Abstract
It is generally believed that late-phase long-term potentiation (L-LTP) and long-term memory (LTM) require new protein synthesis. Although the full complement of proteins mediating the long-lasting changes in synaptic efficacy have yet to be identified, several lines of evidence point to a crucial role for activity-induced brain derived neurotrophic factor (BDNF) expression in generating sustained structural and functional changes at hippocampal synapses thought to underlie some forms of LTM. In particular, BDNF is sufficient to induce the transformation of early to late phase LTP in the presence of protein synthesis inhibitors, and inhibition of BDNF signaling impairs LTM. Despite solid evidence for a critical role of BDNF in L-LTP and LTM, many issues are not resolved. Given that BDNF needs to be processed in Golgi outposts localized at the branch point of one or few dendrites, a conceptually challenging problem is how locally synthesized BDNF in dendrites could ensure synapse-specific modulation of L-LTP. An interesting alternative is that BDNF-TrkB signaling is involved in synaptic tagging, a prominent hypothesis that explains how soma-derived protein could selectively modulate the tetanized (tagged) synapse. Finally, specific roles of BDNF in the acquisition, retention or extinction of LTM remain to be established.
Keywords: synaptic plasticity, TrkB, presynaptic, postsynaptic, local translation, dendritic, tetanic stimulation, synaptic tagging, encoding, consolidation, extinction
Introduction
Synaptic plasticity describes the process by which connections between two neurons, or synapses, change in strength. By definition, it is a functional term referring to an increase or decrease in synaptic efficacy, but we now know that the physiological changes in the strength of transmission are often accompanied by structural alterations of the synapses. Since memories are believed to be stored in synapses of the brain, synaptic plasticity is thought to be the cellular mechanism for learning and memory. LTP in the hippocampus is the most studied form of synaptic plasticity. It has been widely accepted that LTP can be divided into at least two temporally distinct phases that are fundamentally different in their underlying mechanisms. A weak, high frequency tetanus (e.g. a train of 100 pulses at 100 Hz) can trigger an increase in synaptic efficacy that lasts for 1-2 hours. This short-lasting form of LTP is called early phase LTP (E-LTP). E-LTP requires modification of existing proteins and their trafficking at synapses but not de novo protein synthesis (Bliss and Collingridge, 1993; Malenka and Bear, 2004). On the other hand, repeated, strong high frequency stimulations (e.g. multiple trains of 100 pulses at 100 Hz) can induce an increase in synaptic efficacy lasting over 8 hours (Frey, Krug, Reymann, and Matthies, 1988) or even days (Abraham, 2003). L-LTP differs from E-LTP in its requirement for de novo mRNA and in its association with structural changes at synapses (Frey et al., 1988; Harris, Fiala, and Ostroff, 2003; Kandel, 2001; Krug, Lossner, and Ott, 1984; Muller, Nikonenko, Jourdain, and Alberi, 2002; Yuste and Bonhoeffer, 2001). It is generally believed that the E-LTP and L-LTP inducing stimuli trigger very different, albeit partially overlapping, biochemical pathways that lead to distinct changes at synapses. In particular, induction of L-LTP results in activation of cAMP-dependent protein kinase (PKA) and mitogen-associated protein kinase (MAPK, also known as extracellular signal-related protein kinase, ERK) (Kandel, 2001; Pang and Lu, 2004). Subsequently, several constitutively expressed transcription factors (e.g. cAPM/calcium responsive-element binding protein (CREB), and Elk-1) are phosphorylated and activated for the transcription of downstream genes that presumably mediate the changes in the structure and/or function of the synapses (Kandel, 2001; Shaywitz and Greenberg, 1999). One of the downstream genes induced by the L-LTP-inducing tetanus is BDNF.
BDNF is a small dimeric protein that works through high affinity binding with the receptor tyrosine kinase, tropomyosin-related kinase B (TrkB). BDNF and TrkB are widely distributed across subregions of the hippocampus and the adult forebrain (Bramham and Messaoudi, 2005). BDNF containing secretory vesicles are present in both axon terminals (presynaptic site) and dendrites (postsynaptic site) of glutamatergic principal neurons (granule cells and pyramidal cells) (Fawcett, Aloyz, McLean, Pareek, Miller, McPherson, and Murphy, 1997; Haubensak, Narz, Heumann, and Lessmann, 1998; Kohara, Kitamura, Morishima, and Tsumoto, 2001; Kojima, Takei, Numakawa, Ishikawa, Suzuki, Matsumoto, Katoh-Semba, Nawa, and Hatanaka, 2001; Lessmann, Gottmann, and Malcangio, 2003; Lu, 2003). BDNF stands out among all neurotrophins in the activity-dependent regulation of its expression and secretion. Upon high frequency stimulation, BDNF is secreted in a manner dependent on Ca2+ influx through NMDA subtype glutamate receptors or voltage-gated Ca2+ channels (Aicardi, Argilli, Cappello, Santi, Riccio, Thoenen, and Canossa, 2004; Balkowiec and Katz, 2002; Gartner and Staiger, 2002; Hartmann, Heumann, and Lessmann, 2001; Lever, Bradbury, Cunningham, Adelson, Jones, McMahon, Marvizon, and Malcangio, 2001). BDNF can also be secreted from either postsynaptic spines or presynaptic terminals. Possible mechanisms to trigger secretion include activation of N-type Ca2+ channels and mobilization of Ca2+ from intracellular stores (Balkowiec and Katz, 2002). Once secreted into the synaptic cleft, BDNF can bind to TrkB localized at both pre- and postsynaptic sites of glutamatergic synapses (Drake, Milner, and Patterson, 1999). In the postsynaptic density (PSD), TrkB is associated with PSD95 and NMDA receptors (Aoki, Wu, Elste, Len, Lin, McAuliffe, and Black, 2000; Husi, Ward, Choudhary, Blackstock, and Grant, 2000; Ji, Pang, Feng, and Lu, 2005; Yoshii and Constantine-Paton, 2007). In addition, the expression of BDNF, particularly transcription of the BDNF gene through promoter III, is tightly controlled by neuronal activity (Chen, Chang, Lin, Meissner, West, Griffith, Jaenisch, and Greenberg, 2003).
