Abstract
Patterns of behavior were recorded every 10 min during a 2-h period each day from eclosion to death for individual Drosophila melanogaster (both sexes) and Ceratitis capitata (males-only) including walking, preening, feeding, flying, and resting for the former species, and walking, calling (signaling), supine (upside-down), and resting in the latter. Results reveal that, with the exception of preening in D. melanogaster, behavioral patterns are age-specific and the frequency of several behaviors (e.g. supine in medfly; walking and resting in D. melanogaster) are correlated with time-to-death. This is the first set of studies to report the age patterns over a range of behavioral categories throughout the lives of individuals and thus the first that systematically documents the behavior of individuals at advanced ages. We suggest that the new and unique behaviors (e.g. supine) that emerge from the aging process be referred to as degenerative behaviors, not only to distinguish them from the conventional behavioral classifications (innate, learned), but also to reflect their emergent nature.
Keywords: Degenerative behavior, Supine behavior, Disablement, Disability, Impairment, Behavioral gerontology, Insect longevity, Insect life span
1. Introduction
Although behavioral studies of aging in humans have received considerable attention in the geriatric literature (Bergeman and Plomin, 1996; Martin and Bateson, 1993; Plomin et al., 1990), there appears to be no studies on the behavior of old animals or of age-specific behavioral patterns in the context of ethology—the zoological study of animal behavior (Alcock, 1998; Arking, 1998; Bateson, 2002; Futuyma, 1998; Martin and Bateson, 1993). Because of the potential importance of understanding both the extent to which behavior and aging are mutually affecting and the relationship of behavior at young ages to both individual life span and time-to-death, the broad goal of the current research was to consolidate and compare the results of separate studies on the age-specific behavior of both Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Although the specific objectives for each study were slightly different, both were concerned with two general questions: What are the changes in the age patterns of basic behaviors in fruit flies such as walking and feeding? Is there a correlation between changes in activity and both life span and time-to-death? In order to increase empirical continuity and the conceptual coherency of the medfly study, we combine published data on the age patterns of medfly supine and calling behaviors (Papadopoulos et al., 2002, 2004) with unpublished data on walking and resting behavior in this species.
2. Methods
2.1. Background
The original purpose of the medfly behavior study, conducted in Thessaloniki, Greece during 2001, was to measure the frequency of calling (sexual signaling) and other behaviors (resting, walking, feeding) during a 2-h period each day throughout the lives of individual adult males (Papadopoulos et al., 2004). Shortly into this study a behavior unique to older flies was discovered in which individuals would be upside-down for short periods though neither moribund nor in the process of dying (Papadopoulos et al., 2002). The original aim of the Drosophila behavior study, conducted in Davis, California during the summer and fall of 2004, was to record age-specific behaviors of individual flies of both sexes from eclosion to death with the possibility of identifying a supine-like behavior in this species. Although no such behavior was identified, the study yielded detailed new behavioral information on this species.
2.2. Empirical methods
The behavior was recorded for each individual fly of both species at 10-min intervals during a 2-h period each day (total of 12 records) from 1000 to 1200 h for D. melanogaster and from 1200 to 1400 h for the medfly. Observations were conducted daily and continued until each fly died. Each individual fly was observed instantaneously (2–3 s) and its activity was recorded. The behavioral study on D. melanogaster (Oregon RC stock) was conducted on 100 individuals of each sex (but with censoring due to escape or injury) at 25+5 C, 65+5% R.H. and 12:12 (L:D), with photophase beginning at 0900 h from July through November, 2004. Upon emergence adults were placed in inverted 8 dr vials with rubber stoppers consisting of two holes through which short plastic tubing was inserted—one cotton-stoppered tube for ventilation and one with standard Drosophila (banana–agar) diet. The food tube for each cage was changed daily.
