Abstract
Objective
Family-based treatments for pediatric obesity were developed over 25 years ago. Over that time, youth have become more obese and the environment more obesiogenic, which may influence efficacy of pediatric weight control. Mixed-effects regression models were used to compare the efficacy of programs initiated 20 to 25 years ago to current programs through 24-month follow-up, as well as to reanalyze 10-year outcomes of previous research using contemporary measures and analytic strategies.
Main outcome measures
z-BMI and percent overweight.
Results
Results showed significant reductions over time, with no differences in z-BMI change for older versus contemporary studies. Age was a predictor of z-BMI up to 24 months, with younger children showing larger change. Mixed-effects regression models replicated previous long-term effects of family-based interventions. Gender was a predictor of long-term z-BMI change, with girls benefiting more over time than did boys.
Conclusion
The efficacy of the family-based behavioral approach to treating pediatric obesity replicates over a 25-year period. Challenges in evaluating treatment effects over time are discussed. Ideas for studying choice of treatments that vary in effect size and for strengthening family-based behavioral treatments are noted.
Keywords: childhood obesity, obesiogenic environment, diet, physical activity, intervention research
Family-based behavioral pediatric obesity treatment programs were developed over 25 years ago, and both short- and long-term results support their efficacy (Epstein, Myers, Raynor, & Saelens, 1998; Epstein, Valoski, Wing, & McCurley, 1990, 1994; Jelalian & Saelens, 1999). Obesity runs in families (Whitaker, Wright, Pepe, Seidel, & Dietz, 1997), and it has been hypothesized that targeting eating and activity change in the child and parent, along with teaching parents behavioral skills to facilitate child behavior change, could mobilize family resources to improve the efficacy of childhood obesity treatments. Simultaneously treating the parent and child benefits both, and creates positive relationships between child and parent weight change (Wrotniak, Epstein, Paluch, & Roemmich, 2004, 2005).
The efficacy of family-based treatments has been replicated many times since the late 1970s (Epstein, 2003), but there has been no attempt to evaluate changes in efficacy over time. Several variables have changed over that period that may influence the effectiveness of family-based treatments. These include changes in the characteristics of youth who are being treated for pediatric obesity, changes in the environment and family structure that may require conceptual changes in components of family-based treatment, and changes in the analysis and reporting of clinical trials.
There has been an increase in the incidence and prevalence of pediatric obesity over the last 20 years (Ogden, Flegal, Carroll, & Johnson, 2002; Troiano, Flegal, Kuczmarski, Campbell, & Johnson, 1995). The body mass index (BMI) distribution is positively skewed; the average overweight child today is more overweight than the average overweight child in the 1970s and 1980s. More overweight youth may show greater decreases in percentage overweight. It is also possible that the more overweight the child is, the more the environment has influenced the child and the poorer the child’s eating and exercise habits may be. Thus, treatment effectiveness may suffer.
There is general agreement that the increase in obesity is due in part to changes in the environment (Hill, Wyatt, Reed, & Peters, 2003) that can lead to decreased energy expenditure and increased food intake (French, Story, & Jeffery, 2001). Almost all homes have at least one television, and there has been an increase in the percentage of homes with multiple televisions (Neilsen Media Research 2000, 2000) and in the percentage of children with televisions in their bedrooms (Dennison, Erb, & Jenkins, 2002). Television watching has been associated with obesity in youth (Crespo et al., 2001; Gortmaker et al., 1996). Eating is often paired with television watching in youth (Matheson, Killen, Wang, Varady, & Robinson, 2004; Saelens et al., 2002), and television watching is related to energy intake (Epstein, Roemmich, Paluch, & Raynor, 2005b; Taras et al., 1989). Television watching may shift time away from physical activity (Durant, Baranowski, Johnson, & Thompson, 1994; Epstein, Roemmich, Paluch, & Raynor, 2005a; Taras, Sallis, Patterson, Nader, & Nelson, 1989), reducing energy expenditure. Youth make the choice to be active or sedentary, and developments in behavioral choice theory provide a theoretical framework for interventions to reduce sedentary behaviors (Epstein & Roemmich, 2001; Epstein & Saelens, 2000). Two recent studies in our research program have focused on behavioral economic approaches to modify sedentary behavior as part of a comprehensive treatment for pediatric obesity (Epstein, Paluch, Gordy, & Dorn, 2000; Epstein, Paluch, Kilanowski, & Raynor, 2004).
Changes in the environment also influence energy intake (French et al., 2001). There has been an increase in added fats and oils to the food supply (Kantor, 1999). Cheese and pizza consumption (Putnam & Gerrior, 1999) and soda consumption (Tippett & Cleveland, 1999) have increased while milk intake has decreased (Tippett & Cleveland, 1999). In combination with the increase in the number of working mothers and single-parent families (Bowers, 2000), there has been an increase in meals in restaurants (National Restaurant Association, 1998) and energy consumed from eating out (Biing-Hwan, Guthrie, & Frazao, 1999). As people eat out, they experience greater portion sizes (Rolls, 2003), which increases consumption. Behavioral economics also provides ideas for new approaches to reducing energy intake in obese youth. On the basis of research showing that obese youth and adults (Legerski & Epstein, 2006; Saelens & Epstein, 1996) are more motivated to eat than are their leaner peers, in one of our recent studies we attempted to identify nonfood alternatives to compete with the reinforcing value of food (Epstein, Roemmich, Stein, Paluch, & Kilanowski, 2005).
There have been changes in the family since the 1950s. The divorce rate more than doubled from 1950 to 1970 (15/1,000 to 40/1,000 per year) and remained stable from 1970 to 1988 (Shiono & Quinn, 1994). The number of families in which both parents work has increased (Anderson & Butcher, 2006), which has resulted in greater income but made it more challenging for parents to allocate enough time for children in family-based behavioral treatment interventions that focus on teaching behavioral principles and modification of the environment. Changes in family life may increase parents’ distress, which also may affect treatment effectiveness (Zeller, Saelens, Roehrig, Kirk, & Daniels, 2004). We tested an intervention that taught problem-solving skills that may be useful in coping with changes in family life that have evolved over time (Epstein, Paluch, Gordy, Saelens, & Ernst, 2000).
There are methodological reasons for reexamining studies completed in the 1970s and early 1980s. Older studies used height and weight charts for children (Jelliffe, 1966). When children became older than 18, their overweight status was evaluated with the use of adult height and weight charts (Metropolitan Life Insurance Company, 1959, 1983), which were derived using different methods from a different sample than the youth charts. BMI charts were introduced in 1991 (Must, Dallal, & Dietz, 1991) and have been updated (Kuczmarski et al., 2002). BMI charts use the same methods for parents and children, and the BMI curves represent smooth functions between child and young adult age ranges. It is possible that results obtained using older standards, or even different versions of BMI charts, would show different efficacy when current standards are applied.