Given the synaptic localization of TrkB and activity-dependent secretion of BDNF protein and transcription of BDNF mRNA, it is not surprising that BDNF has emerged as a key regulator of synaptic plasticity and memory (Lu, 2003; Pang and Lu, 2004; Poo, 2001). Significant progress has been made in understanding the role of BDNF in E-LTP and short-term memory. BDNF facilitates the induction of E-LTP by enhancing synaptic responses to tetanus stimulation (Figurov, Pozzo-Miller, Olafsson, Wang, and Lu, 1996; Gottschalk, Pozzo-Miller, Figurov, and Lu, 1998; Rex, Lauterborn, Lin, Kramar, Rogers, Gall, and Lynch, 2006; Yano, Ninan, Zhang, Milner, Arancio, and Chao, 2006). This is most likely due to BDNF regulation of synaptic vesicle mobilization and docking, possibly by regulating the distribution and phosphorylation of synaptic proteins (Pozzo-Miller, Gottschalk, Zhang, McDermott, Du, Gopalakrishnan, Oho, Sheng, and Lu, 1999), (Jovanovic, Czernik, Fienberg, Greengard, and Sihra, 2000). BDNF also plays a role in the maintenance (or expression) of E-LTP, possibly by activating “silent synapses” (Shen, Wu, Zhang, Dou, Rao, Chen, and Duan, 2006) and/or by regulating actin motor complex (Rex, Lin, Kramar, Chen, Gall, and Lynch, 2007; Yano et al., 2006). Moreover, studies of the well-known val66met polymorphism in the human BDNF gene (Egan, Kojima, Callicott, Goldberg, Kolachana, Bertolino, Zaitsev, Gold, Goldman, Dean, Lu, and Weinberger, 2003) suggest that activity-dependent secretion of BDNF is critical for short-term, hippocampal dependent episodic memory that is largely dependent on E-LTP. In contrast, progress on the studies of BDNF in L-LTP and LTM is lagging behind. Nevertheless, numerous reports have demonstrated that BDNF is critical for the induction and maintenance of L-LTP. This review will focus on our current knowledge with respect to action sites, sufficiency, and expression of BDNF in hippocampal L-LTP and LTM.
Role of BDNF in hippocampal L-LTP
Is activity-dependent expression of endogenous BDNF sufficient to mediate L-LTP?
A variety of genetic and pharmacological studies suggest that BDNF is necessary for L-LTP to occur. In heterozygous BDNF (+/-) knockout mice, there is a significant deficit in L-LTP induced by several different protocols including theta burst stimulation or forskolin application (Korte, Carroll, Wolf, Brem, Thoenen, and Bonhoeffer, 1995; Pang, Teng, Zaitsev, Woo, Sakata, Zhen, Teng, Yung, Hempstead, and Lu, 2004; Patterson, Pittenger, Morozov, Martin, Scanlin, Drake, and Kandel, 2001). Treatment of hippocampal slices by the BDNF scavenger TrkB-Fc or antibodies against BDNF or TrkB also inhibits L-LTP (Kang, Welcher, Shelton, and Schuman, 1997). Similarly, L-LTP is impaired in TrkB knockout mice (Minichiello, Korte, Wolfer, Kuhn, Unsicker, Cestari, Rossi-Arnaud, Lipp, Bonhoeffer, and Klein, 1999), as well as in mice with a targeted mutation in the phospholipase C-γ (PLC-γ) docking site (but not in the Shc site) on TrkB (Minichiello, Calella, Medina, Bonhoeffer, Klein, and Korte, 2002). Curiously, the classic L-LTP induced by multiple tetani is normal in BDNF+/- mice, suggesting that BDNF is not involved in all forms of long-term synaptic plasticity (Chen, Kolbeck, Barde, Bonhoeffer, and Kossel, 1999; Kang et al., 1997; Patterson et al., 2001).
While these findings suggest that endogenous BDNF is required for the maintenance of L-LTP, a critical question is whether an activity-dependent increase in endogenous BDNF is responsible for the maintenance of L-LTP. Converging experimental results strongly suggest that this is the case. First, BDNF transcription is enhanced by L-LTP-inducing stimuli. With in situ hybridization, BDNF mRNA level is found to increase in the hippocampal CA1 region and dentate gyrus within 2-4 hours after the application of L-LTP-inducing tetanic stimulation (Castren, Pitkanen, Sirvio, Parsadanian, Lindholm, Thoenen, and Riekkinen, 1993; Dragunow, Beilharz, Mason, Lawlor, Abraham, and Gluckman, 1993; Patterson, Grover, Schwartzkroin, and Bothwell, 1992). Thus, the level of BDNF is increased either as a cause or as a result of L-LTP induction. Second, the impairment of L-LTP observed in BDNF+/- mice could be rescued by a perfusion of exogenous BDNF (Korte, Griesbeck, Gravel, Carroll, Staiger, Thoenen, and Bonhoeffer, 1996; Pang et al., 2004), indicating that an increase in BDNF may contribute to the maintenance of L-LTP. Moreover, recent studies from this group found that in a line of mutant mice in which the activity-dependent surge of BDNF mRNA is blocked, L-LTP is selectively disrupted whereas E-LTP is intact (Sakata, Woo, Wu, Shen, and Lu, 2005). These results suggest that without an increase in BDNF levels a strong tetanus is not sufficient to induce L-LTP. Finally, when protein synthesis was completely inhibited by either anisomycin or emitin, L-LTP was blocked in the hippocampus (Frey and Morris, 1997; Scharf, Woo, Lattal, Young, Nguyen, and Abel, 2002). Remarkably, application of exogenous BDNF rescued the L-LTP deficit even when all new protein synthesis is blocked (Pang et al., 2004). Thus, BDNF may be the key (if not the only) protein synthesis product responsible for the long-term maintenance of L-LTP. Taken together, these results support a model in which L-LTP-inducing stimuli increase endogenous BDNF and this increase may support the expression of L-LTP.
Despite its critical role in L-LTP, BDNF by itself is incapable of inducing synaptic potentiation. Earlier reports show that a brief bath application of BDNF for several minutes can trigger a sustained potentiation of synaptic efficacy at CA1 synapses in hippocampal slices (Kang and Schuman, 1995; 1996). Numerous laboratories have attempted to replicate this finding but failed (Figurov et al., 1996; Frerking, Malenka, and Nicoll, 1998; Gottschalk et al., 1998; Huber, Sawtell, and Bear, 1998; Kramar, Lin, Lin, Arai, Gall, and Lynch, 2004; Patterson, Abel, Deuel, Martin, Rose, and Kandel, 1996; Tanaka, Saito, and Matsuki, 1997). The rapid, direct enhancement of synaptic efficacy was later attributed to an unusually high speed of BDNF perfusion (Kang, Jia, Suh, Tang, and Schuman, 1996), the physiological significance of which remains unclear. BDNF-induced LTP was subsequently reported in the medial perforant path-granule cell synapses in the hippcampal dentate (Messaoudi, Bardsen, Srebro, and Bramham, 1998; Messaoudi, Ying, Kanhema, Croll, and Bramham, 2002; Ying, Futter, Rosenblum, Webber, Hunt, Bliss, and Bramham, 2002). In these studies, infusion of BDNF to the dentate was found to elicit a continuous increase in EPSPs over a long period of time (24 hours) (Bramham and Messaoudi, 2005). So far, there is no published report from other laboratories that supports or disputes such results. This appears to be an unusual form of plasticity whose relevance to learning and memory remains to be investigated. Since there is no need for synaptic stimulation, this form of plasticity is clearly activity-independent, making it difficult to conform with the conceptual framework of learning and memory. A surge of BDNF concentration pharmacologically may force synapses to behave out of their physiological norm. Finally, it is difficult to ensure synapse-specificity, a fundamental property of LTP, if BDNF undiscriminatorily potentiates all synapses.