The behavioral study on the medfly was conducted in the laboratory at 25+2 C, 65+5% R.H. and 14:10 (L:D) with photophase beginning at 0600 h (Papadopoulos et al., 2002). The medfly adults were obtained from field-infested applies that were collected during September–October 1999 from orchards near Thessaloniki in northern Greece. Upon emergence, 203 adults (in approximately 50-fly replicates) were placed in individual cages with adult food (4:1 mixture of sugar and yeast hydrolyzate) and water. Individuals cage consisted of a transparent plastic cup, 12-cm high, 5-cm base diameter and 7.5-cm top diameter, which was placed upside-down, and glued to the lid of a plastic Petri dish. Adult food was placed on the floor of the cage, and water was provided by a cotton wick that went through a small hole in the base. A lateral window covered with mesh was perforated on the cup’s side for ventilation.
2.3. Statistical methods
We model the relationship between time-to-death and age-specific behaviors through the Cox proportional hazards model
where coveriates X(t)=(X1(t),…,Xp(t))T are the p age-specific behaviors, measured in the form of counts between 0 and 12, and X̅(t)={X(s):0≤s<t} denotes the covariate history up to time t, β=(β1,…,βp)T is the vector of regression coefficients in the Cox model, and λ0(t) is the unspecified baseline hazard rate function. Note that the ith regression coefficient βi reflects the relationship between time-to-death and the ith age-specific behavior. Coefficients βi significantly less than 0 indicate that the corresponding behavior tends to be associated with increased lifetime while βi that are significantly greater than 0 indicate that the behavior is associated with shortened lifetimes and increased mortality. The estimates of β are derived via maximizing the Cox partial likelihood. The proportional hazards model was also applied to logarithmically transformed behavior counts. Furthermore, the proportionality assumption was assessed by scaled Martingale (l.c.m) residuals to ensure the validity of the Cox model. In this analysis, the log transformed covariate values X′(t)=log(X(t)+1) were seen to provide better fits and therefore these were used in the final analysis.
3. Results
The age-specific behavior data gathered on both the medfly and D. melanogaster are presented in two graphical forms including: (i) event-history charts which show inter- and intra-individual variation as well as patterns relative to longevity (Fig. 1 and Fig. 3) and (ii) proportional changes of cohort behavior at each age (Fig. 2 and Fig. 4). With the exception of preening frequency in D. melanogaster (Fig. 1), which was essentially age independent, virtually all behaviors exhibited distinct age patterns. For example, the walking and flying frequencies in D. melanogaster were high during the first month but decreased at subsequent ages (Fig. 1). Whereas walking constitutes from 25 to 40% of the total behavior activities during the first 30 days of the D. melanogaster’s life, it constitutes only around 10% of the activities after this time (Fig. 2). Flying was nearly non-existent in older D. melanogaster (Fig. 1 and Fig. 2). The frequency of D. melanogaster feeding was high for the first month but tapered off at older ages. Young D. melanogaster spend a greater proportion of their time resting than engaging in other behaviors.
Fig. 1.
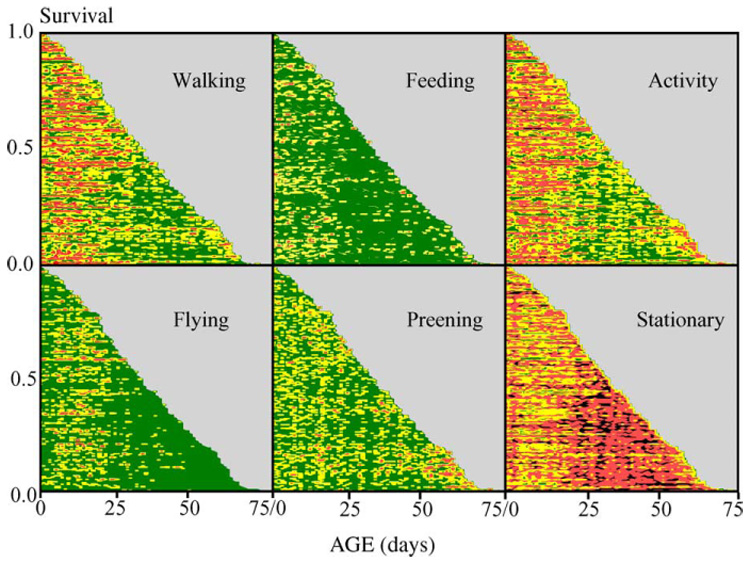
Event-history charts of D. melanogaster (both sexes combined) behavior (Carey et al., 1998). Each horizontal ‘line’ (rank-ordered from shortest to longest from top to bottom) represents the lifeline of an individual fly, the length of which is proportional to its life span. Color-coding indicates the number of times a given behavior was observed for an individual at a specified age with green indicating zero levels in all charts. For walking, flying, feeding, preening, overall activity, and stationary the yellow coding indicates levels (number of times observed) 1–6, 1–2, 1–3, 1–2, 1–6, and 1–6, respectively. Red indicates all levels greater than the range coded for yellow for each of the respective behaviors.