There have been changes in the reporting of randomized clinical trials in obesity during the last 25 years. Studies completed over 2 decades ago generally reported data for study completers, along with the rate of attrition. It is now common to consider intention to treat, whereby everyone who is randomized and begins the study is accounted for. There have also been changes in the analytical approaches to longitudinal data. Mixed-effects regression models can be used to analyze differences in the patterns of between-groups change over time, as well as predictors of the pattern of change over time (Bryk & Raudenbush, 1987; Goldstein, 1995). Mixed-effects regression models use all the data that are available, as these models do not delete participants with missing data and can analyze data obtained at different time points across studies. Mixed-effects regression models take into account serial correlation between repeated observations and changes in the variability over time, which is relevant because increases in variability for weight control over time are commonly observed in obesity treatment studies.
The aims of this study included assessment of changes over time for treatments implemented 25 years ago or current family-based treatments across eight studies (Epstein, Paluch, Gordy, & Dorn, 2000; Epstein, Paluch, Gordy, Saelens, & Ernst, 2000; Epstein, Paluch, Kilanowski, & Raynor, 2004; Epstein, Wing, Koeske, Andrasik, & Ossip, 1981; Epstein, Wing, Koeske, & Valoski, 1984, 1985, 1986; Epstein, Wing, Valoski, & Gooding, 1987) using the same dependent measures (Kuczmarski et al., 2002) and the identification of participant characteristics related to treatment success. The family-based behavioral treatment program for overweight youth is well standardized, with a common core used across all the studies, facilitating the comparison of treatment effects over time. The research program represents a systematic approach to the design of family-based treatments that has focused on different aspects of treatment, including the influence of the family and parent weight, comparison of lifestyle and programmed aerobic activity, the influence of problem solving on treatment outcome, and the influence of methods to reduce sedentary behaviors such as watching television and playing computer games (Epstein, 2003). In addition to evaluating the period during which the study was implemented, we reevaluated long-term (10-year) results for the earlier studies using z-BMI standards and contemporary analytic methods. To identify clinical significance, we also assessed differences in the dichotomous outcomes of achieving BMI values below the overweight (95th BMI percentile) and at risk for overweight (85th BMI percentile) values, as well as reductions greater than 0.5 or 1.0 standard deviation units. This article is unique in the ability to (a) compare a standardized treatment for effectiveness over a long period that overlapped periods of environmental changes that have been hypothesized to increase the prevalence of obesity and to (b) use a large data set to assess how participant characteristics may be related to treatment outcome.
Method
Participants
Participants included 437 overweight children studied in eight randomized, controlled outcome studies initiated in 1978, with studies continuing through the present. The first four studies were completed in Pittsburgh, Pennsylvania (n = 176), with the final four studies completed in Buffalo, New York (n = 261). The description of each study group is presented in Table 1. The number of treatment sessions was similar across all groups in each study. Percentage of attrition relates to how many participants could not be measured at the final follow-up.
Table 1.
Characteristics of Interventions
| Reference | Study group | Description | Sessions | % Attrition |
|---|---|---|---|---|
| Studies 1–4 | ||||
| (Epstein et al., 1981) | 1.1 | Parent and child targeted for behavior change and weight loss | 14 | 24 |
| 1.2 | Child targeted for behavior change and weight loss | |||
| 1.3 | Nonspecific target | |||
| (Epstein et al., 1984) | 2.1 | Diet and activity information | 15 | 8 |
| 2.2 | Diet and targeted lifestyle activity | |||
| (Epstein et al., 1986) | 3.1 | Positive family history for obesity | 18 | 7 |
| 3.2 | Negative family history for obesity | |||
| (Epstein et al., 1985) | 4.1 | Diet and aerobic exercise | 18 | 15 |
| 4.2 | Diet and lifestyle activity | |||
| 4.3 | Diet and calisthenics | |||
|
| ||||
| Studies 5–8 | ||||
| (Epstein, Paluch, Gordy, & Dom, 2000) | 5.1 | Increase activity/low dose | 20 | 16 |
| 5.2 | Reduce sedentary/low dose | |||
| 5.3 | Increase activity/high dose | |||
| 5.4 | Reduce sedentary/high dose | |||
| (Epstein, Paluch, Gordy, Saelens, & Ernst, 2000) | 6.1 | Parent and child problem solving | 18 | 18 |
| 6.2 | Child problem solving | |||
| 6.3 | No problem solving | |||
| (Epstein et al., 2004) | 7.1 | Restrict sedentary behavior | 20 | 5 |
| 7.2 | Reinforce for reduced sedentary behavior | |||
| (Epstein et al., 2005) | 8.1 | Reinforce dietary changes | 16 | 15 |
| 8.2 | Reinforce alternatives to food | |||
Commonalities and Differences Across Studies
There are several common features to the studies. In each study, a parent was treated along with a child, with the exception that in Study 1 (Study Groups 1.1–1.3), parent participation was the independent variable, and only one group had both the parent and child targeted for behavior change and weight loss. All families had a weigh-in and individual meeting with a therapist, followed by separate parent and child groups. All families were provided treatment manuals that included information on dietary changes based on the Traffic Light Diet, a physical activity program, and information on parenting. There have been some changes in content over time, including a shift from the Basic 4 to the Food Guide Pyramid to organize foods in the Traffic Light Diet and a shift from the Cooper Clinic physical activity program in Study 1 to a lifestyle activity program in subsequent studies.
All studies were conceptualized as efficacy studies, and participants who had psychopathology, who could not read the materials, and who could not complete behavioral tasks during screening that were similar to those the youth would do during treatment were excluded from treatment. The upper limit to the degree of adiposity has shifted over time as youth have become more overweight. In general, older studies used an upper cutoff of 100% overweight, whereas the contemporary studies used a cutoff of 120% overweight.