Does L-LTP require pre- or postsynaptic synthesis of BDNF?
There is no dispute that L-LTP requires a sustained increase in endogenous BDNF for its long-lasting maintenance. An interesting and yet unresolved issue is whether the endogenous BDNF is derived pre- or postsynaptically. Early work suggests that E-LTP at the Schaffer collateral-CA1 synapses requires BDNF derived from presynaptic CA3 neurons, but not postsynaptic CA1 neurons (Zakharenko, Patterson, Dragatsis, Zeitlin, Siegelbaum, Kandel, and Morozov, 2003). The initial surge of BDNF may be due to the secretion of preexisting BDNF-containing vesicles from presynaptic terminals of CA3 neurons, induced by high frequency stimulation (HFS). This may be important for the induction of E-LTP. Due to the low expression level of BDNF in neurons, however, the existing BDNF would eventually be exhausted. Thus, for the long-term maintenance of L-LTP, a sustained supply of BDNF may come primarily from new protein synthesis triggered by repeated strong synaptic stimulation. This idea is supported by the findings that BDNF mRNA levels in the hippocampus are significantly increased 1-3 hours after the L-LTP-inducing tetanic stimulation (Castren et al., 1993; Dragunow et al., 1993; Morimoto, Sato, Sato, Yamada, and Hayabara, 1998; Patterson et al., 1992). Moreover, the slow kinetics of BDNF transcription may also serve as a potential mechanism that translates acute high-frequency synaptic activity to long-lasting alteration of synaptic physiology and morphology (Lu, 2003).
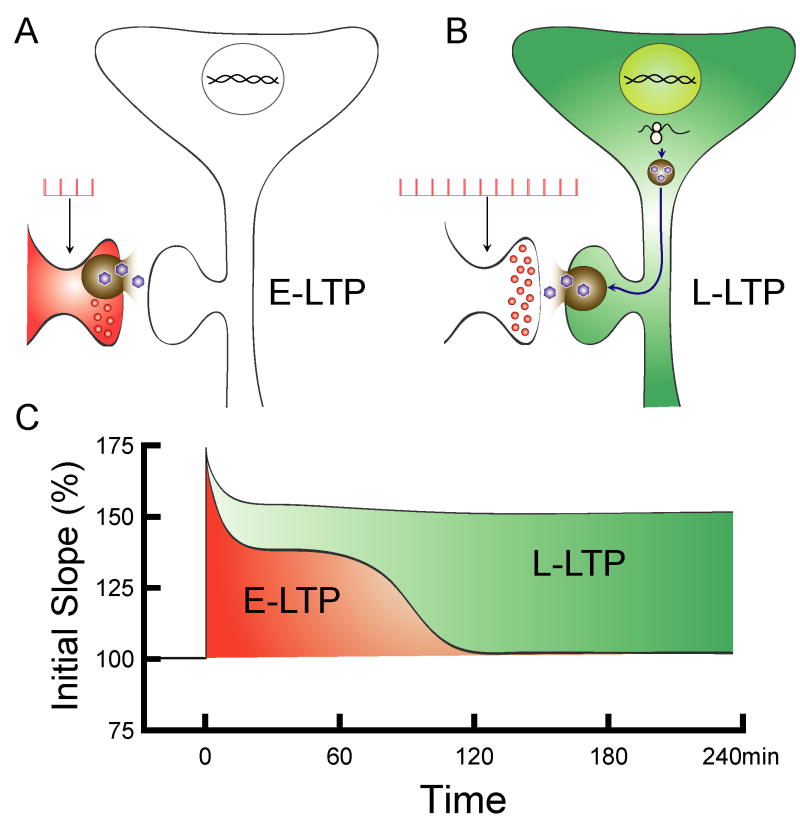
At CA1 synapses, does newly synthesized BDNF come from pre- or postsynaptic neurons? When the Schaffer-collateral pathway is stimulated with HFS, an increase in BDNF mRNA was only observed in postsynaptic CA1, but not in presynaptic CA3, neurons (Patterson et al., 1992). This finding indicates that L-LTP requires postsynaptic BDNF mRNA synthesis. In addition, the activity-dependent up-regulation of postsynaptic BDNF gene expression requires an increase in intracellular Ca2+ concentration through NMDA receptors or L-type Ca2+ channels activated during HFS (Ghosh, Carnahan, and Greenberg, 1994; Sano, Nanba, Tabuchi, Tsuchiya, and Tsuda, 1996; Shieh, Hu, Bobb, Timmusk, and Ghosh, 1998; Tabuchi, Nakaoka, Amano, Yukimine, Andoh, Kuraishi, and Tsuda, 2000; Tao, Finkbeiner, Arnold, Shaywitz, and Greenberg, 1998; Zafra, Lindholm, Castren, Hartikka, and Thoenen, 1992). This is inconsistent with a presynaptic Ca2+ influx, since it is restricted to presynaptic terminals and is mediated primarily through N- or P/Q type Ca2+ channels. Moreover, pairing of a weak presynaptic tetanus with repetitive somatic action potentials in CA1 neurons was sufficient to induce transcription of genes, including BDNF, as well as L-LTP (Dudek & Fields, 2002; Lee, Cohen, Becker & Fields, 2005). Taken together, the existing data support the notion that while activity-dependent secretion of BDNF from presynaptic sites may be necessary for E-LTP, the long-term maintenance of L-LTP requires sustained supply of BDNF through activity-dependent transcription and translation in the postsynaptic neurons (Fig. 1).
Figure 1.
Activity-dependent secretion of BDNF from presynaptic site (red in A and C) may be necessary for E-LTP, while the long-term maintenance of L-LTP requires sustained supply of BDNF through activity-dependent transcription and translation in the postsynaptic neurons (green in B and C).
Does local synthesis of BDNF at dendrites play a role in L-LTP?