Fig. 3.
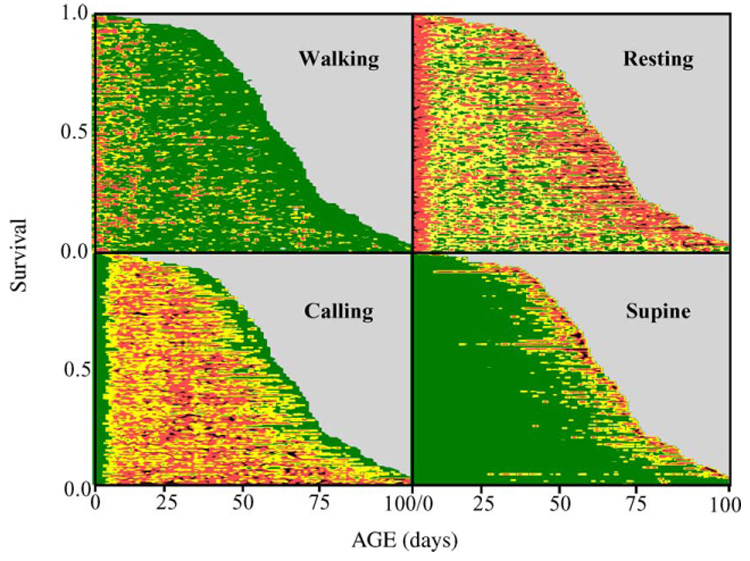
Event-history charts of male medfly behavior (see Fig. 1 legend). For walking, resting, calling and supine behavior the yellow coding indicates levels (number of times observed) 1, 1–5, 1–9, and 1–6, respectively. Red indicates all levels greater than the range coded for yellow for each of the respective behaviors.
Fig. 2.
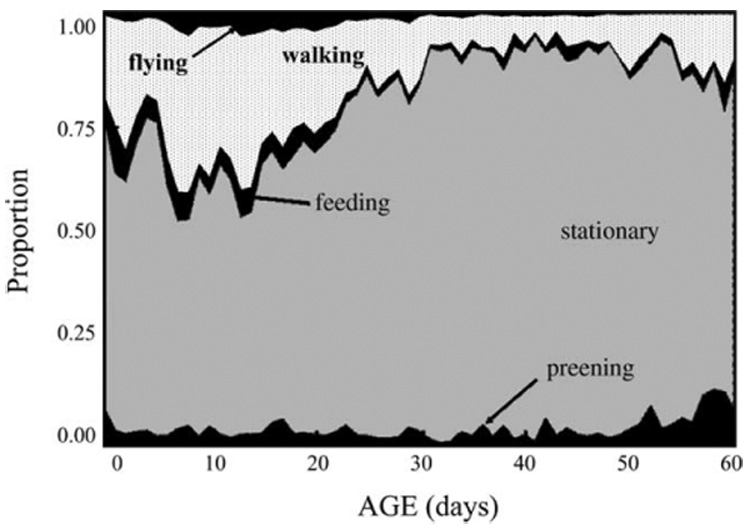
Proportion of individual D. melanogaster alive that exhibited specified behavior by age.
