinal clearance of reovirus and induction of reovirus-specific gut mucosal IgA following oral administration of reovirus.
Fig. 5.
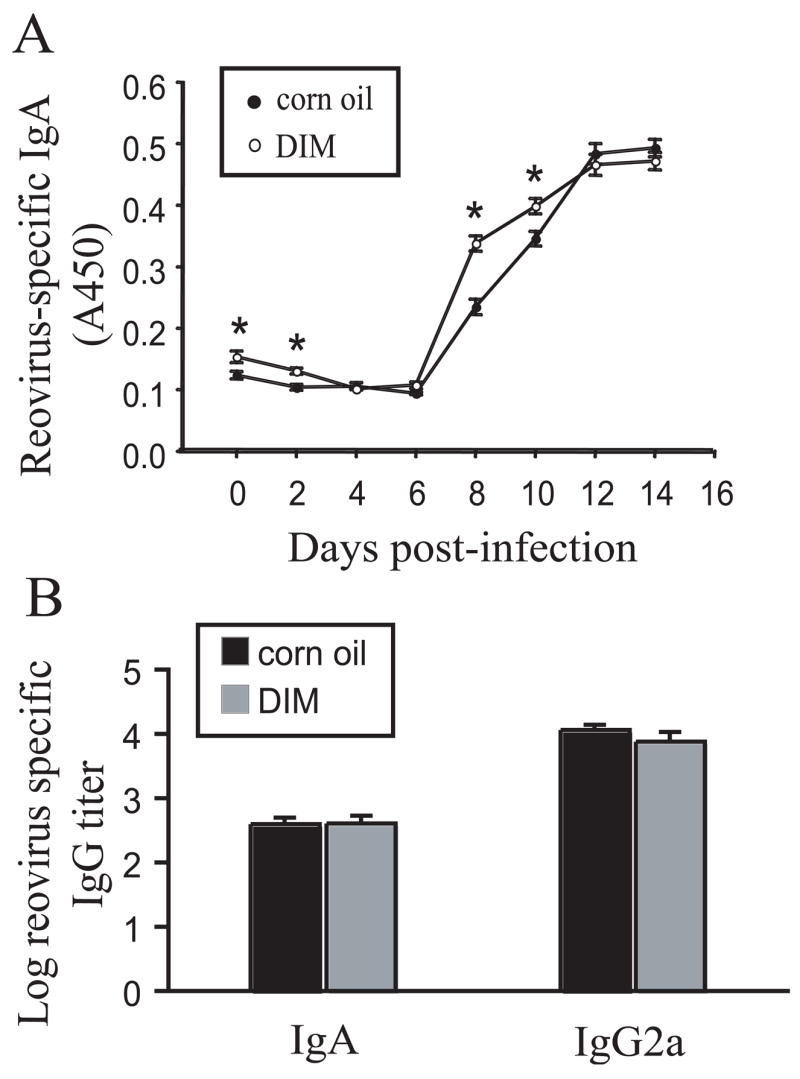
DIM potentiates reovirus-specific intestinal IgA response but not serum IgA or IgG2a responses. (A) Mice were treated as described in Fig. 4A and virus-specific IgA in fecal suspensions determined by ELISA. Data are means ± SEM (n=6). Asterisks indicate significant difference between corn oil-treated group and DIM-treated group (P<0.05). (B) Mice were treated as described in Fig. 4A and serum IgA and IgG2a titers at 10 d were determined by ELISA. Data are means ± SEM (n=6). Results are representative of two separate experiments.
4. Discussion
DIM has been shown to effectively inhibit carcinogen-induced and spontaneous tumor growth in a variety of animal models, but the specific anti-cancer mechanisms remain unresolved. Previous studies in our lab have shown that DIM up-regulates (1) expression of IFN-γ and its receptor, (2) potentiates the IFN-γ signaling pathway and (3) enhances IFN-γ-induced expression of MHC-I complex in MCF-7 cells [15], all of which could stimulate immune responses. In the present study, we investigated the immunological effects of DIM using both in vitro and in vivo functional assays. That DIM significantly increased both unstimulated and mitogen- and IL-2-induced proliferation of splenocytes, as well as induced ROS production in primary macrophage cultures, suggest that this indole can directly affect leukocytes. Moreover, oral administration of DIM stimulated production of several cytokines, G-CSF, IL-6, IL-12, and IFN-γ, each with characteristically different kinetics. Finally, DIM enhanced both clearance of reovirus and the subsequent mucosal IgA response in the model of reovirus infection. Taken together, these data strongly support the premise that DIM augments immune function, an activity that might contribute to in vivo antitumor and antiviral effects of this indole.
