Abstract
The objective of this study was to estimate the annual losses from Mycobacterium avium subspecies paratuberculosis (MAP) for an average, MAP-seropositive, Canadian dairy herd. A partial-budget simulation model was developed with 4 components of direct production losses (decreased milk production, premature voluntary culling, mortality, and reproductive losses). Input values were obtained primarily from a national seroprevalence survey of 373 Canadian dairy farms in 8 of 10 provinces. The model took into account the variability and uncertainty of the required input values; consequently, it produced probability distributions of the estimated losses. For an average Canadian dairy herd with 12.7% of 61 cows seropositive for MAP, the mean loss was $2992 (95% C.I., $143 to $9741) annually, or $49 per cow per year. Additional culling, decreased milk production, mortality, and reproductive losses accounted for 46%, 9%, 16%, and 29% of the losses, respectively. Canadian dairy producers should use best management practices to reduce these substantial annual losses.
Résumé
Estimé des pertes directes de production dans les troupeaux laitiers du Canada à la suite d’infections subcliniques à Mycobacterium avium, sous-espèce paratuberculasis. L’objectif de cette étude était d’évaluer les pertes annuelles causées par Mycobacterium avium sous-espèce paratuberculosis (MAP) pour un troupeau laitier canadien moyen séropositif au MAP. Un modèle de simulation de budget partiel à 4 volets de pertes directes de production (diminution de la production laitière, réforme volontaire anticipée, mortalité et pertes reproductives) a été développé. Les données ont été obtenues principalement à partir d’une enquête nationale sur la séroprévalence effectuée sur 373 fermes laitières canadiennes situées dans 8 des 10 provinces. Le modèle a tenu compte de la variabilité et de l’incertitude des données d’entrées et en conséquence a créé des distributions de probabilités des pertes estimées. Pour un troupeau laitier canadien moyen comprenant 12,7 % de 61 vaches séropositives au MAP, la perte moyenne s’établissait à 2992 $ (95 % I.C., 143 $ à 9741 $) annuellement ou 49 $ par vache, par année. La réforme supplémentaire, la diminution de la production laitière, la mortalité et les pertes reproductives comptaient respectivement pour 46 %, 9 %, 16 % et 29 % des pertes. Les producteurs laitiers au Canada devraient utiliser les meilleures pratiques de gestion pour réduire ces pertes annuelles substantielles.
(Traduit par Docteur André Blouin)
Introduction
Recent international developments in the area of infectious disease control and nontariff trade barriers, along with possible zoonotic concerns, have provoked a revival of interest in Johne’s disease (JD) in Canada and elsewhere. The slow-growing, acid-fast bacterium causing JD, Mycobacterium avium subspecies paratuberculosis (MAP), is distributed worldwide (1–5) and causes chronic, progressive, granulomatous enteritis in domestic and wild ruminants. Dairy cattle infected with MAP have been culled prematurely (6,7) and have had decreased milk production (6,8,9), increased mortality (10), decreased reproductive efficiency (8,11), and, possibly, increased susceptibility to other diseases (12). Also known as paratuberculosis, JD has no known cure (13).
Studies have been done in the past to determine the economic losses from MAP, but the validity of these estimates in the Canadian dairy industry is questionable for the several reasons. Some studies were based on research on a small number of herds that may not have been representative of the wide diversity of herds in the Canadian dairy industry and their differences in management or seroprevalence (6,8,14). Some studies utilized estimation methods, such as regression, or directly multiplied the estimated prevalence with costs of effects associated with MAP (such as decreased milk yield), rather than using stochastic methods, so that the interpretation of these estimates is limited to individual herds (6,15). In an economic study done in dairy herds in Maritime Canada (14), the effects of MAP-seropositivity were estimated, primarily from the scientific literature reporting studies of non-Canadian dairy herds. None of the studies done in the past have utilized either Canadian MAP prevalence estimates or the effects of MAP infection estimated from Canadian dairy herds.
The objective of this study was to determine the estimate and range of annual direct production losses for an average Canadian dairy herd infected with MAP and the Canadian dairy industry as a whole, utilizing Canadian estimates of MAP seroprevalence and impacts of subclinical MAP infection, where available and applicable.
Materials and methods
Partial-budget model
A partial-budget model deals only with those aspects of an enterprise that are affected by a factor being investigated. The partial-budget model used in this study was adapted from that used in previous studies (14,16,17) and included the impacts of MAP infection on milk yield, additional mortality, additional culling, and reproductive losses. Possible effects of MAP on human health, the ability of a farm to market livestock or other products, and other potential indirect costs were not included in the model.
Input parameters
Table 1 lists all of the input parameters used in the partial-budget model, the distribution that was assumed to represent the range of possible values that each parameter might have, the characteristics that defined that distribution, and the source of the information about the parameter and its distribution. Each is discussed in more detail below.
Table 1.