Another important issue is how BDNF, a diffusible factor, achieves synapse-specific modulation during L-LTP? A potential mechanism is activity-dependent translation of BDNF mRNA locally at synapses undergoing L-LTP. Thus, a strong tetanus (but not a weak tetanus) should stimulate the synthesis, processing and secretion BDNF protein right at the tetanized synapses, using BDNF mRNA localized in that dendritic branch. While conceptually attractive, there is, at best, limited evidence supporting this model. In fact, the functional role of dendritic protein synthesis at large remain obscure, despite the fact that numerous experiments have demonstrated local translation of proteins (particularly the cytosolic proteins) and its activity-dependence, in neuronal dendrites (Grossman, Aldridge, Weiler, and Greenough, 2006; Martin, 2004; Sutton and Schuman, 2006). In particular, the function of local dendritic translation in L-LTP has been demonstrated only partially for one protein: CaMKIIα. In a mouse mutant in which the native 3′UTR of CaMKIIα mRNA was replaced with the 3′UTR of bovine growth hormone (Miller, Yasuda, Coats, Jones, Martone, and Mayford, 2002), the translation of CaMKIIα protein is intact, but the dendritic localization of CaMKIIα mRNA is impaired. This is accompanied by deficits in L-LTP and hippocampal-dependent long-term memory, but not E-LTP (Miller et al., 2002). There is no evidence for a role of dendritic BDNF synthesis in L-LTP so far.
If local BDNF synthesis does play a critical role in L-LTP then there must be dendritic localization of BDNF mRNA. Indeed, several early studies have reported the localization of BDNF mRNA in the dendrites of cultured hippocampal pyramidal neurons (Crino and Eberwine, 1996; Tongiorgi, Righi, and Cattaneo, 1997). Subsequent studies showed that neuronal activity enhances the dendritic trafficking of BDNF mRNA both in cultured hippocampal neurons and in CA1 neurons in vivo (Simonato, Bregola, Armellin, Del Piccolo, Rodi, Zucchini, and Tongiorgi, 2002; Tongiorgi et al., 1997), that accumulation of BDNF mRNA in distal dendrites requires Ca2+ influx through NMDA receptor and L-type Ca2+ channels and the activation of PI3 kinase pathway (Righi, Tongiorgi, and Cattaneo, 2000; Tongiorgi et al., 1997), and that exon-IV mRNA is the primary BDNF mRNA translocated to dendrites (Pattabiraman, Tropea, Chiaruttini, Tongiorgi, Cattaneo, and Domenici, 2005). Since BDNF itself can induce dendritic targeting of BDNF mRNA, it is possible that tetanus-induced secretion of BDNF may serve as a signal for the localization of BDNF mRNA to these activated synapses (Righi et al., 2000).
Dendritic BDNF mRNA appears to be associated with polyribosomes, suggesting active translation (Tongiorgi, Armellin, Giulianini, Bregola, Zucchini, Paradiso, Steward, Cattaneo, and Simonato, 2004). Consistent with this, BDNF immuno-reactivity in dendrites was rapidly increased upon neuronal depolarization or seizure (Tongiorgi et al., 2004; Tongiorgi et al., 1997). Moreover, the increase in BDNF immuno-reactivity was blocked by the protein synthesis inhibitor cycloheximide, but not the inhibitor for dendritic transporter nocodazole (Tongiorgi et al., 1997). These results suggest an increase in local translation of BDNF in dendrites, rather than an increase in transport of BDNF protein from soma to dendrites. However, given that the BDNF antibody used in these experiments could detect signals in BDNF-/- mice, further work is necessary to confirm these results. Thus, despite the evidence for dendritic localization of BDNF mRNA, activity-dependent dendritic translation of BDNF protein remains to be firmly established.
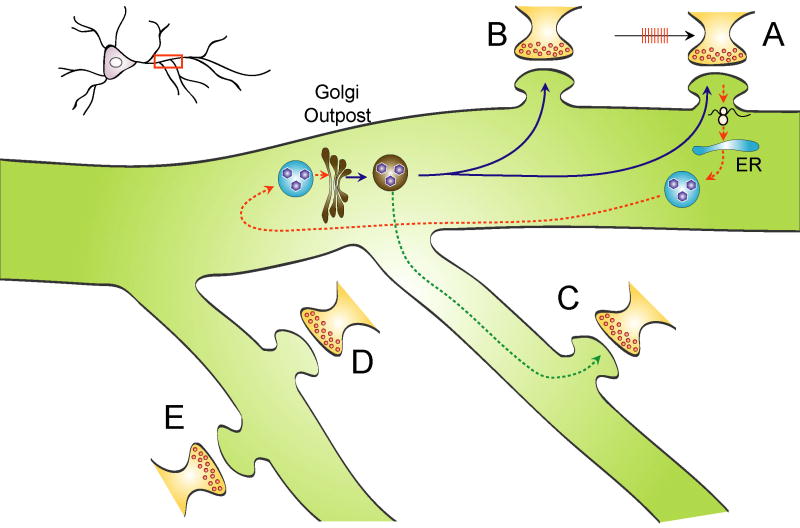
A conceptually more challenging issue is how locally synthesized secretory or transmembrane proteins get processed. There are a number of post-translational modifications for BDNF, including glycosylation, proper folding, cleavage, and sorting to constitutive or regulated secretion pathway. Folding and N-glycosylation are processed in ER whereas cleavage and sorting occur in Golgi apparatus or subsequent organelles. In a provocative paper, Mike Ehlers and colleagues demonstrated that at least in cultured hippocampal neurons, Golgi apparatus are absent in majority of dendrites (Horton, Racz, Monson, Lin, Weinberg, and Ehlers, 2005). Using markers specific for Golgi, they show that small, Golgi-like organelles (so-called Golgi outposts) are selectively localized to dendritic branch points and are typically present in only one of the dendrites (Fig. 2). This study poses a number of important conceptual challenges to local synthesis of BDNF. First, most dendritically synthesized BDNF has to be transported back to the neuronal soma to be processed in Golgi apparatus. Second, even in the long (apical) dendrite, BDNF synthesized in distal dendrites still needs to be transported to the branch point to be sorted in the Golgi outposts. In both cases, a round-trip trafficking of BDNF is implicated, and synapse specificity is lost (Fig. 2). Third, assuming that locally translated BDNF could be secreted after glycosylation and correctly folded in ERs in the distal dendrites without Golgi, it would only be secreted in a constitutive manner in the form of proBDNF, because sorting to the regulated secretion pathway and intracellular cleavage can only happen in Golgi. The requirement of round-trip transport for BDNF processing makes it difficult to ensure a selective modulation of the stimulated synapse (A in Fig. 2) without affecting other synapses (B and C in Fig. 2).
Figure 2. Synaptic specificity is lost after locally synthesized proteins at stimulated synapses are processed at Golgi apparatus elsewhere.