Fig. 4.
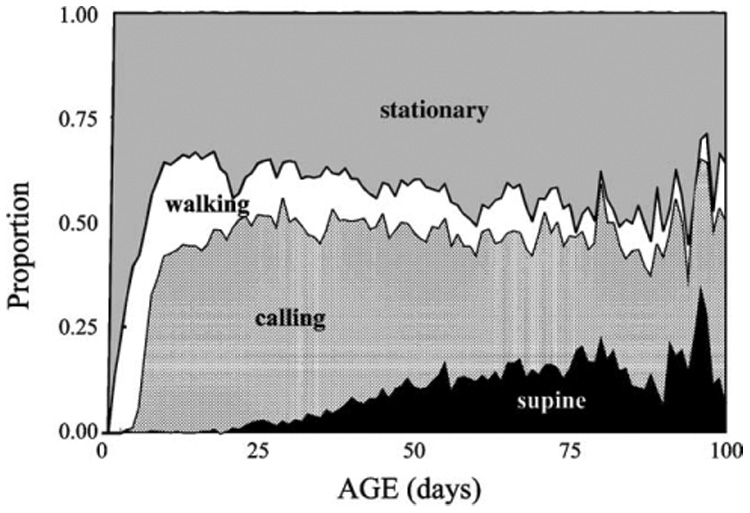
Proportion of individual medfly males alive that exhibited specified behavior by age.
All categories of behavior in the medfly exhibited age-specificity. For example, walking for the medfly was high during the first 3 weeks, sporadic at older ages, and extremely rare a few days before death (Fig. 3). The onset of medfly calling behavior occurred after a maturation period ranging from 3 to 5 days in most males (Fig. 3) but the termination of this behavior in individual flies as well as the onset of supine behavior was time-to-death specific. Because death rate was age-specific but supine behavior was linked to age at death, the proportion of medflies experiencing supine behavior increased with cohort age (Fig. 4). Thus, supine behavior at the individual-level is time-to-death specific but at the cohort level is age-specific. Both very young and very old medflies rest more than do middle-aged individuals.
Statistical analyses of the data sets revealed that the coefficients in the Cox proportional hazards model were negative and significant for resting (p≤10−6), walking (p≤10−4) and calling (p=0) in the medfly and for walking (p≤10−3), and resting (p≤10−5) in D. melanogaster. In other words, greater frequency of walking and resting predicted longer lives in both species and of calling predicted greater longevity in the medfly. Higher frequency of supine behavior in the medfly predicted shorter life spans and was highly significant (p≤10−14). Interactions between behaviors were not significant in either species. Neither flying nor preening in D. melanogaster was discriminatory with respect to longevity but for different reasons. Flying activity was uncorrelated with longevity because this activity was uniformly high in nearly all individuals when they were young and low when they were old. In contrast, preening was uncorrelated with longevity because this behavior was uniform throughout the lives of nearly all individual D. melanogaster.
4. Discussion
We believe the demographic approach to understanding behavior is important for several reasons. First, whereas behavioral data derived from conventional approaches in ethology is typically grouped into broad life history-related categories such as behaviors associated with parental care, reproduction, or foraging (Alcock, 1998; Bateson, 2002; Futuyma, 1998), data derived from behavioral studies that are framed demographically can be classified and analyzed with respect to age dependency. Three subcategories of age-(or time-) dependent behaviors emerged from the current study including those that are correlated with the following: (1) Life span. Calling intensity of young male medflies and the frequency of walking in young D. melanogaster were positively correlated with individual longevity. (2) Time-to-death. Male medflies died an average of 2 weeks after the onset of supine behavior (supine age); and (3) Chronological age. Flight activity in D. melanogaster was negatively correlated with age but not correlated with either longevity or time-to-death. The only behavior that was age-independent (remarkable in itself) was preening in D. melanogaster where young, mid-age, and old flies all exhibited this activity at similar frequencies.