Distribution, parameters, and source of data for the model variables
| Variable # | Variable name (units) | Distribution or formula | Parameters | Source of data |
|---|---|---|---|---|
| 1 | Average cattle population in herd | Fixed | 61 or 100 | (18) |
| 2 | Seroprevalence of infection in the Canadian dairy population | Normal | M = 0.031, σ = 0.004 | (5) |
| 2 | Seroprevalence of infection in seropositive dairy herds | Beta | α = 1.758, β = 21.317, min = 0.06 | (5) |
| 3 | Milk yield (L per cow per y) | Fixed | 9519 | (18) |
| 4 | Milk price ($ per L) | Fixed | 0.59 | (18) |
| 5 | Reduced milk yield (%) | Normal | μ = 2.34, σ = 1.18 | (9) |
| 6 | Losses associated with each low producing animal | (3)*(4)*(5) | ||
| 7 | Percentage of infected animals affected | Triangular | min = 0.20, most likely = 0.25, max = 0.30 | Assigned by author |
| ML | Herd milk loss ($) | (1)*(2)*(6)*(7) | ||
| 8 | Replacement cost of cow ($ per head) | Triangular | min = 1500, most likely = 2000, max = 2500 | Assigned by author |
| 9 | Percentage increased mortality risk in affected cattle (% per y) | Normal | μ = 0.0315, σ = 0.015 | (17) |
| MO | Herd mortality loss ($) | (1)*(2)*(8)*(9) | ||
| 10 | Slaughter value of healthy cattle ($ per head) | Triangular | min = 300, most likely = 500, max = 700 | Assigned by author |
| 11 | Percentage of affected cattle with reduced slaughter value | Triangular | min = 0.2, most likely = 0.25, max = 0.3 | Assigned by author |
| 12 | Losses associated with each culled animal ($) | (8)-[10-{(11)*(10)}] | ||
| 13 | Excess culling risk for infected cattle | Normal | μ = 0.109, σ = 0.04 | (7) |
| CL | Herd additional premature culling loss ($) | (1)*(2)*(12)*(13) | ||
| 14 | Increased calving interval (d) | Normal | μ = 27.9, σ = 11.4 | (11) |
| 15 | Cost of increased calving interval ($ per d) | Triangular | min = 2.5, most likely = 4.4, max = 6.25 | (24–26) |
| RL | Herd reproductive loss ($) | {(1)*(2)*(14)*(15)}*0.923 | ||
| AL | Herd annual losses ($) | ML + MO + CL + RL |
— multiplied by
Farm characteristics and prices
Two herd sizes were used in the analyses. To estimate the average annual losses for the Canadian dairy industry, a herd size of 100 cows was used and the losses were expressed as being “per 100 cows” or “per cow.” For estimating the range of possible losses at the level of the individual infected herd, the average size of a Canadian dairy herd, as reported by Dairy Farmers of Canada in 2002 (n = 61 cows), was used. The average milk production per cow per 305-day lactation (9519 L) and average milk price ($0.59 per L) were obtained from the Canadian Dairy Information Centre (18). Herd sizes, production levels, and milk price were all treated as fixed values, so that all estimates of MAP-associated losses were independent of differences in those parameters across herds.
Data about the seroprevalence of MAP were obtained from a stratified 2-stage random sample of 373 herds in 8 Canadian provinces from 1998 to 2003, with details of the sampling protocol found elsewhere (5). An ELISA was utilized to determine whether cows were seropositive for MAP. In total, 10 578 cows were tested, for which 3.1% (95% C.I., 2.3% to 3.8%) had positive tests for antibodies to MAP. For the model estimating overall economic losses for the Canadian dairy industry, the overall seroprevalence of MAP was assumed to fall within a normal distribution, with a mean of 0.031 and standard deviation (s) of 0.004.
A much wider distribution for the within-herd seroprevalence was utilized for the estimate of the range of possible economic losses that individual infected Canadian herds might encounter. The average within-herd seroprevalence in herds having at least 2 MAP-seropositive cows was 12.7%, and 95% of the values were < 33.3% (5). Consequently, a beta distribution (α = 1.758, β = 21.317) was utilized for estimating the range of infected herd losses.
Impact of MAP on milk yield
Based on a Canadian study of the effectc of MAP-seroprevalence on milk production, an interaction was found between MAP-seropositivity and lactation number (9), with a statistically significant reduction in milk yield being observed only in the 4th or higher lactation animals. This effect (loss of 212 kg [standard error of the mean (sx̄ ) 106] in a 305-day lactation) would represent 2.34% of the average yield used in this study. In our partial budget analysis (Table 1), the losses associated with 4-plus lactation animals was estimated by multiplying together the average milk yield (9519 L/cow/y) for Canadian dairy herds subscribing to Dairy Herd Improvement (DHI) for monthly milk testing (18), milk price ($0.59 per L) for all Canadian dairy herds (18), and reduced milk yield (2.34%). A triangular distribution (min = 0.20, most likely = 0.25, and max = 0.30) was utilized for the proportion of 4-plus lactation animals in a herd (9). The herd milk losses were estimated by multiplying together the herd size, the within herd seroprevalence of infection, the losses associated with 4-plus lactation animals, and the proportion of animals in this parity group.
Mortality
No Canadian data on mortality risk associated with MAP infection was available. Based on a study of 121 dairy herds in Michigan, USA (17), where mortality risk of cows within herds positive for paratuberculosis was 3.15% higher than in negative herds, the percentage of increased mortality risk (death on the farm) in affected cattle was added to the model as a normal distribution with μ = 0.0315 and σ = 0.015. Cow value at death was set to be equal to the cost of replacement of the cow, because no carcass value was assumed for dead animals. Replacement cost of a cow (triangular distribution, min = $1500, most likely = $2000, max = $2500 per head) was assigned by the authors following consultation with dairy clinicians familiar with the normal characteristics of these costs in Canada. Therefore, the herd mortality losses were estimated by multiplying together the herd size, the within herd seroprevalence of infection, the percentage of increased mortality risk in affected cattle, and the replacement cost of a cow.