When synapse A is stimulated with L-LTP inducing protocol, local protein synthesis is initiated. Since no Golgi apparatus is present at synapses, these proteins have to be delivered to Golgi in other places for processing, such as Glogi outpost at dendritic branch point. The newly processed proteins could be shipped back from Golgi to synapse A as well as synapse B on the same dendrite or synapse C on a different dendrite that share the same Golgi outpost. Synapses D and E are less likely to be affected.
Does BDNF serve as a plasticity-related protein in synaptic tagging?
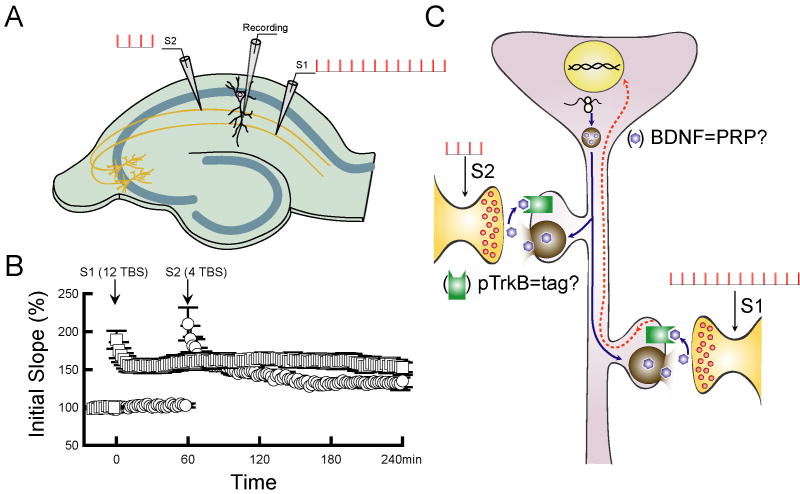
In addition to de novo protein synthesis, maintenance of L-LTP, like that of LTM, also requires activity-dependent gene transcription, which occurs in the nucleus located in the cell body of neurons. Another fundamental question then is how the newly synthesized protein(s) are specifically targeted to, or “captured” by, the tetanized synapses without affecting nearby un-tetanized synapses. The synaptic “tagging” hypothesis is a prominent model that accounts for synapse-specificity of L-LTP (Frey and Morris, 1997). In this hypothesis, a strong tetanus induces protein synthesis in the soma, these “plasticity-related proteins (PRPs)” are then transported to dendrites. The PRPs can only be captured by the synaptic tags previously created by tetanic stimulation, leading to modification of only the tetanized (activated) synapses (Fig. 3C). A weak tetanus is sufficient to generate a tag, but the tag is generally short-lived. The best demonstration of synaptic tagging is the two-pathway experiment in which two independent synaptic inputs to the same neuronal population are activated by two stimulating electrodes, while field EPSPs are monitored by a single recording electrode in the Schaffer collateral region of the CA1 (Fig. 3A and 3B). When one afferent pathway (S1) to a specific population of CA1 neurons is activated by strong tetanus, a weak tetanus applied to a second independent pathway (S2) usually induces L-LTP because it creates a tag that captures the PRP induced by the strong tetanus in S1.
Figure 3. Synaptic tagging model.
A, Two independent synaptic inputs to the same neuronal population are activated by two stimulating electrodes (S1 and S2). Field EPSPs are monitored by a single recording electrode in the Schaffer collateral region of the CA1. B, When one afferent pathway (S1) is activated by strong tetanus (12 theta burst at 100 Hz), a weak tetanus (4 theta burst at 100 Hz) applied to a second independent pathway (S2) to the same neuron usually induces L-LTP. C, Short-lived synaptic tag can be generated by strong tetanus and weak tetanus, while the PRPs can only be induced by strong tetanus. When one afferent pathway (S1) is activated by strong tetanus, a weak tetanus applied to a second independent pathway (S2) usually induces L-LTP because it creates a tag that captures the PRP induced by the strong tetanus in S1.
The nature and identity of the PRPs and synaptic tags remain to be established (Reymann and Frey, 2007). Several lines of evidence suggest that BDNF could serve as a PRP. First, substantial experimental data support the fact that strong tetani enhance the expression of BDNF as a PRP in the soma of CA1 pyramidal neurons (Castren et al., 1993; Dragunow et al., 1993; Patterson et al., 1992). This is possibly mediated by BDNF transcription through promoter III (Lee, Cohen, Becker, and Fields, 2005). Microarray experiments demonstrate that strong, L-LTP inducing stimulation results in an increase in the expression of 100 genes, but only three genes qualify statistically as L-LTP specific genes. Among the three, BDNF fits the best as a PRP (Barco, Patterson, Alarcon, Gromova, Mata-Roig, Morozov, and Kandel, 2005). Direct proof of the trafficking of newly synthesized BDNF and its capture at the tetanized synapse has yet to be reported. Second, coupling of weak tetanic stimulation, which creates a “synaptic tag” but not PRP expression, and elevated BDNF levels can induce L-LTP. It has been shown that pairing BDNF perfusion with a set of sub-threshold tetani could induce LTP which otherwise would not occur with stimulation alone (Figurov et al., 1996; Kovalchuk, Hanse, Kafitz, and Konnerth, 2002). Pang et al performed a similar experiment but examined L-LTP explicitly. They showed that a set of weak theta bursts (3 sets of TBS, or 3TBS), which normally could induce E-LTP but not L-LTP, elicited reliable L-LTP that lasts more than 3 hours when paired with a perfusion of BDNF (Pang et al., 2004). In VP16-CREB mice in which BDNF levels are elevated, a weak tetanus could induce L-LTP, which is reversed by TrkB-IgG (Barco et al., 2005). Third, when PRP production is completely blocked by protein synthesis inhibitors, strong tetani no longer induce L-LTP. Remarkably, application of exogenous mBDNF completely rescues the deficit in L-LTP (Pang et al., 2004). If the synaptic tag is short lived, then the newly generated PRP should only work within a finite time window after delivery of strong tetani. Indeed, application of TrkB-IgG, a BDNF scavenger, to hippocampal slices within 70 min after the tetanus can reverse an already established LTP (Kang et al., 1997). Finally, the capture of activity-induced BDNF by a weakly tetanized synapse has been demonstrated in the “two pathway” experiment (Barco et al., 2005). In wild type mice, a weak tetanus to the second pathway (S2) could induce L-LTP if it is preceded by a strong tetanus to the first pathway (S1) (Fig. 3B). In mice in which the BDNF gene is deleted in the hippocampus (or more specifically in postsynaptic CA1 neurons which could produce PRPs), a weak tetanus can no longer capture PRPs to sustain L-LTP in S2 (Barco et al., 2005).