T and B lymphocytes play critical regulatory and effector roles in the immune system. Immune response amplification typically involves proliferation of lymphocytes that are normally in a resting state [38]. Thus, proliferation assays using spleen or lymph node cell cultures can be used to assess overall immune competence[39]. DIM’s capacity to stimulate proliferation in naïve splenocytes suggests an inherent potential for this compound to stimulate immune responses. T cell activation can result in cytokine production, cytokine receptor expression and finally proliferation of the activated T cells [39]. Con A activates T cells by binding TCR and specifically induces proliferation of T cells. The strong amplification of the Con A response by DIM suggests that T cells are a possible target of DIM. Finally, IL-2 can stimulate both T cells and B cells and proliferation is dependent on IL-2 receptors [38]. While only modest stimulation of splenocyte proliferation by IL-2 was noted here, it was remarkably magnified by DIM. The mechanisms driving DIM-induced or –augmented cell proliferation are unclear at this time but might involve alterations in receptor signaling, intracellular kinase activity, gene transcription translation, as well as cell division. Future understanding of these processes and their downstream impact will require assessment of DIM’s effects in purified lymphocyte populations and extension of these effects to specific in vivo endpoints.
Macrophages constitute a major part of the host defense system against infection and cancer. Accumulating evidence indicates that activated macrophages carry out their microbicidal and tumoricidal activities by oxygen-dependent killing via products of oxidative metabolism such as hydrogen peroxide, superoxide anion, and hydroxyl radical, and the oxygen-independent killing via cytokines and hydrolytic enzymes [40]. Since DIM dose-dependently increased ROS production in primary macrophage cell cultures, this indole has the potential to promote microbiocidal and anti-tumor activities via oxygen-dependent mechanisms. Further insight is needed on how DIM directly affects macrophages, as well as on what relevant downstream immune events might be affected by this signaling change.
Cytokines are major mediators of host defense because they regulate communication between antigen-presenting cells, lymphocytes and other host cells in the course of an immune response [36]. The cytokine repertoire present at a tissue site determines the types of host response directed against a tumor or locus of infection. Cytokines promoting the development of T-cell-mediated immunity can induce or enhance the antitumor and antimicrobial immunity [41]. Here, we demonstrated that DIM increases the level of several critical cytokines in vivo, including G-CSF, IL-6, IL-12 and IFN-γ. G-CSF is a growth factor that induces the bone marrow to produce more leukocytes, which are essential for fighting infection and cancer [42,43]. IL-6 is a pleuripotent cytokine demonstrated to act as a growth factor in T cells and a differentiation factor in B cells [44–47]. The antitumor, antimetastatic and antiviral activities of IFN-γ and IL-12 also have been well-documented in many different murine models [48–53]. The combined effects of these cytokines on overall immunity is not readily predictable, as yet. Thus, their specific role in the release of other cytokines and mediation of anti-cancer effects of DIM awaits further investigation.
It should be noted that in vivo peritoneal administration of DIM did not result in changes in serum cytokines, while oral administration increased cytokine levels. In studies with peritoneal macrophage cultures, we were similarly unable to observe increases in IFN-γ, IL-6, IL-12 and G-CSF (data not shown). This is consistent with the lack of in vivo cytokine production that we observed following peritoneal administration. The differences observed for cytokine production between oral and intraperitoneal exposure might reflect different tissue distribution of DIM, a requirement for metabolism of the compound and /or different cell targets within the peritoneum, systemic compartment and gut mucosa.
The ultimate test of a putative immune modulator is to evaluate its effects on host resistance to an infectious agent. The mechanisms for host resistance to viruses are multi-factorial, including innate and acquired immune components. Immune responses to enteric reovirus infection in murine gastrointestinal-associated lymphoid tissues (GALT) have been well-characterized relative to: (1) cell-mediated immunity [27,54], (2) expression of virus-specific intestinal IgA [25,27,55], (3) serum IgG level [55,56], and (4) cytokine production [57,58]. These characteristics make reovirus useful to study the effects of immunomodulatory compounds on viral infection in vivo [59,60]. As demonstrated here, DIM markedly interfered with reovirus infection. The observation that DIM suppressed reovirus infection at a very early time point (2 d PI) and also stimulated IgA response at 8 and 10 d, suggests that both the innate and acquired arms of the immune responses, respectively, might be enhanced by this compound.