Premature culling and reduced slaughter value
Input values included slaughter value of healthy cattle (triangular distribution, min = $300, most likely = $500, and max = $700 per head) and percentage reduced slaughter value of affected MAP-seropositive cattle (triangular distribution, min = 0.20, most likely = 0.25 and max = 0.30). With limited Canadian research data on these parameters, these values were assigned by the authors following consultation with dairy clinicians familiar with the normal characteristics of these costs in Canada.
The percentage reduced slaughter value of affected cattle was multiplied with the slaughter value of healthy cattle, and this total was subtracted from the slaughter value of healthy cattle to calculate the average slaughter value of a cull cow with subclinical MAP infection. Therefore, the losses associated with each culled animal was estimated by subtracting the average slaughter value of a cull cow with MAP infection from the replacement cost of a cow.
The hazard of culling for MAP-seropositive cows in 134 Canadian dairy herds was 1.38 (95% C.I., 1.05 to 1.81) times that for MAP-seronegative cows (7). Interpreting the hazard ratio as a risk ratio, assuming an average follow-up period of 2 y, and using a within herd seroprevalence of MAP of 2.4%, the risk difference associated with MAP seropositivity was estimated to be 0.109 (sx̄ = 0.04). Consequently, the excess culling risk for infected cattle was added to the model as a normal distribution with μ = 0.109 and σ = 0.04. Therefore, the herd additional premature culling losses were estimated by multiplying together the herd size, the within herd seroprevalence of infection, the losses associated with each culled animal, and the excess culling risk for infected cattle.
Reproductive losses
With no Canadian estimate available, an increased calving interval of 27.9 d for a MAP-seropositive cow was added to the model as a normal distribution with μ = 27.9 and σ = 11.4, based on data from 7 dairy herds in Michigan, USA (11). The cost of a day open (days open = calving-to-conception interval) was added to the model as a triangular distribution with min = 2.5, most likely = 4.4, and max = 6.25.
The herd reproductive losses were estimated by multiplying together the herd size, the within herd seroprevalence of infection, the increased calving interval, and the cost of a day of increased calving interval. The objectives of the partial budget were to determine economic losses on an annual basis; however, reproductive losses were estimated on a lactation basis due to their inherent relationship with consecutive calvings. To convert this to an annual loss, an average calving interval of 13 mo was assumed (based on consultation with dairy clinicians familiar with the normal calving interval in Canada) and the losses per lactation were multiplied by 0.923 (12/13 mo).
Statistical analysis and stochastic simulation of the partial budget model
Overall annual herd losses were the sum of the 4 categories of production losses. A stochastic simulation model that combined the values presented in Table 1 into an overall estimate of the MAP-associated losses was developed, using simulation software (@RISK 2002, Version 4.5.2; Palisade Corporation, Ithaca, New York, USA) with Latin hypercube sampling and 10 000 iterations for each analysis. The estimated distributions of total losses for the Canadian dairy industry overall, as well as for the infected individual dairy herds, were determined and graphed. Mean and median values were determined, along with the range of values that encompassed 95% of the estimates. The percentages of losses for each of the 4 main components of the model were determined.
A sensitivity analysis was carried out on key parameters for which solid evidence was lacking. The sensitivity analysis involved a reanalysis of the data, but utilizing expected values that were either 10% lower or 10% higher than estimated for each of the following parameters: seroprevalence, reduction in milk yield, risk difference for mortality, risk difference for culling, and difference in calving interval. For example, in the model evaluating overall effects in the Canadian dairy population, increased culling was changed from 10.9% to 9.81% (10% lower) and 11.99% (10% higher), with the standard deviations of each of these distributions being left unchanged at 4%. The impacts of each of these 10% changes on the overall estimates of annual economic loss were compared in order to determine which factors were most influential in the model.
Results
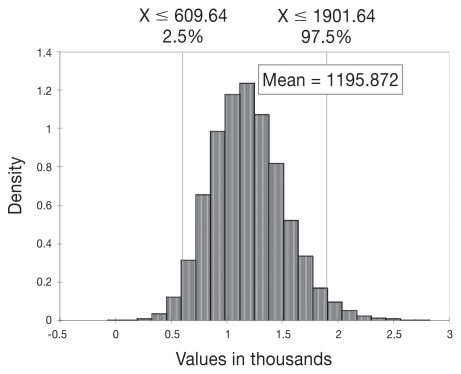
For the Canadian dairy industry as a whole, the mean economic loss per 100 cows was estimated to be $1196 annually ($12/cow and $385/seropositive cow), assuming a seroprevalence of 3.1% for this group of 100 cows (Table 2). Additional culling costs, and reproductive, mortality, and milk losses associated with seroprevalence for MAP were responsible for 45.8%, 29.2%, 16.2%, and 8.8% of the losses, respectively. Figure 1 shows the distribution and potential variability around the estimate, which could be as low as $610 and as high as $1901 (not including the upper and lower 2.5% of estimates), based on the variability of the input estimates into the model and the stochastic nature of the model.
Table 2.