Characteristics of the Pittsburgh and Buffalo communities in 1980 and 2000 were similar. For example, between 1980 and 2000, the population of Pittsburgh decreased from 423,938 to 334,563, whereas Buffalo’s population decreased from 357,870 to 292,648 over the same period. The percentage of racial/ethnic minorities in Pittsburgh and Buffalo was 25.6% and 30.2%, respectively, in 1980 and 24.8% and 24.8%, respectively, in 2000. The average temperature in Pittsburgh in 1980 and 2000 was 50° and 51°, respectively, whereas the average temperatures for Buffalo in those years was 47° and 48°, respectively. Buffalo did have more snow, with 64 in. and 146 in. (162 cm and 371 cm) in 1980 and 2000, respectively, compared with 48 in. and 32 in. (122 cm and 81 cm) during those years in Pittsburgh.1
Measurement
BMI (in kilograms per square meter) was calculated from height and weight. Entry criteria for participants in the initial studies were greater than 20% overweight, whereas entry criteria for contemporary studies were greater than the 85th BMI percentile (Kuczmarski et al., 2002). The primary dependent measure was z-score BMI (z-BMI), calculated on the basis of the mean and standard deviation from the sample (Kuczmarski et al., 2002) that was used to develop the BMI percentiles (BMI – BMI 50th percentile/BMI SD). Percentage overweight was also calculated with the following formula: [(BMI – BMI 50th percentile/BMI 50th percentile) × 100].2,3
Analytic Plan
Analysis of variance (ANOVA) and chi-square were used to compare differences in participant characteristics between studies done in the 1970s (Studies 1–4) and late 1990s–2000s (Studies 5–8). Mixed-effects regression models (Bryk & Raudenbush, 1987; Goldstein, 1995) were used to assess z-BMI values up to 24 months across all studies, with months as the time-variant Level 1 variable and gender, age, and older and contemporary studies (studies in the 1970s–1980s and 1990s–2000s) as time-invariant Level 2 predictor variables. The models included a random intercept, with other variables entered as fixed effects. The first step in the analysis was to use linear and quadratic models to explore significant changes. Subsequent models added gender, age, older and contemporary studies, and parental z-BMI change as predictors of the linear and quadratic changes in child z-BMI. Both significant improvements in the log-likelihood ratios and significant effects for new terms in the model were used to determine what model should be used. Nonsignificant covariates were removed from final models. We used only participants with at least baseline and two of the three subsequent data points (6, 12, and 24 months), which included 389 of the 437 participants who were initially randomized (89%).
Separate mixed-effects regression models were used to compare treatments within each of the initial long-term studies (Studies 1–4) to provide analysis of long-term changes using intention to treat, contemporary dependent measures, and newer analytic approaches that may be better suited to the analysis of longitudinal data than ANOVA. Treatment was dummy coded in each model to make comparisons between study groups. Each model also tested whether gender and age interacted with time to influence z-BMI. The data were combined across studies to assess the best predictors of z-BMI over the 10 years of observation across the studies, including child gender, age, and parental z-BMI change.
Study 1 provided follow-up data at 8, 21, 60, and 120 months; Study 2 at 6, 12, 60, and 120 months; Study 3 at 6, 12, 36, 60, and 120 months; and Study 4 at 6, 12, 24, 60, and 120 months. The four recent studies provided data at 6, 12, and 24 months.4 End censored data points were replaced by returning the participant to baseline or to the highest values obtained during treatment. The usual pattern of change is for participants to show maximal change at 6 months, and missing values at 6 months were replaced by the last measurement taken during treatment. Missing values between 6 months and a final value were replaced with the linear imputation of that time point based on follow-up data points that were available, as z-BMI changes generally deteriorate from 6 months through subsequent follow-up. These methods were based on research comparing alternative methods of imputing missing data (Engels & Diehr, 2003).
The percentage of youth below the 95th (overweight) and 85th (at risk for obesity) BMI percentiles and reductions of 0.5 and 1.0 z-BMI units were determined at 6, 12, 24, 60, and 120 months using chi-square analyses. Effect size for each study group was calculated. The typical method to calculate effect sizes is to compare treatments to a common control. The studies reported used different active control conditions, which precludes meaningful comparison of different treatments to different controls. One alternative is to standardize the treatment response for each group by dividing the treatment response by the pooled standard deviation of the pre- and postvalues, as follows: {[z-BMI at time point (6, 12, 24, 60, 120 months) – baseline z-BMI]/pooled SD of baseline and follow-up z-BMI values}. This is the effect size for a within-subject comparison across conditions, with the effect sizes standardized on the basis of within-group variability. Analyses were conducted with SYSTAT (SYSTAT Software, 2004).
Results
Baseline Characteristics
The specifics of participants in the older versus contemporary studies, including sample size, baseline percentage overweight and z-BMI, gender distribution, parental age and percentage overweight, are shown in Table 2. Children in the contemporary studies were 9.3 lb (4.2 kg) heavier, had 0.9 greater z-BMI units, had 2.4 BMI units, and were 15.3% more overweight than children in the older studies (ps < .0001). The percentage of boys participating increased from 25.6% in the older studies to 39.1% in the contemporary studies (p < .003). Mothers and fathers in the contemporary studies were heavier, with a greater percentage overweight and with greater z-BMI units (ps < .01). Although mothers and fathers in contemporary studies were heavier, there were no significant differences in weight, percentage overweight, BMI, or z-BMI for parents who participated in treatment. The percentage of participating fathers decreased from 26.1% in the older studies to 14.6% in contemporary studies, χ2(1, N = 437) = 9.07, p = .003.
Table 2.
Participant Characteristics at Baseline in Studies 1–4 and 5–8
| Studies | n | % male | % female | Age (years) | Height (cm) | Weight (kg) | % overweight | BMI | z-BMI |
|---|---|---|---|---|---|---|---|---|---|
| Child | |||||||||
| 1–4 | 176 | 25.6* | 74.4* | 10.4 ± 1.5 | 146.6 ± 11.4 | 55.7 ± 13.1** | 49.9 ± 17.2** | 25.6 ± 3.2** | 2.8 ± 1.1** |
| 5–8 | 261 | 39.0 | 61.0 | 10.3 ± 1.3 | 145.8 ± 8.1 | 56.0 ± 11.2 | 65.2 ± 16.8 | 28.0 ± 3.1 | 3.7 ± 1.1 |
|
| |||||||||
| Mother | |||||||||
| 1–4 | 176 | 39.1 ± 6.2* | 163.1 ± 6.3 | 74.0 ± 15.4** | 28.0 ± 24.7** | 27.8 ± 5.4** | 1.0 ± 1.1** | ||
| 5–8 | 261 | 40.4 ± 5.1 | 163.8 ± 6.1 | 79.7 ± 18.2 | 36.3 ± 28.4 | 29.6 ± 6.2 | 1.4 ± 1.3 | ||
|
| |||||||||
| Father | |||||||||
| 1–4 | 172 | 41.6 ± 7.1 | 176.3 ± 6.6 | 91.5 ± 15.1** | 27.6 ± 18.7** | 29.4 ± 4.3** | 1.6 ± 1.1** | ||
| 5–8 | 261 | 42.2 ± 6.5 | 176.8 ± 6.6 | 96.9 ± 17.7 | 34.5 ± 21.2 | 30.9 ± 4.9 | 2.0 ± 1.3 | ||
|
| |||||||||
| Participating parent | |||||||||
| 1–4 | 176 | 26.1** | 73.9** | 40.0 ± 6.5 | 167.1 ± 9.1 | 84.1 ± 16.8 | 36.0 ± 21.7 | 30.0 ± 4.8 | 1.6 ± 1.1 |
| 5–8 | 261 | 14.6 | 85.4 | 40.9 ± 5.9 | 165.9 ± 7.6 | 83.4 ± 19.1 | 37.7 ± 26.6 | 30.2 ± 5.9 | 1.6 ± 1.3 |
Note. BMI = body mass index; z-BMI = BMI z-score.
p < .05.
p < .01, for the difference between Studies 1–4 and Studies 5–8.