If BDNF is a PRP, a natural question is whether TrkB, the receptor for BDNF, can serve as a synaptic tag. A synaptic tag has to satisfy several criteria (Kelleher, Govindarajan, and Tonegawa, 2004): 1) the tag can be activated by weak tetanus that induces only E-LTP; 2) the lifetime of the tag is about 1–2 hours; 3) the activation of the tag does not require protein synthesis; 4) the tag is induced in an input-specific and physically immobile manner; and 5) the tag interacts with PRP for L-LTP. A twist of the “tagging” model here is that the PRP (BDNF) needs to be secreted before it can be captured by the tag (TrkB). Demonstrating that TrkB is a “synaptic tag” represents the ultimate challenge in the hypothesis that BDNF-TrkB is a PRP-tag pair in synaptic tagging.
Role of BDNF in hippocampal-dependent long-term memory
As the putative cellular mechanism underlying memory formation, LTP has been correlated with the acquisition and retention of learned behaviors. Distinct forms of LTP, defined by unique temporal and molecular characteristics, mirror many temporal and mechanistic aspects of memory formation. Hippocampal-dependent memory can also be divided into at least two phases, short-term and long-term. Like E-LTP, short-term memory (STM) lasts from minutes to a few hours and does not require protein or mRNA synthesis. In contrast, long-term memory (LTM) lasts from many hours to days, weeks, or even longer. In addition, both LTM and L-LTP are dependent on mRNA and protein synthesis (Dudai, 2004; McGaugh, 2000). It is tempting to map early and late phase LTP onto these short and long-term forms of memory and indeed there is much evidence to support this delineation. Several downstream effectors of Ca2+ and cAMP-mediated signaling have been shown to regulate both L-LTP and LTM in a similar manner (Abel, Nguyen, Barad, Deuel, Kandel, and Bourtchouladze, 1997; Costa-Mattioli, Gobert, Stern, Gamache, Colina, Cuello, Sossin, Kaufman, Pelletier, Rosenblum, Krnjevic, Lacaille, Nader, and Sonenberg, 2007; Kang, Sun, Atkins, Soderling, Wilson, and Tonegawa, 2001). However, it is not clear to what extent the molecular mechanisms underlying LTP are applicable to different forms of memory – even when those memories depend on the same anatomical structure, such as the hippocampus. Furthermore, it is not certain whether induction and maintenance of L-LTP in the hours immediately following memory acquisition is itself sufficient to maintain memory weeks or months after initial learning. In order to fully understand how BDNF regulates LTM, it will be important to determine when BDNF-dependent L-LTP is required for successful memory acquisition, retention, or retrieval.
Formation (acquisition or encoding) of LTM
Despite the fact that L-LTP inducing stimuli or learning turns on (or off) the expression of a set of common genes in the hippocampal neurons involved, the specific genes that mediate specific aspects of LTM have not been fully established (Ingi, Worley, and Lanahan, 2001; Matsuo, Murayama, Saitoh, Sakaki, and Inokuchi, 2000; Qian, Gilbert, Colicos, Kandel, and Kuhl, 1993). Nevertheless, evidence seems quite strong that BDNF is critically involved in LTM. An increase in BDNF mRNA in the hippocampus has been observed following acquisition of spatial tasks such as the Morris water maze and the radial maze (Kesslak, So, Choi, Cotman, and Gomez-Pinilla, 1998; Mizuno, Yamada, Olariu, Nawa, and Nabeshima, 2000); aversive conditioning in the form of inhibitory avoidance (Alonso, Vianna, Depino, Mello e Souza, Pereira, Szapiro, Viola, Pitossi, Izquierdo, and Medina, 2002; Ma, Wang, Wu, Wei, and Lee, 1998), contextual fear conditioning (Hall, Thomas, and Everitt, 2000); and olfactory recognition (Broad, Mimmack, Keverne, and Kendrick, 2002). In parallel, blocking BDNF synthesis or TrkB availability has been shown to impair LTM formation: BDNF+/- mice are impaired in Morris water maze acquisition (Linnarsson, Bjorklund, and Ernfors, 1997) and contextual fear conditioning (Liu, Lyons, Mamounas, and Thompson, 2004); deletion of the TrkB gene in the forebrain results in severe behavioral deficits in a spatial water maze task and moderate deficits in a radial arm maze task (Minichiello et al., 1999); inhibition of BDNF mRNA expression via hippocampal infusion of BDNF antisense oligos or anti-BDNF antibody before training blocks acquisition in inhibitory avoidance and radial arm maze tasks (Alonso et al., 2002; Ma et al., 1998; Mizuno et al., 2000). Genetic and pharmacological manipulations of BDNF signaling prior to training have also been shown to impair LTM (Gorski, Balogh, Wehner, and Jones, 2003; Heldt, Stanek, Chhatwal, and Ressler, 2007; Johnston, Clements, and Rose, 1999; Koponen, Voikar, Riekki, Saarelainen, Rauramaa, Rauvala, Taira, and Castren, 2004; Monteggia, Barrot, Powell, Berton, Galanis, Gemelli, Meuth, Nagy, Greene, and Nestler, 2004; Mu, Li, Yao, and Zhou, 1999; Saarelainen, Pussinen, Koponen, Alhonen, Wong, Sirvio, and Castren, 2000).
The genetic and pharmacological manipulations described above alter BDNF signaling before learning. Thus, it is difficult to dissociate the role of BDNF in initial acquisition from that in long-term memory retention. Moreover, BDNF is involved in E-LTP and short-term memory (Alonso et al., 2002; Figurov et al., 1996), and therefore is likely to contribute to hippocampal processing of learning-related stimuli from the onset of training. Although studies of memory formation suggest that temporal phases of memory are dissociable mechanistically, it is unclear whether and how BDNF is involved in temporally distinct memory phases. An explicit test of BDNF in memory encoding would require a transient disruption of BDNF signaling at the time of acquisition or sequential manipulations in which BDNF levels are first decreased and then restored to baseline levels by the time of L-LTP onset.