Average and range of annual economic losses from MAP seropositivity for the entire Canadian dairy industry, per 100 cows
| 95% C.I.
|
|||||
|---|---|---|---|---|---|
| Variable # | Variable name (units) | Distribution or formula | Mean | Lower limit | Upper limit |
| 1 | Average cattle population in herd | Fixed | 100 | ||
| 2 | Prevalence of infection among cows in the Canadian dairy population | Normal | 0.031 | 0.023 | 0.038 |
| 3 | Milk yield (L per cow per y) | Fixed | 9519 | — | — |
| 4 | Milk price ($ per L) | Fixed | 0.59 | — | — |
| 5 | Reduced milk yield (%) | Normal | 0.023 | 0.0012 | 0.0472 |
| 6 | Losses associated with each low producing animal | (3)*(4)*(5) | 132 | 1.5 | 262 |
| 7 | Percentage of infected animals affected | Triangular | 0.25 | — | — |
| ML | Herd milk loss ($) | (1)*(2)*(6)*(7) | 102 | 1 | 216 |
| 8 | Replacement cost of cow ($ per head) | Triangular | 2000 | — | — |
| 9 | Percentage increased mortality risk in affected cattle (% per y) | Normal | 3.15 | 0.21 | 6.09 |
| MO | Herd mortality loss ($) | (1)*(2)*(8)*(9) | 195 | 12 | 404 |
| 10 | Slaughter value of healthy cattle ($ per head) | Triangular | 500 | — | — |
| 11 | Percent of affected cattle with reduced slaughter value (%) | Triangular | 25 | — | — |
| 12 | Losses associated with each culled animal | (8)-[10-{(11)*(10)}] | 1625 | 1215 | 2034 |
| 13 | Excess culling risk for infected cattle (%) | Normal | 10.9 | 3.1 | 18.7 |
| CL | Herd additional culling loss ($) | (1)*(2)*(12)*(13) | 549 | 143 | 1035 |
| 14 | Increased calving interval (d) | Normal | 28 | 6 | 50 |
| 15 | Cost of increased calving interval ($ per d) | Triangular | 4 | — | — |
| RL | Herd reproductive losses | {(1)*(2)*(14)*(15)}*0.923 | 350 | 64 | 718 |
| AL | Herd annual losses | ML + MO + CL + RL | 1196 | 610 | 1901 |
— multiplied by
Figure 1.
Distribution of annual economic losses (in Canadian dollars) from MAP-seropositivity for the entire Canadian dairy industry, per 100 cows.
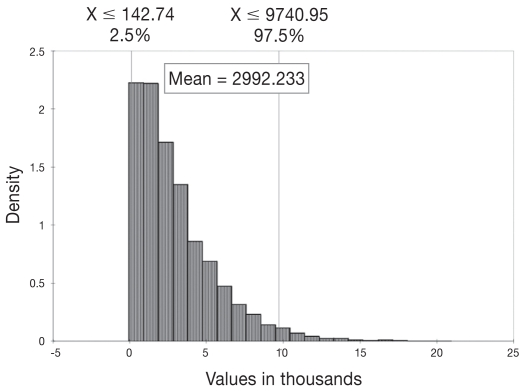
In an average, Canadian, MAP-seropositive, dairy herd of 61 cows, the mean economic loss was estimated to be $2992 annually ($49/cow and $409/seropositive cow), assuming an average within herd seroprevalence of 12.7% (Table 3). Costs due to culling losses were responsible for 46% ($1374) of the total herd losses from MAP. Decreased milk production, mortality, and reproductive losses accounted for 9% ($254), 16% ($488), and 29% ($875) of the total herd losses, respectively. Figure 2 demonstrates that the mean loss could be as low as $143 and as high as $9741 (again, not including the upper and lower 2.5% of estimates), based on the variability of the input estimates into the model and the stochastic nature of the model.
Table 3.
Average and range of annual economic losses in a MAP-seropositive, 61-cow dairy herd in Canada
| 95% C.I.
|
|||||
|---|---|---|---|---|---|
| Variable # | Variable name (units) | Distribution or formula | Mean | Lower limit | Upper limit |
| 1 | Average cattle population in herd | Fixed | 61 | — | — |
| 2 | Seroprevalence of infection in individual seropositive dairy herds | Beta | 12.7 | 0.65 | 38.47 |
| 3 | Milk yield (L per cow per y) | Fixed | 9519 | — | — |
| 4 | Milk price ($ per L) | Fixed | 0.59 | — | — |
| 5 | Reduced milk yield | Normal | 0.023 | 0.0012 | 0.0472 |
| 6 | Losses associated with each low producing animal | (3)*(4)*(5) | 102 | 1 | 215 |
| 7 | Percentage of infected animals affected | Triangular | 0.25 | — | — |
| ML | Herd milk loss ($) | (1)*(2)*(6)*(7) | 254 | 0.5 | 975 |
| 8 | Replacement cost of cow ($ per head) | Triangular | 2000 | — | — |
| 9 | Percentage increased mortality risk in affected cattle (% per y) | Normal | 3.15 | 0.21 | 6.09 |
| MO | Herd mortality loss ($) | (1)*(2)*(8)*(9) | 488 | 5 | 1828 |
| 10 | Slaughter value of healthy cattle ($ per head) | Triangular | 500 | — | — |
| 11 | Percent of affected cattle with reduced slaughter value (%) | Triangular | 25 | — | — |
| 12 | Losses associated with each culled animal | (8)-[10-{(11)*(10)}] | 1625 | 1215 | 2034 |
| 13 | Excess culling risk for infected cattle (%) | Normal | 10.9 | 3.1 | 18.7 |
| CL | Herd additional culling loss ($) | (1)*(2)*(12)*(13) | 1374 | 46 | 4833 |
| 14 | Increased calving interval (d) | Normal | 28 | 6 | 50 |
| 15 | Cost of increased calving interval ($ per d) | Triangular | 4 | — | — |
| RL | Herd reproductive losses ($) | {(1)*(2)*(14)*(15)}*0.923 | 875 | 23 | 3259 |
| AL | Herd annual losses ($) | ML + MO + CC + RL | 2992 | 143 | 9741 |
— multiplied by
Figure 2.