Comparison of Changes Through 24 Months
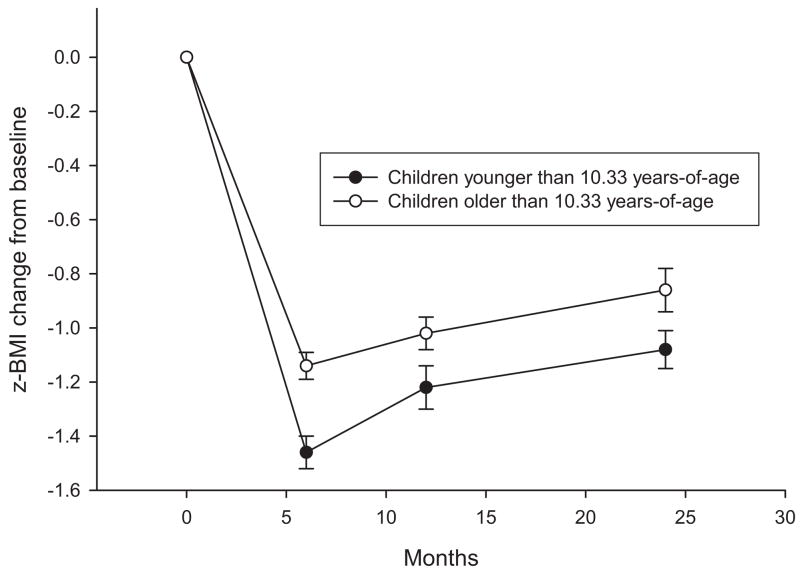
The best-fitting mixed-effects regression model to predict the pattern of change in z-BMI values over 2 years included the linear (estimate = −.435, z = 8.94, p < .001) and quadratic (estimate = .016, z = 8.07, p < .001) function for months, as well as the main effect for age (estimate = −.248, z = 5.25, p < .001) and parental weight (estimate = .431, z = 11.36, p < .001), the interactions of age with the linear (estimate = −.026, z = 5.69, p < .001) and quadratic (estimate = −.001, z = 5.19, p < .001) functions of months, and the interactions of parental weight with the linear (estimate = .016, z = 3.17, p = .015) and quadratic (estimate = −.0007, z = 3.40, p < .001) functions of months. As shown in Figure 1, younger children (less than or equal to the median age of 10.33) showed reductions of 1.46 and 1.08 z-BMI units at 6 and 12 months, respectively, versus 1.14 and 0.86 z-BMI units for the older (greater than 10.33 years of age) children. Youth who had parents with greater z-BMI change had larger decreases in z-BMI. Neither child gender nor older or contemporary studies were predictors of z-BMI values over time.
Figure 1.
z-body mass index (z-BMI) change (M ± SEM) for younger (≤ 10.33 years of age) or older (> 10.33 years of age) children in Studies 1–8.
Reanalysis of Long-Term z-BMI Change
The mixed-effects regression model for Study 1 showed significantly greater reduction in z-BMI for the group comparing parent plus child targeted for behavior and weight change (Study Group 1.1) versus the nonspecific control group (Study Group 1.3) through 10 years (estimate = −.009, z = 2.99, p = .003), as well as a significant difference in the pattern of change over time for Study Group 1.1 versus the child-only group (Study Group 1.2; estimate = −.008, z = 2.50, p < .012). Intention-to-treat analyses also showed differences in the pattern of change over 10 years for Study Group 1.1 versus Study Group 1.3 (estimate = −.008, z = 2.95, p = .003), as well as a significant difference in the pattern of change over time for Study Group 1.1 versus Study Group 1.2 (estimate = −.007, z = 2.47, p = .014). Similarly, significant differences in the pattern of z-BMI were observed between lifestyle (Study Group 4.2; estimate = −.01, z = 2.50, p = .013) and programmed aerobic activity (Study Group 4.1; estimate = −.01, z = 2.41, p = .016) versus the calisthenics control group, with no differences between the exercise protocols. Intention to treat also showed that lifestyle exercise (estimate = −.008, z = 2.40, p = .017) and programmed aerobic activity (estimate = −.008, z = 2.36, p = .018) had significantly greater reductions than the calisthenics control group. Gender or age did not influence the pattern of change over time in either study. No significant between-groups differences were observed for groups in Studies 2 or 3, consistent with previous analysis of these studies using ANOVA (Epstein et al., 1994).
When considering Studies 1–4, we found that mixed-effects regression models showed a significant linear (estimate = .0053, z = 2.88, p = .0039) change over time and an interaction of child gender with linear change (estimate = −.0053, z = 3.09, p = .0020), as well as a relationship between parental weight and child weight (estimate = .50, z = 9.27, p < .001) and an interaction of parent weight by time (estimate = −.0027, z = 4.57, p < .001). As shown in Figure 2, although the z-BMI changes were similar during the initial stages of treatment, girls showed z-BMI decreases of −.80 and −.85 at 5 and 10 years, respectively, whereas boys showed z-BMI decreases of −.27 and −.38 at 5 and 10 years, respectively. Children who had parents with the largest z-BMI changes had larger z-BMI changes than children who had parents with smaller z-BMI changes.
Figure 2.
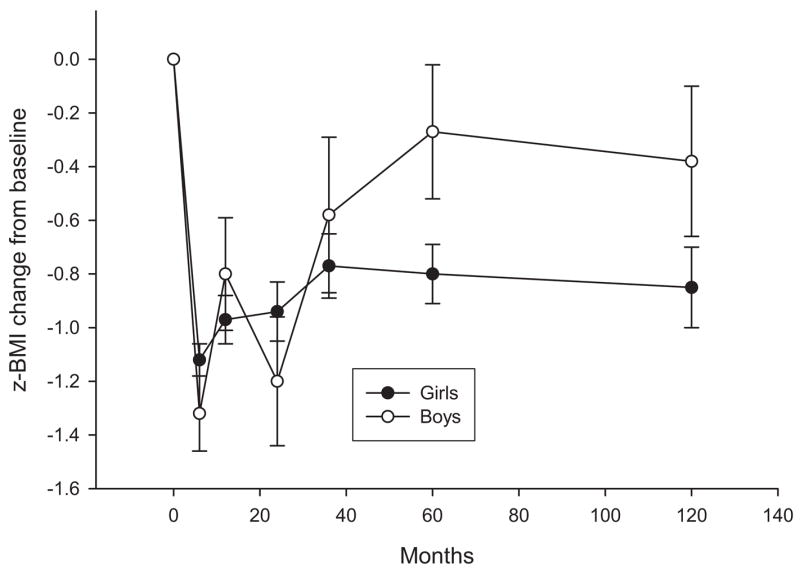
z-body mass index (z-BMI) change (M ± SEM) for boys and girls in Studies 1–4.