Second, inasmuch as mortality often runs counter to constitutional frailty because of behavior, knowledge of how an individual’s susceptibility of death is filtered through behavior to produce a certain probability of death is fundamental to understanding aging and longevity (Kannisto, 1991). For example, behavior plays a major role in sex-mortality differentials in humans (Hazzard, 1986; Zuk, 1990) and other species (Trivers, 1972) and the inactivity of older medflies may partly account for deceleration of mortality at advanced ages (Carey et al., 1992). Similarly, the relationship between longevity and behavior can have far-reaching evolutionary implications. For example, wasps that practice progressive (as needed) provisioning to larva capable of eliciting a strong feeding response may sometimes ‘overfeed’ their young which then become longer-lived adults, and set the stage for the evolution of incipient sociality due to generation overlap (Evans, 1958; Wilson, 1971). This is an example of how specific behaviors (larval feeding response) can sometimes have far-reaching evolutionary ramifications (Alcock, 1998; Futuyma, 1998).
Third, in light of the discovery of supine behavior in medflies (Papadopoulos et al., 2002), a behavior unique to older individuals that had not been previously described, it is likely that new, equally unique behaviors of older individuals in other species will be discovered in the future. Inasmuch as these behaviors cannot be classified into either of the two standard categories of innate or learned behaviors (Alcock, 1998; Bateson, 2002), a new category may need to be considered in ethology and gerontology that will accommodate this new group. We suggest that the set of behaviors that are common in or unique to individuals at older ages be referred to as degenerative behaviors. This new category will provide needed coherency in the development of concepts concerned with disability processes (Albrecht et al., 2001; Bickenbach et al., 1999; Verbrugge and Jette, 1994), active life span (Katz et al., 1983; Manton and Land, 2000), and morbidity dynamics (Crimmins, 2004; Crimmins et al., 1994; Manton et al., 1997) in both humans and model organisms.
Although there are numerous studies on various aspects of behavior in both the medfly and D. melanogaster (Arita and Kaneshiro, 1983; Prokopy et al., 1984; Tomaru and Oguma, 2000; Whittier et al., 1992) the studies described here on lifetime behavior in these two species appear to be the first to systematically document lifetime changes in individual-level activity and behavior for any species. The findings establish a preliminary framework for investigations concerned with lifetime behavioral patterns, correlations of different behaviors with respect to each other as well as to longevity and time-to-death, and the emergence of new types of behaviors that are unique either to older individuals in particular or to the aging process in general. We believe that the results of studies using model animals will help to identify common threads in the disablement process across species as well as at different biological levels, will shed new and important light on the identification of preclinical signals of disablement in nonhuman species, encourage development of novel concepts and approaches for understanding fundamental aspects of the disablement process rather than characteristics that are idiosyncratic to humans, provide context and perspectives on mechanisms underlying aging as well as deeper insights into the manifestations of aging, and foster the creation of a set of general principles of disablement that are as relevant to insects and other invertebrates as they are to humans and other vertebrates.
Acknowledgements
We thank N. Hopelian, D. Nguyen, L. Zhang, E. Wendt, M. Hosseinion, L. Quon, N. Marosky, S. Ng and A. Sivagnanasundaram for technical assistance on the Drosophila behavioral research. Supported by grants from the National Institute on Aging (P01-AG022500-01; P01-AG08761-10).