The capacity of DIM to induce G-CSF, IL-12 and IFN-γ might enhance cell-mediated immune responses to viruses. G-CSF stimulates cytolytic function of NKT cells [61], whereas IL-12 can induce IFN-γ production in both NK and NKT cells [62]. IFN-γ mediates antiviral immunity by multiple mechanisms including suppression of viral replication, macrophage activation, inducible nitric oxide synthase expression and stimulation of specific cytotoxic immunity via cell-surface-bound antigen associated MHC proteins [18,63]. Elevated IFN-γ expression might have enhanced one or more of these mechanisms, thus impairing reovirus replication and survival.
The observation that DIM markedly increased reovirus-induced IgA response is also notable because this antibody provides protection and mediates clearance of viruses that invade the mucosal route [64]. IL-6 appears to be critical to IgA production based on in vitro [65] and knockout studies [66]. Therefore, IL-6 upregulation by DIM could contribute to the enhanced IgA responses.
DIM might be included in the very small group of known low molecular weight immune enhancers. Other compounds with related activities include imiquimod and its homologs, which modulate innate immune responses by activating dendritic cells, macrophages and other cells via Toll-like receptor 7 (TLR-7). Imiquimod is used topically and has been shown to effectively clear genital warts and decrease the recurrence rates of human papillomavirus (HPV)-associated diseases [67–69]. That I3C and DIM are active orally with low toxicity apparently have made them the most popular adjunct therapies for the treatment of recurrent respiratory papillomatosis (RRP) [4]. RRP is caused by certain types of human papilloma viruses (HPVs) [70,71], and a hallmark of this disease is the tendency of the papillomas to recur after surgical removal [72,73]. One report indicated that most patients (55.4%) responded to the treatment of I3C/DIM by slowing down the recurrence rate. Recurrence of the disease was completely inhibited in 19% of patients [4]. Previously suggested modes of action of I3C/DIM in the control of RRP include induction of a better estrogen metabolite balance [74], inhibition of cell proliferation [75], and apoptosis induction [9]. Our results suggest that DIM can also enhance viral clearance.
Taken together, the results presented herein indicate that DIM is a potent stimulator of immune function and can directly affect splenocyte and macrophage function. These properties might contribute to its well-documented antitumor and antiviral effects. In vitro studies are ongoing in our laboratories to identify specific target cells and mechanisms for DIM immuno-stimulation. Additional investigations are underway to characterize the DIM immuno-modulation in vivo relative to dose response and duration. Such data will provide essential insight into DIMs comparative efficacy as a food constituent or as a nutraceutical. Ultimately, if such immunostimulatory effects can be confirmed in human subjects, it might explain DIM’s efficacy in treatment of papillomatosis and furthermore, suggest the use this indole as an antiviral chemotherapeutic, as well as in the chemoprevention of malignant conversion of a broad range of tumor types.
Acknowledgments
We would like to thank Dr. Chris Cuff (West Virginia University) for kindly supplying reovirus and technical advice.