Comparison of Effect Sizes
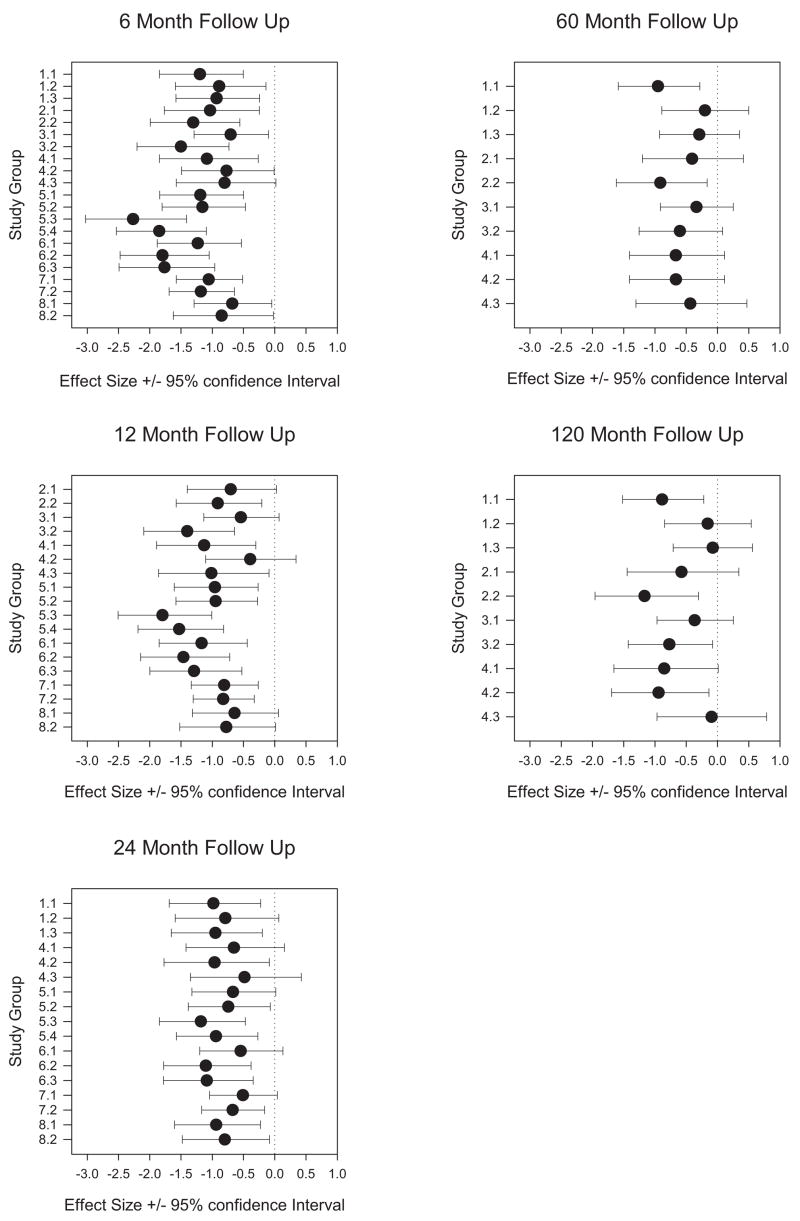
Effect sizes and the 95% confidence intervals for each group across all eight studies are shown in Figure 3. There was wide variability in effect size, with twofold differences between groups. In general, effect sizes decreased over time (−1.20, −1.02, −0.82, −0.55, and −0.67 at 6, 12, 24, 60, and 120 months, respectively).
Figure 3.
Forest plot of effect sizes and 95% confidence intervals for studies that provided 6-, 12-, 24-, 60-, and 120-month follow-up.
Comparing Percentages of Youth With Different Degrees of Success After Treatment
As shown in Table 3, at 6 months, χ2(1, N = 361) = 12.69, p < .001, and 24 months, χ2(1, N = 280) = 10.88, p = .001, a greater percentage of children in the earlier studies achieved a BMI value below the 95th BMI percentile. Similarly, at 6 months, χ2(1, N = 410) = 25.19, p < .001; 12 months, χ2(1, N = 343) = 10.48, p = .001; and 24 months, χ2(1, N = 304) = 5.85, p = .016, a greater percentage of children in the older studies achieved a BMI value below the 85th BMI percentile. The pattern was reversed when the magnitude of z-BMI changes was considered. At 6 months, χ2(1, N = 410) = 11.48, p = .001, and at 12 months, χ2(1, N = 343) = 5.48, p = .019, a greater percentage of children in the contemporary studies achieved at least 1.0 z-BMI unit reduction.
Table 3.
Percentage of Children Who Met Success Criteria
| Study group
|
Study group
|
|||||
|---|---|---|---|---|---|---|
| Month | 1–4 | 5–8 | p | 1–4 | 5–8 | P |
| < 95th | < 85th | |||||
| 6 | 52.3 | 33.2 | .001 | 27.5 | 8.8 | < .001 |
| 12 | 39.1 | 28.1 | .060 | 23.7 | 10.5 | .001 |
| 24 | 41.3 | 20.7 | .001 | 19.0 | 8.9 | .016 |
| 80 | 42.1 | 18.5 | ||||
| 120 | 47.5 | 22.2 | ||||
|
| ||||||
| > 0.5 SD | > 1 SD | |||||
| 6 | 79.5 | 85.8 | .10 | 48.5 | 65.3 | .0007 |
| 12 | 73.7 | 75.5 | .71 | 46.5 | 59.8 | .019 |
| 24 | 65.8 | 68.4 | .67 | 45.6 | 43.6 | .76 |
| 60 | 59.3 | 38.9 | ||||
| 120 | 66.7 | 44.4 | ||||
Note. BMI = body mass index; z-BMI = BMI z-score. Success criteria were < 95th or < 85th BMI percentile or change > 0.5 or 1.0 z-BMI units.
Discussion
This article shows that the degree of obesity in children participating in a childhood weight control program has increased over the last 2 decades, consistent with the increase in the prevalence of obesity during this period (Ogden et al., 2002; Strauss & Pollack, 2001). Despite the increases in degree of overweight, there have been no differences in the pattern of z-BMI over the first 2 years of treatment for programs that were implemented over 2 decades ago versus contemporary programs. Because children are becoming more overweight, it is not surprising that more children in the earlier studies were below the criteria for being at risk for overweight or overweight after treatment.