Retention (storage) and recall (retrieval) of LTM
Investigating the role of BDNF during retention or recall of hippocampal dependent memory also requires an inducible or transient disruption of BDNF signaling. As discussed above, this cannot be determined using conventional BDNF or TrkB knockouts since deletion of these genes compromise initial acquisition. It has been shown that recall of some forms of hippocampal dependent memory increase BDNF mRNA expression, namely following contextual fear conditioning and Morris water maze training (Hall et al., 2000; Kesslak et al., 1998). Several studies have been designed to address the role of BDNF in the consolidation or recall of more remotely formed memories. Infusion of antisense BDNF oligonucleotides in rats that had been trained for the reference memory task for 28 days in the radial arm maze disrupts recall of a well-established spatial memory (Mizuno et al., 2000). In an inhibitory avoidance test, infusion of BDNF into the hippocampus 1 hr or 4 hrs after training facilitates, whereas that of anti-BDNF antibody at the same time after training impairs, LTM tested 24 hrs later (Alonso et al., 2002). Interestingly, infusion of BDNF antibodies or antisense oligonucleotide 6 hrs post-training no longer affects memory retention tested at 24 hrs (Alonso et al., 2002; Ma et al., 1998). However, one recent study showed that intra-hippocampal infusion of BDNF antisense oligonucleotide 12 hours after inhibitory avoidance training impairs memory retention 7 days later (Bekinschtein, Cammarota, Igaz, Bevilaqua, Izquierdo, and Medina, 2007). Thus, there are two time windows for LTM that require BDNF: one at 1-4 hours after encoding and this is critical for LTM lasting for 1-2 days, whereas the other at 12 hours after memory formation and is important for persistence of LTM 7 days later. Previous work has demonstrated the existence of two critical periods for memory consolidation, both of which require protein synthesis (Bourtchouladze, Abel, Berman, Gordon, Lapidus, and Kandel, 1998; Grecksch and Matthies, 1980; Igaz, Vianna, Medina, and Izquierdo, 2002). By extending the investigation of LTM beyond 24 hours, the authors revealed a previously unknown, time-delayed effect of BDNF expression on memory recall. This should encourage further investigation into the temporal parameters of BDNF-mediated plasticity and its role in LTM retention.
How could BDNF synthesis be induced 12 hours after memory acquisition when there is no obvious trigger? Replay of synaptic activity during consolidation (such as that during sleep) may be sufficient to elicit activity-dependent expression of BDNF (Foster and Wilson, 2006; Louie and Wilson, 2001). In fact, it is possible that the BDNF antisense oligos interrupted this replay of learning-related activity (Bekinschtein et al., 2007). On the other hand, there may be distinct phases of consolidation mediated by BDNF activity that are independent of experience and synaptic replay and are instead contingent on the induction of BDNF-dependent L-LTP. In other words, there are at least two distinct possibilities for post-acquisition involvement of BDNF signaling in the hippocampus: 1) BDNF synthesis and signaling is triggered by subsequent synaptic activity similar to that induced by initial learning or 2) the initial upregulation of BDNF during acquisition triggers a cascade of events resulting in subsequent BDNF-mediated consolidation processes that itself may recapitulate the learning-related activity. Further work is necessary to distinguish these possibilities.
Memory extinction
Extinction of previously acquired memories is a new form of learning and a potential target of BDNF-mediated plasticity. It has been shown that extinction of conditioned fear is accompanied by a significant increase in BDNF gene expression, particularly transcription mediated by BDNF promoters I and III in the prefrontal cortex (Bredy, Wu, Crego, Zellhoefer, Sun, and Barad, 2007) and amygdala (Chhatwal, Stanek-Rattiner, Davis, and Ressler, 2006). Moreover, extinction of fear-potentiated startle was impaired in mice with a site-specific knockout of the BDNF gene in the dorsal hippocampus through targeted injections of a cre-recombinase lentivirus (Heldt et al., 2007). Although it has not been tested, another potential target of BDNF modulation is the context-specific acquisition or retrieval of extinction memory (Corcoran and Maren, 2004). Many questions remain to be addressed. First, the role of BDNF in the encoding versus consolidation of extinction memory has not been determined and requires temporally restricted and inducible manipulations of BDNF signaling. As with initial learning, if BDNF plays a role in both, it will be difficult to observe the effect on consolidation if acquisition is compromised. Second, it is unclear whether BDNF transcription, or other aspects of BDNF signaling (trafficking, secretion, etc) is required, and if so, whether specific BDNF transcripts (exon I or III) are involved. Third, BDNF may contribute to specific aspect(s) of memory extinction by modulating synaptic function in specific brain regions. For example, amygdala-specific expression of a dominant-negative truncated TrkB receptor impairs consolidation but not encoding of conditioned fear extinction (Chhatwal et al., 2006). It is possible that hippocampal BDNF is required for acquisition of extinction memory. In contrast, BDNF in the amygdala or prefrontal cortex may be necessary for the consolidation and retention of extinction memory (Monfils, Cowansage, and Ledoux, 2007).
Dissociation of L-LTP and hippocampal dependent LTM
Although converging evidence demonstrates an important role for BDNF in the formation and maintenance of hippocampal-dependent memory traces, a few studies show inconsistent or contradictory results: some report significant impairments in spatial memory tested by Morris water maze, but not in rapidly acquired contextual memory of conditioned fear, in BDNF or TrkB mutant mice (Gorski et al., 2003; Heldt et al., 2007; Minichiello et al., 1999). Since it has also been shown that L-LTP can be induced without BDNF if multiple high frequency trains are used (Patterson et al., 2001), it is possible that BDNF-independent memory could form using signaling mechanisms that lead to BDNF-independent L-LTP in the hippocampus. While the stimulation protocol used to induce BDNF-dependent L-LTP (theta burst stimulation or TBS) is presumed to reflect physiologically relevant patterns of activation, there is very little data regarding the specific patterns of learning-related activity and how it differs in terms of resultant plasticity and memory formation. It is unknown whether or not BDNF-independent memory formation is driven by unique activity patterns or stronger activation of the hippocampus.