Distribution of annual economic losses (in Canadian dollars) in a MAP-seropositive, 61-cow dairy herd in Canada.
Table 4 shows the results from the sensitivity analyses with the 10% changes in input estimates. The changes in MAP-seroprevalence lead to the largest differences (9.7%) in the overall estimate of economic impact, from $1196 to $1076 when MAP-seroprevalence was reduced by 10% and from $1196 to $1315 when MAP-seroprevalence was increased by 10% in the Canadian dairy industry. Similarly, for a MAP-seropositive herd, the overall estimate of economic impact changed from $2992 to $2691 when MAP-seroprevalence was reduced by 10% and from $2992 to $3289 when MAP-seroprevalence was increased by 10%. A 10% change in additional culling, mortality risk, increased calving interval, and reduced milk yield resulted in a 4.6%, 1.6%, 2.9%, and 1.2% change in annual losses due to MAP seropositivity, respectively.
Table 4.
Range of annual economic losses due to MAP seropositivity with a 10% change in seroprevalence, or a 10% change in losses associated with milk production, mortality, additional culling, and reproductive losses
| Range of annual losses ($) due to MAP seropositivity
|
||||
|---|---|---|---|---|
| In entire Canadian industry (per 100 cows)
|
In infected herds (per herd)
|
|||
| Variables | Less 10% | More 10% | Less 10% | More 10% |
| Changes in input estimates | ||||
| Prevalence (%) | 1076 | 1315 | 2691 | 3289 |
| Milk loss ($) | 1188 | 1209 | 2675 | 2722 |
| Mortality loss ($) | 1176 | 1215 | 2647 | 2735 |
| Additional culling loss ($) | 1141 | 1251 | 2568 | 2815 |
| Reproductive losses ($) | 1161 | 1231 | 2612 | 2770 |
Discussion
Calculations of costs due to MAP infection in the Canadian dairy industry require an estimation of the MAP infection prevalence and a quantification of the losses that can be attributed to MAP infection. Clinical effects of paratuberculosis are well documented (6). However, due to the long incubation period of MAP, very few cattle show clinical signs of paratuberculosis (diarrhea) before being culled (7). While the productivity costs due to clinical paratuberculosis are significant for that animal and can be observed by the producer, the costs due to subclinical paratuberculosis may be more devastating due to the effects occurring on a larger number of cattle. Therefore, the costs due to subclinical paratuberculosis may be far more damaging at the herd and industry levels.
The following explanations provide rationales for some of the numbers used in this study. Regarding milk production effects of MAP seropositivity, a 6% reduction in milk volume in the second to last lactation and a 16% reduction in the final lactation prior to culling were reported in histopathologically positive, subclinically infected cows compared with culled cows without histopathological evidence of MAP infection (6). Similar results have been reported elsewhere, with a 15% (835 kg) reduction in mean annual milk yield in fecal-culture positive, subclinically infected cows compared with fecal-culture negative cows (8). A 4% (376 kg) reduction in mature equivalent milk production in ELISA-positive cows compared with ELISA-negative cows (19) has been reported. In contrast, others have reported no significant decrease in milk volume in culled, asymptomatic, fecal-culture positive or histopathologically positive cows compared with test-negative culled cows (20), or ELISA-seropositive and fecal-culture positive cows (21), compared with test-negative cows.
Some studies (21,22) have suggested that the observed reduction in milk volume may not occur across all lactations. In the largest evaluation of the effect of MAP-seroprevalence on milk production (22 665 lactations from 9834 cows in 342 Canadian dairy herds), an interaction was found between MAP seropositivity and lactation number (9), with a statistically significant reduction in milk yield being observed only in 4th or higher lactation animals. Therefore, in our study, we incorporated a milk volume effect, but only for 4 + lactation, MAP-seropositive cows.
There is limited literature on the reduced slaughter value of cattle seropositive for MAP. For Holstein cows that were clinically affected by JD in the Netherlands, the reported slaughter value of infected cows was 30% lower than the usual slaughter value (6). A 10% increase in proportion of cows positive per herd for paratuberculosis was associated with a 33.4 kg decrease in mean weight of culled cows in 121 dairy herds in Michigan, USA (17). However, there has been considerable research regarding what proportion of MAP-infected cattle are prematurely culled. Internationally, a higher cull rate has been reported in MAP fecal-culture positive cows than in negative cows in a 210-cow Holstein herd in New York (22). In another 900-cow Guernsey herd with subclinically infected cows, 22.6% of fecal-culture positive cows and 3.6% of fecal-culture negative cows were reported culled due to mastitis, and 68.8% of fecal-culture positive versus 60.2% of fecal-culture negative were culled due to infertility, respectively (23). The hazard of culling for MAP-seropositive cows in 134 Canadian dairy herds was 1.38 (95% C.I., 1.05 to 1.81) times that of MAP-seronegative cows (7). These Canadian numbers were deemed more representative of the Canadian dairy industry and, therefore, were utilized in our study.