The similarity in treatment effects over such an extended time interval suggests that the treatments that were implemented in the 1970s and 1980s were at least as powerful as the treatments used today. However, youth today are heavier and may live in a more obesiogenic environment. Taking these things into account, we think it is possible that the contemporary studies are in fact more powerful, as new interventions were designed in part in response to changes in the environment that may be related to pediatric obesity. Overweight youth of today are faced with temptations to eat larger portion sizes (Rolls, Morris, & Roe, 2002) and to be sedentary (Crespo et al., 2001), compared with youth in the environment of over 20 years age. Because these variables are also related to the increase in overweight, it is difficult to disentangle the correlated effects of an obesiogenic environment and greater degree of obesity. The best way to compare the treatments would be to test the protocols used in older versus contemporary studies in environments that are equivalent to the 1970s and early 1980s versus contemporary environments. Without access to a time machine, this comparison will have to involve analyses using data collected across the two periods.
These data are based on a consistent pattern of results for efficacy studies over an extended period, which is the first step in translation of randomized, controlled clinical trials into clinical protocols for widespread use. Efficacy research is needed to guide effectiveness studies, which extend the interventions tested in efficacy research on a broader base of participants, which may be more overweight, have greater diversity, have lower income and educational levels, and consist of youth and parents who may have comorbid psychological problems. Effectiveness research may indicate that the treatments are ready for broad application or may indicate characteristics of participants who do not benefit from treatment. These problems would be addressed in more efficacy research that may tailor the treatment or devise new treatments for populations who are less responsive to established interventions.
Evaluation of Long-Term Treatment Effectiveness Using z-BMI and Intention-to-Treat Methods
A secondary goal of the present study was to assess long-term changes in treatment effectiveness using more contemporary dependent measures and analytic approaches. Results for the mixed-effects regression models show the same pattern as previous analyses using ANOVA methods. Targeting both parents is superior to a nontargeted control group, and both lifestyle and aerobic physical activity programs are superior to a calisthenics control group (Epstein et al., 1994).
Variability in Treatment Effects
One unique aspect of this analysis was the opportunity to compare effect sizes for groups within studies in one research program. Effect sizes can provide a guide to the choice of effective interventions. There are group differences in both the magnitude and variability of change, which provide the opportunity for considering multiple factors in choosing treatments. The choice of a treatment must be balanced by considering the magnitude of change and the variability in treatment response. For example, compare Study Groups 1.1 and 3.2 after 6 months of treatment. They had similar magnitude of change (−1.21 vs. −1.18), but the standard deviation of Study Group 1.1 was greater than that of Study Group 3.2 (1.00 vs. 0.79), which resulted in differences in effect sizes of 1.20 versus 1.50. The lower variability in treatment response for Study Group 3.2 than for Study Group 1.1 might suggest this would be the preferred treatment, because the response for youth would be more predictable given the similar change in z-BMI. Consider two treatments with the same effect sizes, Study Groups 1.1 and 5.1. In this case, the magnitude of change was 26% greater for Study Group 5.1 versus Study Group 1.1 (1.62 vs. 1.21), whereas the standard deviation was 34% larger (1.35 vs. 1.01). From the perspective of a family who is considering entering a treatment program, would it be preferable to enter a treatment that may produce a larger response but is riskier because the variability in treatment effects is greater?
Comparing treatments with similar effect sizes, but one treatment has double the degree of change and double the variability, represents a choice between a treatment that produces smaller but more reliable change for most people provided the treatment and a treatment that can produce greater but more variable change. This is similar to choosing between treatments that differ in their degree of risk (Armstrong, Schwartz, Fitzgerald, Putt, & Ubel, 2002; Mayhorn, Fisk, & Whittle, 2002). To our knowledge, there has been no research evaluating how overweight families or their therapists would decide which treatment to choose, but as more is known about the magnitude and variability of different pediatric obesity treatments, this type of evaluation may become increasingly important.
Clinical Significance of Treatment Effects
The usual analysis of treatment effectiveness over time is based on inferential statistics that provide limited insight into clinical effectiveness. Two different ways to evaluate clinical effectiveness were presented. First, the percentage of youth who do not meet diagnostic criteria of being overweight or at risk for being overweight was higher for the earlier studies that treated less overweight children. At 5 and 10 years after treatment, over 40% of the youth did not meet obesity criteria, and 18.5% to 22% did not meet the more restrictive requirement of not at risk for obesity. It is worthwhile to consider the use of the more restrictive criteria, because when youth become adults they will be evaluated by the more restrictive criteria, and a 17-year-old who would be labeled at risk for obesity would be labeled an obese adult if he or she maintained the same degree of z-BMI status. Using the most restrictive criteria, about 9% of youth treated in the contemporary studies met criteria for not at risk for obesity 2 years after treatment.
A second criterion is to compare treatments by amount of change. Treatment success in adults can be conceptualized as a reduction in body weight of 5% to 10% of initial weight (NHLBI Obesity Education Initiative Expert Panel, 1998). Adults who achieve these amounts of weight loss may still be very obese and may not be satisfied with these outcomes (Foster et al., 2004). To our knowledge, there has been no theoretical or experimental work to indicate how much weight for height change should be the goal in pediatric treatment programs. We arbitrarily chose to evaluate treatments that were associated with 0.5 and 1.0 standard deviation units of change, and a large percentage of youth met these goals. Because the 95th BMI percentile is equivalent to about 2.0 standard deviations above the mean (see Footnote 2), a change of 1.0 standard deviation unit for a child who is 3.0 standard deviations above the mean moves them from above to below the 95th BMI percentile, but a change of 1.0 standard deviation represents only a small degree of change in youth overweight for a child who may be 4.0 or more z-BMI units over the mean z-BMI values for children of the same age and gender. There are likely to be health advantages to youth who show a 1.0-standard deviation reduction even if they remain well above the 95th BMI percentile. However, the long-term benefits of reducing a child from 5.0 to 4.0 z-BMI units is unknown, if the change means that child would enter adulthood 50 lb (23 kg) or more overweight. It is likely that the benefits would be greater if children could reduce their BMI to below the 85th percentile, into the not-at-risk-for-overweight group, and thus become adults who are not obese.
The current family-based treatments have a strong evidence base, and the translation of these research-based programs to clinical interventions is long overdue. However, it may be worthwhile to conceptualize different treatment programs or intensities of treatment for youth who are more overweight and who are overweight in combination with comorbidities, such as diabetes (Fagot-Campagna et al., 2000). One option is to have longer treatments, as longer treatments are associated with better treatment outcome in both adults and children (Goldfield, Raynor, & Epstein, 2002; Perri, Nezu, Patti, & McCann, 1989). However, longer treatments create challenges in maintaining participants in treatment. In adults, the longer the treatment, the greater the proportion of participants who do not attend (Wing, Blair, Marcus, Epstein, & Harvey, 1994), and this problem may be magnified with families, who may have more challenges in scheduling than individual adults, and because there are multiple people who may want to drop out of treatment. A second option is to consider more powerful treatments, such as portion-controlled foods and pharmacological interventions (Berkowitz, Wadden, Tershakovec, & Cronquist, 2003; Wadden, Berkowitz, Sarwer, Prus-Wisniewski, & Steinberg, 2001). As the intensity of treatments is increased, it may be the time to consider inpatient treatments for selected obese youth (Braet, Tanghe, Decaluwe, Moens, & Rosseel, 2004), in which intensive state-of-the-art interventions can be used to drastically reduce weight and provide the opportunity for entering adulthood within a normal weight range. Likewise, it may be possible to develop intensive year-round interventions that use local summer camp programs for the initial intensive intervention and supplemental booster sessions and camps during weekends and school vacations to maintain treatment effects. Finally, traditional clinical treatments that focus on the child and family may need to be combined with public health approaches that focus on the school and the community to change the environment that promotes unhealthy eating and activity behaviors.