Abbreviations
- DIM
3, 3′-Diindolylmethane
- I3C
indole-3-carbinol
- IFN-γ
interferon-gamma
- ROS
reactive oxygen species
- i.p
intraperitoneal injection
- IL-6
interleukin
- G-CSF
granulocyte-colony stimulating factor
Footnotes
This study was supported by the Department of Defense, Army Breast Cancer Research Program grant (DAMDI7-96-1-6149), by National Institutes of Health grants (CA69056 and CA102360), and in part by a Strategic Partnership Research Grant from the Michigan State University Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grose KR, Bjeldanes LF. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992;5:188–193. doi: 10.1021/tx00026a007. [DOI] [PubMed] [Google Scholar]
- 2.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 3.Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38:1410–1413. [PubMed] [Google Scholar]
- 4.Auborn KJ. Therapy for recurrent respiratory papillomatosis. Antivir Ther. 2002;7:1–9. [PubMed] [Google Scholar]
- 5.Wiatrak BJ. Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg. 2003;11:433–441. doi: 10.1097/00020840-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem. 2001;276:22332–22340. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 7.Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr. 2003;133:2448S–2455S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 8.Gong Y, Sohn H, Xue L, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane is a novel mitochondrial H(+)-ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006;66:4880–4887. doi: 10.1158/0008-5472.CAN-05-4162. [DOI] [PubMed] [Google Scholar]
- 9.Hong C, Firestone GL, Bjeldanes LF. Bcl-2 family-mediated apoptotic effects of 3,3′-diindolylmethane (DIM) in human breast cancer cells. Biochem Pharmacol. 2002;63:1085–1097. doi: 10.1016/s0006-2952(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 10.Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23:1297–1305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 11.Riby JE, Chang GH, Firestone GL, Bjeldanes LF. Ligand-independent activation of estrogen receptor function by 3, 3′-diindolylmethane in human breast cancer cells. Biochem Pharmacol. 2000;60:167–177. doi: 10.1016/s0006-2952(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 12.Exon JH, South EH. Dietary indole-3-carbinol alters immune functions in rats. J Toxicol Environ Health A. 2000;59:271–279. doi: 10.1080/009841000156934. [DOI] [PubMed] [Google Scholar]
- 13.Exon JH, South EH, Magnuson BA, Hendrix K. Effects of indole-3-carbinol on immune responses, aberrant crypt foci, and colonic crypt cell proliferation in rats. J Toxicol Environ Health A. 2001;62:561–573. doi: 10.1080/152873901300007842. [DOI] [PubMed] [Google Scholar]
- 14.Riby JE, Xue L, Chatterji U, Bjeldanes EL, Firestone GL, Bjeldanes LF. Activation and potentiation of interferon-gamma signaling by 3,3′-diindolylmethane in MCF-7 breast cancer cells. Mol Pharmacol. 2006;69:430–439. doi: 10.1124/mol.105.017053. [DOI] [PubMed] [Google Scholar]
- 15.Xue L, Firestone GL, Bjeldanes LF. DIM stimulates IFNgamma gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene. 2005;24:2343–2353. doi: 10.1038/sj.onc.1208434. [DOI] [PubMed] [Google Scholar]
- 16.Chesler DA, Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13:441–454. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 17.Hurlock EC. Interferons: potential roles in affect. Med Hypotheses. 2001;56:558–566. doi: 10.1054/mehy.2000.1218. [DOI] [PubMed] [Google Scholar]
- 18.Novelli F, Casanova JL. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev. 2004;15:367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunda MJ. Interleukin-12. J Leukoc Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 20.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 22.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 23.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blod. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 24.Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26:771–778. doi: 10.1093/carcin/bgi018. [DOI] [PubMed] [Google Scholar]
- 25.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer’s patches. J Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuff CF, Lavi E, Cebra CK, Cebra JJ, Rubin DH. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J Virol. 1990;64:1256–1263. doi: 10.1128/jvi.64.3.1256-1263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.London SD, Rubin DH, Cebra JJ. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer’s patches. J Exp Med. 1987;165:830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Cuff CF, Pestka J. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol Sci. 2005;87:134–145. doi: 10.1093/toxsci/kfi225. [DOI] [PubMed] [Google Scholar]