Regarding reproductive losses, in a sample of 533 animals from 7 dairy herds in Michigan, USA, ELISA-positive cows had a 27.9-day increase in calving interval compared with ELISA-negative cows (11). Because this reproductive loss was the average loss for all ELISA-positive cows, there was no need to determine the proportion of infected cattle undergoing this loss in the partial-budget model. Reported costs of increased days open range from US $2.00 to $5.00/d (24,25), which was converted to CD $2.5 to $6.25/d by using an exchange rate of 1.25, an approximation of the appropriate exchange rate for the study data at that time. A Canadian estimate of the cost of a day open was $4.7 (26), suggesting that our range was likely appropriate.
This paper includes major updates on previous results regarding the effects of MAP infection in Maritime Canadian dairy herds (mean loss of $2472) (14). The methodology in that study was based on a deterministic spreadsheet model (16), used sero-prevalence data only from the 3 Maritime provinces of Canada collected in 1998 (27,28), and used production effects of MAP seropositivity based on literature sources outside Canada. In the current study, estimates of milk losses and risk of culling associated with subclinical MAP infections were based on the newly developed Canadian seroprevalence data based on 8 of the 10 provinces in Canada. Furthermore, the partial-budget model used previously was revised to be almost fully stochastic in nature, allowing us to incorporate the variability of the input estimates into the model. From the current study, an average infected herd would have a mean economic loss of $2992 annually. By comparison, in the deterministic partial-budget model of infectious diseases in dairy cattle in the Maritime provinces of Canada, total annual herd costs associated with seropositivity for MAP, bovine viral diarrhea virus, Neospora caninum, and bovine leukosis virus were $2472, $2421, $2304, and $806, respectively (14).
In herds with MAP-seropositive cows, our current results suggested a range (95% C.I.) of $2 to $160/cow/y (mean was $49/cow/y) in costs. In the USA, a similar range (90% C.I.) of US $2 to $120/cow/y was reported in MAP-seropositive dairy herds (in which < 10% of cows were culled due to clinical signs similar to JD) (15). Those American estimates can be considered as similar to our results, because more than 90% of MAP-seropositive herds in our database had < 10% of cows culled due to clinical signs representative of JD (unpublished data).
Our results also suggested that the annual cost estimates due to subclinical MAP infection ranged (95% C.I.) from $197 to $613/infected cow (mean was $385/infected cow/y). In a similar American study, utilizing similar reduced milk yields and replacement costs, MAP infection costs ranged from US $123 to $696/subclinically infected cow/y (15).
The herd-level cost estimates due to subclinical MAP infection from our study should be considered as conservative estimates. The effects of subclinical MAP infection on the susceptibility of dairy cattle to other diseases have not been determined (9). Also, the low sensitivity of the current ELISA tests to identify all subclinically infected cattle (29–31) leads to substantial misclassification and underestimation of the prevalence of infection. However, because the estimation of MAP infection costs were based mostly on the ELISA results, the economic costs to individual animals may be close to accurate. Cows that are ELISA positive are more likely to be in the later stages of MAP infection (32). The impact of infection on animals in the early stages of MAP infection (when they are ELISA negative) is likely small or null. Inclusion of cows in the early stage of MAP infection might increase the herd-level costs attributable to infection, but it could lower the average cow-level costs of infection, because inclusion of infected cows with small or no effects would lower the average for cow-level costs.
Another reason why our estimates of economic impact of MAP seropositivity were likely conservative in nature would be the small number of test-positive cows in the Canadian study investigating milk effects of MAP seropositivity (9). This low number (3.1%) resulted in reduced power to detect milk productivity impacts within different lactations due to the limited number of MAP-seropositive cows within each of the lactation categories, especially with the substantial misdiagnosis described above. The Canadian study on culling effects of MAP seropositivity also had limited power to detect an association between MAP-seropositivity and specific reasons for culling due to the limited number of culled MAP-seropositive cows within each of the categories for reasons of culling (7).
Mitigating this conservative estimate somewhat was the utilization of “normal” replacement cost and slaughter value of a healthy cow from the year before the discovery of bovine spongiform encephalopathy in Canada. The period during which the Canada-US border was closed to the crossing of live cattle resulted in much lower prices for replacements and culled cattle.
Furthermore, the mortality risk among herds positive for MAP-infection was assumed to be 3% higher than that of negative herds. However, most MAP-infected cows are culled before they develop clinical signs (7); therefore, the mortality costs due to MAP infection utilized in our study may be overestimated. However, due to the lack of any other published estimate of MAP’s impact on mortality risk, we were not able to adjust for this possible overestimation. It is possible that farms with clinical cases of JD could have substantially higher economic costs from MAP infection, particularly if there is a higher prevalence of infection on the farm. For example, in 2002, Hendrick et al (33) examined the effect of paratuberculosis on culling, milk production, and milk quality in 9 dairy herds in Ontario that had a reported clinical case of JD. Of the tested cattle (of all ages), 19% were seropositive on ELISA, whereas in our study, only 3.1% of tested cattle were seropositive. Therefore, estimates of productivity impacts from cattle on those farms from Ontario would be higher than those from the average MAP-seropositive farm in Canada. Mortality was not examined in that study (33).