Behavioral tagging
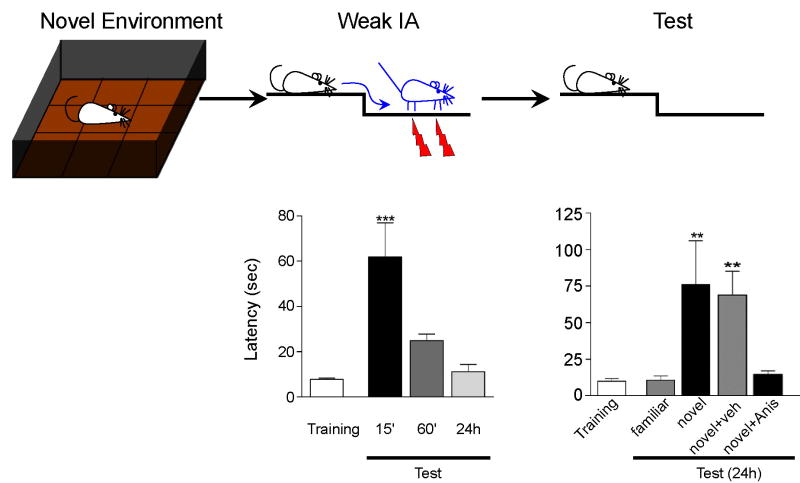
Based on the investigations of BDNF regulation of L-LTP, there are several hypotheses that remain to be tested behaviorally. One of these is that BDNF-TrkB signaling may serve as a PRP-synaptic tag pair (Fig. 3). As discussed above, synaptic tagging allows for synapse specificity of plasticity and, under appropriate conditions, can generate heterosynaptic L-LTP if weak stimulation, sufficient to produce a synaptic tag, can capture PRPs induced by stimulation of an independent pathway. Evidence for “behavioral tagging” was recently reported in a study designed to test whether novelty exposure before or after weak training could provide the PRPs necessary to convert STM to LTM (Moncada and Viola, 2007). Weak inhibitory avoidance conditioning (IA) normally results in a STM memory detectable at 15 minutes, but not 1 or 24 hrs, after training. The same training produces LTM if coupled with novelty exposure. This effect was dependent on protein synthesis as infusion of anisomycin, a protein synthesis inhibitor, immediately after the pretraining exposure to novelty blocked the formation of LTM (Fig. 4). Because BDNF has been suggested to be a critical PRP, it would be interesting to test the sufficiency of this protein to induce LTM by replacing novelty exposure with a transient increase in BDNF expression. Likewise, the role of TrkB in synaptic tagging could be evaluated with a temporally restricted disruption of the receptor at the time of the weak training.
Figure 4. Behavioral tagging.
A, Schematic diagram of experimental protocol. A rat was placed in a novel environment (open field) for 5 min within 1 hour before training for weak inhibitory avoidance conditioning (IA) and memory was tested at different time after weak IA conditioning. B, Weak inhibitory avoidance conditioning normally results in a STM detectable at 15 minutes, but not 1 or 24 hrs, after training. C, The same weak IA training produces LTM if coupled with novelty exposure. This effect was dependent on protein synthesis, since infusion of anisomycin, a protein synthesis inhibitorimmediately after the pretraining exposure to novelty blocked the formation of LTM, while vehicle control (novel + veh) still produces LTM. When the rat was familiarized with the novel environment before, the open field did not produce LTM when coupled with weak IA (familiar). (Modified from Moncada and Viola, J Neuroscience, 2007)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Mimmack ML, Keverne EB, Kendrick KM. Increased BDNF and trk-B mRNA expression in cortical and limbic regions following formation of a social recognition memory. Eur J Neurosci. 2002;16:2166–2174. doi: 10.1046/j.1460-9568.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Fields RD. Somatic action potentials are sufficient for late-phase LTP-related signaling. Proc Natl Acad Sci U S A. 2002;99:3962–3967. doi: 10.1073/pnas.062510599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Aloyz R, McLean JH, Pareek S, Miller FD, McPherson PS, Murphy RA. Detection of brain-derived neurotrophic factor in a vesicular fraction of brain synaptosomes. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci U S A. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Harris KM, Fiala JC, Ostroff L. Structural changes at dendritic spine synapses during long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:745–748. doi: 10.1098/rstb.2002.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. Embo J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37:571–579. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Vianna MR, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingi T, Worley PF, Lanahan AA. Regulation of SSAT expression by synaptic activity. Eur J Neurosci. 2001;13:1459–1463. doi: 10.1046/j.0953-816x.2001.01529.x. [DOI] [PubMed] [Google Scholar]
- Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- Johnston AN, Clements MP, Rose SP. Role of brain-derived neurotrophic factor and presynaptic proteins in passive avoidance learning in day-old domestic chicks. Neuroscience. 1999;88:1033–1042. doi: 10.1016/s0306-4522(98)00362-5. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kang H, Jia LZ, Suh KY, Tang L, Schuman EM. Determinants of BDNF-induced hippocampal synaptic plasticity: role of the Trk B receptor and the kinetics of neurotrophin delivery. Learn Mem. 1996;3:188–196. doi: 10.1101/lm.3.2-3.188. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Koponen E, Voikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castren E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Lee PR, Cohen JE, Becker KG, Fields RD. Gene expression in the conversion of early-phase to late-phase long-term potentiation. Ann N Y Acad Sci. 2005;1048:259–271. doi: 10.1196/annals.1342.023. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin KC. Local protein synthesis during axon guidance and synaptic plasticity. Curr Opin Neurobiol. 2004;14:305–310. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Matsuo R, Murayama A, Saitoh Y, Sakaki Y, Inokuchi K. Identification and cataloging of genes induced by long-lasting long-term potentiation in awake rats. J Neurochem. 2000;74:2239–2249. doi: 10.1046/j.1471-4159.2000.0742239.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Ledoux JE. Brain-derived neurotrophic factor: linking fear learning to memory consolidation. Mol Pharmacol. 2007;72:235–237. doi: 10.1124/mol.107.038232. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Yamada N, Hayabara T. Time-dependent changes in neurotrophic factor mRNA expression after kindling and long-term potentiation in rats. Brain Res Bull. 1998;45:599–605. doi: 10.1016/s0361-9230(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Muller D, Nikonenko I, Jourdain P, Alberi S. LTP, memory and structural plasticity. Curr Mol Med. 2002;2:605–611. doi: 10.2174/1566524023362041. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Righi M, Tongiorgi E, Cattaneo A. Brain-derived neurotrophic factor (BDNF) induces dendritic targeting of BDNF and tyrosine kinase B mRNAs in hippocampal neurons through a phosphatidylinositol-3 kinase-dependent pathway. J Neurosci. 2000;20:3165–3174. doi: 10.1523/JNEUROSCI.20-09-03165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirvio J, Castren E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sakata K, Woo N, Wu J, Shen L, Lu B. Activity-dependent transcription of BDNF through promoter 3 contributes selectively to long-term hippocampal plasticity and long-term memory. SFN 2005.2005. [Google Scholar]
- Sano K, Nanba H, Tabuchi A, Tsuchiya T, Tsuda M. BDNF gene can Be activated by Ca2+ signals without involvement of de novo AP-1 synthesis. Biochem Biophys Res Commun. 1996;229:788–793. doi: 10.1006/bbrc.1996.1881. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, Duan S. Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron. 2006;50:401–414. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Simonato M, Bregola G, Armellin M, Del Piccolo P, Rodi D, Zucchini S, Tongiorgi E. Dendritic targeting of mRNAs for plasticity genes in experimental models of temporal lobe epilepsy. Epilepsia. 2002;43 5:153–158. doi: 10.1046/j.1528-1157.43.s.5.32.x. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Armellin M, Giulianini PG, Bregola G, Zucchini S, Paradiso B, Steward O, Cattaneo A, Simonato M. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. J Neurosci. 2004;24:6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Righi M, Cattaneo A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci. 2006;9:1009–1018. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]