- 29.Major AS, Cuff CF. Enhanced mucosal and systemic immune responses to intestinal reovirus infection in beta2-microglobulin-deficient mice. J Virol. 1997;71:5782–5789. doi: 10.1128/jvi.71.8.5782-5789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohly H, Jenkins J, Skelton T, Meydrech E, Markov AK. Fructose-1,6-diphosphate suppresses T-lymphocyte proliferation, promotes apoptosis and inhibits interleukins-1, 6, beta-actin mRNAs, and transcription factors expression. Immunol Invest. 2004;33:407–421. doi: 10.1081/imm-200038668. [DOI] [PubMed] [Google Scholar]
- 31.Khanna AK, Plummer M, Nilakantan V, Pieper GM. Recombinant p21 protein inhibits lymphocyte proliferation and transcription factors. J Immunol. 2005;174:7610–7617. doi: 10.4049/jimmunol.174.12.7610. [DOI] [PubMed] [Google Scholar]
- 32.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 33.Keller R, Keist R, Frei K. Lymphokines and bacteria, that induce tumoricidal activity, trigger a different secretory response in macrophages. Eur J Immunol. 1990;20:695–698. doi: 10.1002/eji.1830200334. [DOI] [PubMed] [Google Scholar]
- 34.Mavier P, Edgington TS. Human monocyte-mediated tumor cytotoxicity. I Demonstration of an oxygen-dependent myeloperoxidase-independent mechanism. J Immunol. 1984;132:1980–1986. [PubMed] [Google Scholar]
- 35.Beadling C, Slifka MK. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp (Warsz ) 2006;54:15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- 36.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 37.Tyler KL, Clarke P, DeBiasi RL, Kominsky D, Poggioli GJ. Reoviruses and the host cell. Trends Microbiol. 2001;9:560–564. doi: 10.1016/s0966-842x(01)02103-5. [DOI] [PubMed] [Google Scholar]
- 38.James SP. Measurement of proliferative responses of cultured lymphocytes. Current Protocols in Immunology. 1994:7.10.1–7.10.10. doi: 10.1002/0471142735.im0710s11. [DOI] [PubMed] [Google Scholar]
- 39.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays of T cell function. Current Protocols in Immunology. 2004:3.12.1–3.12.20. doi: 10.1002/0471142735.im0312s60. [DOI] [PubMed] [Google Scholar]
- 40.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 41.Chouaib S, sselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493–497. doi: 10.1016/s0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 42.Adachi Y, Imagawa J, Suzuki Y, Yogo K, Fukazawa M, Kuromaru O, Saito Y. G-CSF treatment increases side population cell infiltration after myocardial infarction in mice. J Mol Cell Cardiol. 2004;36:707–710. doi: 10.1016/j.yjmcc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Pentheroudakis G, Malamou-Mitsi V, Briasoulis E, Damala K, Vassou A, Vartholomatos G, Kolaitis N, Pavlidis N. The neutrophil, not the tumor: serum CA 15-3 elevation as a result of granulocyte--colony-stimulating factor-induced neutrophil MU1C overexpression and neutrophilia in patients with breast carcinoma receiving adjuvant chemotherapy. Cancer. 2004;101:1767–1775. doi: 10.1002/cncr.20581. [DOI] [PubMed] [Google Scholar]
- 44.Galandrini R, Cernetti C, Albi N, Dembech C, Terenzi A, Grignani F, Velardi A. Interleukin-6 is constitutively produced by human CTL clones and is required to maintain their cytolytic function. Cell Immunol. 1991;138:11–23. doi: 10.1016/0008-8749(91)90128-x. [DOI] [PubMed] [Google Scholar]
- 45.Ming JE, Cernetti C, Steinman RM, Granelli-Piperno A. Interleukin 6 is the principal cytolytic T lymphocyte differentiation factor for thymocytes in human leukocyte conditioned medium. J Mol Cell Immunol. 1989;4:203–211. [PubMed] [Google Scholar]
- 46.Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167:332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takai Y, Wong GG, Clark SC, Burakoff SJ, Herrmann SH. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988;140:508–512. [PubMed] [Google Scholar]
- 48.Barth RJ, Jr, Mule JJ, Spiess PJ, Rosenberg SA. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boggio K, Di CE, Rovero S, Cavallo F, Quaglino E, Lollini PL, Nanni P, Nicoletti G, Wolf S, Musiani P, Forni G. Ability of systemic interleukin-12 to hamper progressive stages of mammary carcinogenesis in HER2/neu transgenic mice. Cancer Res. 2000;60:359–364. [PubMed] [Google Scholar]
- 50.Lollini PL, Forni G. Specific and nonspecific immunity in the prevention of spontaneous tumours. Immunol Today. 1999;20:347–350. doi: 10.1016/s0167-5699(99)01450-4. [DOI] [PubMed] [Google Scholar]