Future research should focus on more detailed estimates of impacts on productivity and culling from representative farms in order to develop more precise estimates of direct losses. Monitoring farms enrolled in JD control programs could provide such additional economic data for this purpose.
In conclusion, the mean annual production costs for an average Canadian dairy herd with 12.7% of 61 cows seropositive for MAP totalled $2992. Culling losses were responsible for 46% ($1374) of the total losses attributable to MAP status, with decreased milk production, mortality, and reproductive losses accounting for the remainder of the losses. MAP seroprevalence was the most influential parameter in the sensitivity analysis. Mycobacterium avium subspecies paratuberculosis seropositive dairy farms sustain substantial, although somewhat variable, economic costs associated with this infection.
Footnotes
This research was made possible by the following funding sources (monetary or in-kind contributions): the Food Safety Division of Alberta Agriculture, Food and Rural Development, Atlantic Veterinary College, Canadian Food Inspection Agency, Canadian Dairy Herd Management Services, Dairy Farmers of Canada, Dairy Farmers of PEI, Dairy Herd Improvement companies, Fédération des Producteurs de Lait du Québec, Dairy Farmers of Manitoba, Manitoba Agriculture and Food, PEI Agricultural Research Investment Fund, Production Limiting Diseases Committee, Saskatchewan Agriculture, Food and Rural Revitalization, and the Western Economic Partnership Agreement.
Author contributions
Drs. Dohoo, Keefe, Tiwari, and VanLeeuwen were involved in the data collection, data analysis, interpretation of results, and writing of the manuscript. Dr. Weersink was involved in the data analysis, interpretation of results, and writing of the manuscript. CVJ
References
- 1.US Department of Agriculture, Animal and Plant Health Inspection Service, 1997. Johne’s Disease on US Dairy Operations, NAHMS Dairy ’96. Center for Animal Health Monitoring. 1997; Report N245,797.
- 2.Boelaert F, Walravens K, Biront P, Vermeersch JP, Berkvens D, Godfroid J. Prevalence of paratuberculosis (Johne’s disease) in the Belgian cattle population. Vet Microbiol. 2000;77:269–281. doi: 10.1016/s0378-1135(00)00312-6. [DOI] [PubMed] [Google Scholar]
- 3.Gasteiner J, Wenzl H, Fuchs K, Jark U, Baumgartner W. Serological cross-sectional study of paratuberculosis in cattle in Austria. Zentralbl Veterinarmed B. 1999;46:457–466. doi: 10.1046/j.1439-0450.1999.00256.x. [DOI] [PubMed] [Google Scholar]
- 4.Muskens J, Barkema HW, Russchen E, Van Maanen K, Schukken YH, Bakker D. Prevalence and regional distribution of paratuberculosis in dairy herds in The Netherlands. Vet Microbiol. 2000;77:253–261. doi: 10.1016/s0378-1135(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari A. Charlottetown, Prince Edward Island: University of Prince Edward Island; 2005. Seroprevalence, production impacts, economics, and risk factors of Mycobacterium avium subspecies paratuberculosis in Canadian dairy cattle [PhD dissertation] [Google Scholar]
- 6.Benedictus G, Dijkhuizen AA, Stelwagen J. Economic losses due to paratuberculosis in dairy cattle. Vet Rec. 1987;121:142–146. doi: 10.1136/vr.121.7.142. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari A, VanLeeuwen JA, Dohoo IR, Stryhn H, Keefe GP, Haddad JP. Effects of seropositivity for bovine leukemia virus, bovine viral diarrhoea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum on culling in dairy cattle in four Canadian provinces. Vet Microbiol. 2005;109:147–158. doi: 10.1016/j.vetmic.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Abbas B, Riemann HP, Hird DW. Diagnosis of Johne’s disease (para-tuberculosis) in Northern California cattle and a note of it’s economic significance. Calif Vet. 1983;37:20–24. [Google Scholar]
- 9.Tiwari A, VanLeeuwen JA, Haddad JP, et al. Production effects of seropositivity of pathogens causing bovine leucosis, bovine viral diarrhea, paratuberculosis, and neosporosis. J Dairy Sci. 2007;90:659–669. doi: 10.3168/jds.S0022-0302(07)71548-5. [DOI] [PubMed] [Google Scholar]
- 10.Kreeger JM. Ruminant paratuberculosis — a century of progress and frustration. J Vet Diagn Invest. 1991;3:373–382. doi: 10.1177/104063879100300425. [DOI] [PubMed] [Google Scholar]
- 11.Johnson-Ifearulundu YJ, Kaneene JB, Sprecher DJ, Gardiner JC, Lloyd JW. The effect of subclinical Mycobacterium paratuberculosis infection on days open in Michigan, USA, dairy cows. Prev Vet Med. 2000;46:171–181. doi: 10.1016/s0167-5877(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari A, VanLeeuwen JA, Dohoo IR, et al. Management risk factors associated with Mycobacterium avium subspecies paratuberculosis in Canadian dairy herds. Prev Vet Med. 2008 doi: 10.1016/j.prevetmed.2008.06.019. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne’s disease): The current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 14.Chi J, VanLeeuwen JA, Weersink A, Keefe GP. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev Vet Med. 2002;55:137–153. doi: 10.1016/s0167-5877(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 15.Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev Vet Med. 1999;40:179–192. doi: 10.1016/s0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 16.Bennett R, Christiansen K, Clifton-Hadley R. Preliminary estimates of the direct costs associated with endemic diseases of livestock in Great Britain. Prev Vet Med. 1999;39:155–171. doi: 10.1016/s0167-5877(99)00003-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnson-Ifearulundu Y, Kaneene JB, Lloyd JW. Herd-level economic analysis of the impact of paratuberculosis on dairy herds. J Am Vet Med Assoc. 1999;214:822–825. [PubMed] [Google Scholar]
- 18.CDIC — Canadian Dairy Information Centre [page on the Internet] [Last accessed 20/02/2008];Publications and Bulletins, Annual Publications, Statistics of the Canadian Dairy Industry. 2005 Available from http://www.dairyinfo.gc.ca/pdf/publication2005.pdf.