References
- Albrecht GL, Seelman KD, Bury M. Handbook of Disability Studies. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Alcock J. Animal Behavior: An Evolutionary Approach. sixth ed. Sunderland: Sinauer; 1998. [Google Scholar]
- Arita LH, Kaneshiro KY. Pseudomale courtship behavior of the female Mediterranean fruit fly, Ceratitis capitata (Wiedemann); Proceedings of the Hawaiian Entomological Society; 1983. pp. 205–210. [Google Scholar]
- Arking R. Biology of Aging. Sunderland: Sinauer Associates Inc.; 1998. [Google Scholar]
- Bateson P. Ethology. In: Pagel M, editor. Encyclopedia of Evolution. Oxford: Oxford University Press; 2002. pp. 310–317. [Google Scholar]
- Bergeman CS, Plomin R. Behavioral genetics. In: Birren JE, editor. Encyclopedia of Gerontology. San Diego, CA: Academic Press; 1996. pp. 163–172. [Google Scholar]
- Bickenbach JE, Chatterji S, Badley EM, Ustun TB. Models of disablement, universalism and the international classification of impairments, disabilities and handicaps. Social Science and Medicine. 1999;48:1173–1187. doi: 10.1016/s0277-9536(98)00441-9. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Orozco D, Vaupel JW. Slowing of mortality rates at older ages in large medfly cohorts. Science. 1992;258:457–461. doi: 10.1126/science.1411540. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller H-G, Wang J-L, Vaupel JW. A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Functional Ecology. 1998;12:359–363. [Google Scholar]
- Crimmins EM. Trends in the health of the elderly. Annual Review of Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Hayward MD, Saito Y. Changing mortality and morbidity rates and the health status and life expectancy of the older population. Demography. 1994;31:159–175. [PubMed] [Google Scholar]
- Evans HE. The evolution of social life in wasps; Proceedings of the 10th International Congress of Entomology; 1958. pp. 449–457. [Google Scholar]
- Futuyma DJ. Evolutionary Biology. third ed. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- Hazzard WR. Biological basis of the sex differential in longevity. Journal of American Geriatrics. 1986;34:455–471. doi: 10.1111/j.1532-5415.1986.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Kannisto V. Frailty and survival. Genus. 1991;47:101–118. [PubMed] [Google Scholar]
- Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. The New England Journal of Medicine. 1983;309:1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- Manton KG, Land KC. Active life expectancy estimates for the US elderly population: a multidimensional continuous-mixture model of functional change applied to completed cohorts, 1982–1996. Demography. 2000;37:253–265. [PubMed] [Google Scholar]
- Manton KG, Corder L, Stallard E. Chronic disability trends in elderly United States populations: 1982–1994. Proceedings of the National Academy of Science. 1997;94:2593–2598. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Papadopoulos NT, Carey JR, Katsoyannos BI, Kouloussis NA, Müller H-G, Liu X. Supine behaviour predicts time-to-death in male Mediterranean fruit flies; Proceedings of the Royal Society of London: Biological Sciences; 2002. pp. 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Carey JR, Müller H-G, Zhang Y. High sexual calling rates predicts extended life span in male Mediterranean fruit flies. Oecologia. 2004;138:127–134. doi: 10.1007/s00442-003-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JE, McClearn GE. Behavioral Genetics: A Primer. New York: W.H. Freeman; 1990. [Google Scholar]
- Prokopy RJ, McDonald PT, Wong TTY. Inter-population variation among Ceratitis capitata flies in host acceptance pattern. Entomologia experimentalis et applications. 1984;35:65–69. [Google Scholar]
- Tomaru M, Oguma Y. Mate choice in Drosophila melanogaster and D. sechellia: criteria and their variation depending on courtship song. Animal Behavior. 2000;60:797–804. doi: 10.1006/anbe.2000.1543. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871–1971. Chicago, IL: Aldine; 1972. pp. 136–179. [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Whittier TS, Kaneshiro KY, Prescott LD. Mating behavior of Mediterranean fruit fly (Diptera: Tephritida) in a natural environment. Annals of the Entomological Society of America. 1992;85:214–218. [Google Scholar]
- Wilson EO. The Insect Societies. Cambridge: The Belknap Press of Harvard University Press; 1971. [Google Scholar]
- Zuk M. Reproductive strategies and disease susceptibility: an evolutionary viewpoint. Parasitology Today. 1990;6:231–233. doi: 10.1016/0169-4758(90)90202-f. [DOI] [PubMed] [Google Scholar]