- 51.McEarchern JA, Besselsen DG, Akporiaye ET. Interferon gamma and antisense transforming growth factor beta transgenes synergize to enhance the immunogenicity of a murine mammary carcinoma. Cancer Immunol Immunother. 1999;48:63–70. doi: 10.1007/s002620050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melero I, Mazzolini G, Narvaiza I, Qian C, Chen L, Prieto J. IL-12 gene therapy for cancer: in synergy with other immunotherapies. Trends Immunol. 2001;22:113–115. doi: 10.1016/s1471-4906(00)01824-x. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Curr Top Microbiol Immunol. 1999;238:57–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 54.Cuff CF, Cebra CK, Rubin DH, Cebra JJ. Developmental relationship between cytotoxic alpha/beta T cell receptor-positive intraepithelial lymphocytes and Peyer’s patch lymphocytes. Eur J Immunol. 1993;23:1333–1339. doi: 10.1002/eji.1830230622. [DOI] [PubMed] [Google Scholar]
- 55.Major AS, Cuff CF. Effects of the route of infection on immunoglobulin G subclasses and specificity of the reovirus-specific humoral immune response. J Virol. 1996;70:5968–5974. doi: 10.1128/jvi.70.9.5968-5974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virgin HW, Bassel-Duby R, Fields BN, Tyler KL. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan JY, Boyce CS, Cuff CF. T-Helper 1 and T-helper 2 cytokine responses in gut-associated lymphoid tissue following enteric reovirus infection. Cell Immunol. 1998;188:55–63. doi: 10.1006/cimm.1998.1350. [DOI] [PubMed] [Google Scholar]
- 58.Mathers AR, Cuff CF. Role of interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic reovirus infection. J Virol. 2004;78:3352–3360. doi: 10.1128/JVI.78.7.3352-3360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuff CF, Fulton JR, Barnett JB, Boyce CS. Enteric reovirus infection as a probe to study immunotoxicity of the gastrointestinal tract. Toxicol Sci. 1998;42:99–108. doi: 10.1006/toxs.1998.2425. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Cuff CF, Pestka JJ. T-2 toxin impairment of enteric reovirus clearance in the mouse associated with suppressed immunoglobulin and IFN-gamma responses. Toxicol Appl Pharmacol. 2006;214:318–325. doi: 10.1016/j.taap.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005;175:7085–7091. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 62.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 63.Shtrichman R, Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001;4:251–259. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 64.Kato H, Fujihashi K, Kato R, Yuki Y, McGhee JR. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J Immunol. 2001;166:3114–3121. doi: 10.4049/jimmunol.166.5.3114. [DOI] [PubMed] [Google Scholar]
- 65.Beagley KW, Eldridge JH, Lee F, Kiyono H, Everson MP, Koopman WJ, Hirano T, Kishimoto T, McGhee JR. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsay AJ, Leong KH, Boyle D, Ruby J, Ramshaw IA. Enhancement of mucosal IgA responses by interleukins 5 and 6 encoded in recombinant vaccine vectors. Reprod Fertil Dev. 1994;6:389–392. doi: 10.1071/rd9940389. [DOI] [PubMed] [Google Scholar]
- 67.Beutner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM., Jr Treatment of genital warts with an immune-response modifier (imiquimod) J Am Acad Dermatol. 1998;38:230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- 68.Beutner KR, Tyring SK, Trofatter KF, Jr, Douglas JM, Jr, Spruance S, Owens ML, Fox TL, Hougham AJ, Schmitt KA. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789–794. doi: 10.1128/aac.42.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, Hougham AJ, Schmitt KA. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human PapillomaVirus Arch Dermatol. 1998;134:25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 70.Coll DA, Rosen CA, Auborn K, Potsic WP, Bradlow HL. Treatment of recurrent respiratory papillomatosis with indole-3-carbinol. Am J Otolaryngol. 1997;18:283–285. doi: 10.1016/s0196-0709(97)90012-0. [DOI] [PubMed] [Google Scholar]
- 71.Rosen CA, Woodson GE, Thompson JW, Hengesteg AP, Bradlow HL. Preliminary results of the use of indole-3-carbinol for recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 1998;118:810–815. doi: 10.1016/S0194-5998(98)70274-8. [DOI] [PubMed] [Google Scholar]
- 72.Grissmann L, Diehl V, Schultzcoulon HJ, Hausen HZ. J Virol. 1982;44:393–400. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kashima HK, Mounts P, Shah K. Recurrent respiratory papillomatosis. Obstet Gynecol Clin North Am. 1996;23:699–706. [PubMed] [Google Scholar]
- 74.Yuan F, Chen Z, Liu K, Sepkovic DW, Bradlow HL, Auborn K. Anti-estrogenic activities of indole-3-carbinol in cervical cells: implication for prevention of cervical cancer. Anticancer Res. 1999;19:1673–1680. [PubMed] [Google Scholar]
- 75.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]