If current trends are predictive of the future, there is likely to be an increase in prevalence of very overweight youth who require more powerful interventions than those that are currently available. Replication of the treatment effects observed in efficacy studies to clinical populations in effectiveness studies and development of more powerful treatments for pediatric obesity represent challenges for the future. These new treatments may be stimulated by changes in the environment and advances in biobehavioral theory that further our understanding of how to change behavior and maintain those changes (Epstein, 1992).
Acknowledgments
We thank Rena R. Wing, who helped develop the initial research program at the University of Pittsburgh; Alice Valoski, who coordinated multiple treatment outcome studies at the University of Pittsburgh; Dominca Vito, CeCe Gordy, and Colleen Kilanowski, who coordinated studies at the University at Buffalo; and the research assistants, therapists, and families who made this research possible.
The development of this article was funded in part by National Institute of Child Health and Human Development Grant R01 HD 39778 to Leonard H. Epstein. Leonard H. Epstein is a member of the scientific advisory board of Kraft Foods.
Footnotes
Weather data were obtained from the National Weather Service Forecast Offices in Pittsburgh and Buffalo, respectively.
The standard deviations referred to are based on traditional standard deviation units, lambda mu sigma z-BMI units that do not increase linearly as obesity increases (Cole, 1990; Cole, Faith, Pietrobelli, & Heo, 2005).
We have established that both percentage overweight and z-BMI are linearly related to weight loss, whereas the lambda mu sigma z-BMI values are not linear across levels of change for overweight youth and are not recommended as a dependent measure to evaluate pediatric obesity treatments (Cole et al., 2005).
Data collection for the first four studies began over 25 years ago, and some of these data, which were not on retrievable computer files, were recreated from archived computer databases and archived patient records. Given the use of archival files, there may be some slight differences in analyses reported for the initial studies and the analyses used in the newer analyses.
References
- Anderson PM, Butcher KF. Childhood obesity: Trends and potential causes. Future of Children. 2006;16:19–45. doi: 10.1353/foc.2006.0001. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Schwartz JS, Fitzgerald G, Putt M, Ubel PA. Effect of framing as gain versus loss on understanding and hypothetical treatment choices: Survival and mortality curves. Medical Decision Making. 2002;22:76–83. doi: 10.1177/0272989X0202200108. [DOI] [PubMed] [Google Scholar]
- Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: A randomized controlled trial. Journal of the American Medical Association. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- Biing-Hwan L, Guthrie JF, Frazao E. America’s changing eating habits: Changes and consequences. Washington, DC: U.S. Department of Agriculture, Economic Research Division; 1999. Nutrient contribution of food away from home; pp. 213–242. [Google Scholar]
- Bowers DE. Cooking trends echo changing roles of women. Food Review. 2000;23:23–29. [Google Scholar]
- Braet C, Tanghe A, Decaluwe V, Moens E, Rosseel Y. Inpatient treatment for children with obesity: Weight loss, psychological well-being, and eating behavior. Journal of Pediatric Psychology. 2004;29:519–529. doi: 10.1093/jpepsy/jsh054. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchal linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Cole TJ. The LMS method for constructing normalized growth standards. European Journal of Clinical Nutrition. 1990;44:45–60. [PubMed] [Google Scholar]
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z-score or BMI centile? European Journal of Clinical Nutrition. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in US children: Results from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine. 2001;155:360–365. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- Dennison BA, Erb TA, Jenkins PL. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics. 2002;109:1028–1035. doi: 10.1542/peds.109.6.1028. [DOI] [PubMed] [Google Scholar]
- Durant RH, Baranowski T, Johnson M, Thompson WO. The relationship among television watching, physical activity, and body composition of young children. Pediatrics. 1994;94:449–455. [PubMed] [Google Scholar]
- Engels JM, Diehr P. Imputation of missing longitudinal data: A comparison of methods. Journal of Clinical Epidemiology. 2003;56:968–976. doi: 10.1016/s0895-4356(03)00170-7. [DOI] [PubMed] [Google Scholar]
- Epstein LH. Role of behavior theory in behavioral medicine. Journal of Consulting and Clinical Psychology. 1992;60:493–498. doi: 10.1037//0022-006x.60.4.493. [DOI] [PubMed] [Google Scholar]
- Epstein LH. Development of evidence-based treatments for pediatric obesity. In: Kazdin AE, Weisz JR, editors. Evidence-based psychotherapies for children and adolescents. New York: Guilford Press; 2003. pp. 374–388. [Google Scholar]
- Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101:554–570. [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Gordy CC, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Archives of Pediatrics and Adolescent Medicine. 2000;154:220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Gordy CC, Saelens BE, Ernst MM. Problem solving in the treatment of childhood obesity. Journal of Consulting and Clinical Psychology. 2000;68:717–721. [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychology. 2004;23:371–380. doi: 10.1037/0278-6133.23.4.371. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN. Reducing sedentary behavior: Role in modifying physical activity. Exercise and Sport Science Reviews. 2001;29:103–108. doi: 10.1097/00003677-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Physical activity as a substitute for sedentary behavior in youth. Annals of Behavioral Medicine. 2005a;29:200–209. doi: 10.1207/s15324796abm2903_6. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Paluch RA, Raynor HA. The influence of changes in sedentary behavior on energy and macronutrient intake in youth. American Journal of Clinical Nutrition. 2005b;81:361–366. doi: 10.1093/ajcn.81.2.361. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: Clinic and field studies. Annals of Behavioral Medicine. 2005;30:201–209. doi: 10.1207/s15324796abm3003_4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saelens BE. Behavioral economics of obesity: Food intake and energy expenditure. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. Mahwah, NJ: Erlbaum; 2000. pp. 293–311. [Google Scholar]
- Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral family-based treatment for obese children. Journal of the American Medical Association. 1990;264:2519–2523. [PubMed] [Google Scholar]
- Epstein LH, Valoski AM, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychology. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wing RR, Koeske R, Andrasik F, Ossip DJ. Child and parent weight loss in family-based behavior modification programs. Journal of Consulting and Clinical Psychology. 1981;49:674–685. doi: 10.1037//0022-006x.49.5.674. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wing RR, Koeske R, Valoski A. Effects of diet plus exercise on weight change in parents and children. Journal of Consulting and Clinical Psychology. 1984;52:429–437. doi: 10.1037//0022-006x.52.3.429. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wing RR, Koeske R, Valoski A. A comparison of lifestyle exercise, aerobic exercise and calisthenics on weight loss in obese children. Behavior Therapy. 1985;16:345–356. [Google Scholar]