- 19.Nordlund KV, Goodger WJ, Pelletier J, Collins MT. Associations between subclinical paratuberculosis and milk production, milk components, and somatic cell counts in dairy herds. J Am Vet Med Assoc. 1996;208:1872–1876. [PubMed] [Google Scholar]
- 20.Buergelt CD, Duncan JR. Age and milk production data of cattle culled from a dairy herd with paratuberculosis. J Am Vet Med Assoc. 1978;173:478–480. [PubMed] [Google Scholar]
- 21.Johnson YJ, Kaneene JB, Gardiner JC, Lloyd JW, Sprecher DJ, Coe PH. The effect of subclinical Mycobacterium paratuberculosis infection on milk production in Michigan dairy cows. J Dairy Sci. 2001;84:2188–2194. doi: 10.3168/jds.S0022-0302(01)74665-6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DJ, Rossiter C, Han HR, Sears PM. Association of Mycobacterium paratuberculosis infection with reduced mastitis, but with decreased milk production and increased cull rate in clinically normal dairy cows. Am J Vet Res. 1993;54:1851–1857. [PubMed] [Google Scholar]
- 23.Merkal RS, Larsen AB, Booth GD. Analysis of the effect of inapparent bovine paratuberculosis. Am J Vet Res. 1975;36:837–838. [PubMed] [Google Scholar]
- 24.De Vries A. Economic value of pregnancy in dairy cattle. J Dairy Sci. 2006;80:3876–3885. doi: 10.3168/jds.S0022-0302(06)72430-4. [DOI] [PubMed] [Google Scholar]
- 25.Holmann FJ, Shumway CR, Blake RW, Schwart RB, Sudweeks EM. Economic value of days open for Holstein cows of alternative milk yields. J Dairy Sci. 1984;67:636. [Google Scholar]
- 26.Plaizier JC, King GJ, Dekkers JC, Lissemore K. Estimation of economic values of indices for reproductive performance in dairy herds using computer simulation. J Dairy Sci. 1997;80:2775–2783. doi: 10.3168/jds.S0022-0302(97)76240-4. [DOI] [PubMed] [Google Scholar]
- 27.VanLeeuwen JA, Keefe GP, Tremblay R, Power C, Wichtel JJ. Seroprevalence of infection with Mycobacterium avium subspecies paratuberculosis, bovine leukemia virus, and bovine viral diarrhea virus in maritime Canada dairy cattle. Can Vet J. 2001;42:193–198. [PMC free article] [PubMed] [Google Scholar]
- 28.Keefe GP, VanLeeuwen JA. Neospora then and now: prevalence of Neospora caninum in Maritime Canada in 1979, 1989, and 1998. Can Vet J. 2000;41:864–866. [PMC free article] [PubMed] [Google Scholar]
- 29.McKenna SLB, Keefe GP, Barkema HW, Sockett DC. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet Microbiol. 2005;110:105–111. doi: 10.1016/j.vetmic.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Dargatz DA, Byrum BA, Barber LK, et al. Evaluation of a commercial ELISA for diagnosis of paratuberculosis in cattle. J Am Vet Med Assoc. 2001;218:1163–1166. doi: 10.2460/javma.2001.218.1163. [DOI] [PubMed] [Google Scholar]
- 31.Sockett DC, Carr DJ, Collins MT. Evaluation of conventional and radiometric fecal culture and a commercial DNA probe for diagnosis of Mycobacterium paratuberculosis infections in cattle. Can J Vet Res. 1992;56:148–153. [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney RW, Whitlock RH, Buckley CL, Spencer PA. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J Vet Diagn Invest. 1995;7:488–493. doi: 10.1177/104063879500700411. [DOI] [PubMed] [Google Scholar]
- 33.Hendrick SH, Kelton DF, Leslie KE, Lissemore KD, Archambault M, Duffield TF. Effect of paratuberculosis on culling, milk production, and milk quality in dairy herds. J Am Vet Med Assoc. 2005;227:1302–1308. doi: 10.2460/javma.2005.227.1302. [DOI] [PubMed] [Google Scholar]