- Epstein LH, Wing RR, Koeske R, Valoski A. Effect of parent weight on weight loss in obese children. Journal of Consulting and Clinical Psychology. 1986;54:400–401. doi: 10.1037//0022-006x.54.3.400. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wing RR, Valoski A, Gooding W. Long term effects of parent weight on child weight loss. Behavior Therapy. 1987;18:219–226. [Google Scholar]
- Fagot-Campagna A, Pettitt DJ, Engelgau MM, Burrows NR, Geiss LS, Valdez R, et al. Type 2 diabetes among North American children and adolescents: An epidemiologic review and a public health perspective. Journal of Pediatrics. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- Foster GD, Phelan S, Wadden TA, Gill D, Ermold J, Didie E. Promoting more modest weight losses: A pilot study. Obesity Research. 2004;12:1271–1277. doi: 10.1038/oby.2004.161. [DOI] [PubMed] [Google Scholar]
- French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annual Review of Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Raynor HA, Epstein LH. Treatment of pediatric obesity. In: Stunkard AJ, Wadden TA, editors. Obesity: Theory and therapy. 3. New York: Guilford Press; 2002. pp. 532–555. [Google Scholar]
- Goldstein H. Multilevel statistical models. New York: Halstead Press; 1995. [Google Scholar]
- Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television watching as a cause of increasing obesity among children in the United States, 1986–1990. Archives of Pediatrics and Adolescent Medicine. 1996;150:356–362. doi: 10.1001/archpedi.1996.02170290022003. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003 February 7;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Jelalian E, Saelens BE. Empirically supported treatments in pediatric psychology: Pediatric obesity. Journal of Pediatric Psychology. 1999;24:223–248. doi: 10.1093/jpepsy/24.3.223. [DOI] [PubMed] [Google Scholar]
- Jelliffe DB. The assessment of the nutritional status of the community. Geneva, Switzerland: World Health Organization; 1966. [PubMed] [Google Scholar]
- Kantor LS. A comparison of the US food supply with the Food Guide Pyramid recommendations. In: Frazo E, editor. America’s eating habits: Changes and consequences. Washington, DC: U.S. Department of Agriculture, Economic Research Division; 1999. pp. 71–95. [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. Vital health statistics Series 11. Vol. 246. Hyattsville, MD: National Center for Health Statistics; 2002. CDC growth charts for the United States: Methods and development; pp. 1–90. [PubMed] [Google Scholar]
- Legerski C, Epstein LH. Food is more reinforcing for overweight than lean children. 2006. Unpublished manuscript. [Google Scholar]
- Matheson DM, Killen JD, Wang Y, Varady A, Robinson TN. Children’s food consumption during television viewing. American Journal of Clinical Nutrition. 2004;79:1088–1094. doi: 10.1093/ajcn/79.6.1088. [DOI] [PubMed] [Google Scholar]
- Mayhorn CB, Fisk AD, Whittle JD. Decisions, decisions: Analysis of age, cohort, and time of testing on framing of risky decision options. Human Factors. 2002;44:515–521. doi: 10.1518/0018720024496935. [DOI] [PubMed] [Google Scholar]
- Metropolitan Life Insurance Company. New weight standards for men and women. Statistical Bulletin. 1959;40:1–4. [Google Scholar]
- Metropolitan Life Insurance Company. Metropolitan height and weight tables. Statistical Bulletin. 1983;64:1–9. [PubMed] [Google Scholar]
- Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) and triceps skinfold thickness. American Journal of Clinical Nutrition. 1991;53:839–846. doi: 10.1093/ajcn/53.4.839. [DOI] [PubMed] [Google Scholar]
- National Restaurant Association. Restaurant industry pocket fact-book. Washington, DC: Author; 1998. [Google Scholar]
- Neilsen Media Research 2000. 2000 report on television: The first 50 years. New York: AC Neilsen; 2000. [Google Scholar]
- NHLBI Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obesity Research. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Journal of the American Medical Association. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. Journal of Consulting and Clinical Psychology. 1989;57:450–452. [PubMed] [Google Scholar]
- Putnam J, Gerrior S. Trends in the US food supply, 1970–1997. In: Frazo E, editor. America’s eating habits: Changes and consequences. Washington, DC: U.S. Department of Agriculture, Economic Research Division; 1999. pp. 133–160. [Google Scholar]
- Rolls BJ. The supersizing of America: Portion size and the obesity epidemic. Nutrition Today. 2003;38:42–53. doi: 10.1097/00017285-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. American Journal of Clinical Nutrition. 2002;76:1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Sallis JF, Nader PR, Broyles SL, Berry CC, Taras HL. Home environmental influences on children’s television watching from early to middle childhood. Journal of Developmental and Behavioral Pediatrics. 2002;23:127–132. doi: 10.1097/00004703-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Shiono PH, Quinn LS. Epidemiology of divorce. Children and Divorce. 1994;4:15–28. [PubMed] [Google Scholar]
- Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. Journal of the American Medical Association. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- SYSTAT Software. SYSTAT 11.0. Richmond, CA: SYSTAT Software; 2004. [Google Scholar]
- Taras HL, Sallis JF, Patterson PR, Nader PR, Nelson JA. Television’s influence on children’s diet and physical activity. Developmental and Behavioral Pediatrics. 1989;10:176–180. [PubMed] [Google Scholar]
- Tippett KS, Cleveland LE. How current snacks stack up: Comparison with dietary guidelines. In: Frazao E, editor. America’s eating habits: Changes and consequences. Washington, DC: U.S. Department of Agriculture Economic Research Division; 1999. pp. 51–70. [Google Scholar]
- Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The national health and nutrition examination surveys, 1963 to 1991. Archives of Pediatrics and Adolescent Medicine. 1995;149:1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: A randomized trial. Archives of Internal Medicine. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with Type II diabetes: Does including an intermittent very-low-calorie diet improve outcome? American Journal of Medicine. 1994;97:354–362. doi: 10.1016/0002-9343(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Archives of Pediatric and Adolescent Medicine. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]
- Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. The relationship between parent and child self-reported adherence and weight loss. Obesity Research. 2005;13:1089–1096. doi: 10.1038/oby.2005.127. [DOI] [PubMed] [Google Scholar]
- Zeller MH, Saelens BE, Roehrig H, Kirk S, Daniels SR. Psychological adjustment of obese youth presenting for weight management treatment. Obesity Research. 2004;12:1576–1586. doi: 10.1038/oby.2004.197. [DOI] [PubMed] [Google Scholar]