Abstract
The transcriptional coactivator MN1 has been identified as a gene overexpressed in certain types of human acute myeloid leukemia. Upregulation is invariantly associated with inv(16) AML but is also found in other AML subtypes. Overexpression of this gene is also associated with a worse prognosis and a shorter survival in AML patients with a normal karyotype. In this short review I will discuss the role of MN1 in myeloid leukemia.
MN1 is a gene highly conserved among vertebrates
Although many genes are involved in human acute myeloid leukemia, few seem more efficient in triggering malignant myeloid disease than the transcriptional coactivator MN1. The MN1 gene has been identified as the target of a sporadic balanced t(4;22) in a patient with meningioma. Therefore the gene was thought to be a candidate for the meningioma tumor suppressor gene on chromosome 22[1], although its relation to meningioma remains unresolved. The encoded protein of 136 kDa is highly conserved among vertebrates but shows no homologies to other proteins. Its sequence suggested a role in transcription[1], which was confirmed by the observation that it activates transcription of the moloney sarcoma virus long terminal repeat (MSV-LTR) in transient transcription assays and the protein comprises several transactivating domains[2]. MN1 activates transcription of the MSV-LTR via the nuclear receptor dimers RAR-RXR binding to direct repeat sequences (DR5) in the LTR; it interacts with RAR-RXR most probably via the protein intermediates p300 and RAC3 (also known as nuclear receptor coactivator 3, NCOA3)[3]. Like MN1, p300 and RAC3 are transcription coactivators[4,5], and coexpression of MN1 with p300 or RAC3 synergistically activates the transcriptional activity of RAR-RXR dimers in the presence of retinoic acid[3]. MN1’s co-activation activity is not restricted to the RAR-RXR nuclear receptor, as MN1 expression inhibits proliferation of an osteoblast cell line via coactivation of the vitamin D receptor[6]. Inhibition of growth of epithelial cell proliferation, is also associated with induction of MN1 expression[7,8]. MN1 can also bind to a transcription factor, which recognizes the CACCCAC sequence that together with MN1 transactivates transcription of the IGFBP5 promoter[9], but the identity of this transcription factor has not been determined yet.
MN1 is the target of a recurrent chromosomal translocation in human AML
MN1 is the target of the balanced chromosome translocation t(12;22)(p13;q12) associated with human myeloid disease, including acute myeloid leukemia (AML), myelodysplasia (MDS), and chronic myeloid leukemia (CML)[10]. The translocation encodes a fusion protein, MN1-TEL, consisting of almost the entire MN1 open reading frame fused to the ETS transcription factor TEL (ETV6). We showed that MN1-TEL has weak transforming activity in NIH3T3 fibroblasts, in which this activity depends on DNA binding via TEL’s ETS domain and on the presence of the N-terminal 500 amino acids (aa) of MN1[2]. By generating a conditional MN1-TEL knock-in mouse, we showed that MN1-TEL is a hematopoietic oncogene. Its expression in both the myeloid and lymphoid compartments in these mice resulted in T-cell lymphoma as well as AML, depending on the nature of the cooperating mutations[11,12]. Overexpression of MN1-TEL in mouse bone marrow (BM) cells also generated myeloid cell lines, an activity dependent on MN1’s N-terminal 500 amino acids but not on the presence of an intact TEL DNA binding domain[13].
The connection of MN1 with myeloid malignancy goes beyond MN1’s involvement in the t(12;22): it is also overexpressed in inv(16) AML[14,15], a core binding factor (CBF) leukemia in which the fusion protein CBFb-MYH11 is expressed[16]. In addition, MN1 is overexpressed[15] in myeloid leukemia in which the immortalizing transcription factor EVI1 is overexpressed[17], an AML subtype with a poor prognosis[18] and in some adult AMLs without karyotypic abnormalities[19]. In the latter case overexpression of MN1 was associated with a worse prognosis and a shorter survival rate[19]. Together these data suggested that upregulation of MN1 contributes to these malignancies.
MN1 overexpression induces myeloid malignancy in mice
To test this possibility we showed that mice receiving transplants of BM overexpressing MN1 rapidly developed a malignant myeloid disease that was readily transplantable to secondary recipients[20]. The peripheral blood contained large numbers of granulocytes and granulocyte progenitors and few blast-like cells, whereas the BM contained more than 20% blasts. Heuser and coworkers[21] reported similar results. Combined these 2 reports suggested that MN1 is a highly effective myeloid oncoprotein. Also, mice receiving transplants of BM of CBFb-MYH11 knock-in chimeric mice overexpressing MN1 developed AML[20], whereas CBFb-MYH11 knock-in chimeric mice do not[16]. Malignant cells of a primitive phenotype (cKit+) in BM and peripheral blood expressed both MN1 and CBFb-MYH11 in the affected transplanted mice. This suggests that in comparison with MN1-induced myeloid disease the combination of the growth promoting MN1 protein with the differentiation inhibiting CBFb-MYH11 changed the phenotype of the disease, although the latency was longer. This latter might be caused by the growth inhibiting effect of CBFb-MYH11[22]. Analysis of MN1 expression in a group of 41 AML patients by Q-RT-PCR of all subtypes (FAB subtypes (M0–M7) suggested that in approximately 50% of patient samples MN1 expression is 2–3 fold higher than in normal BM. But significant over expression was found in all inv16 patient samples and in M1-AML patient samples[20].
MN1 expression in normal BM
Given the detrimental hyperproliferative effects of MN1 overexpression on myelopoiesis, it was unknown which progenitors in normal BM express this gene. Quantitative-RT-PCR analysis of Mn1 expression in sorted fractions representing the HSC, CMP, CLP, GMP, and MEP of mouse BM showed that the gene is expressed at a low level in the HSC but not in the CMP, CLP or MEP[20]. However, strong upregulation of Mn1 was found in the GMP, suggesting a specific role for Mn1 in GMP-derived myelopoiesis. Although an Mn1 knockout mouse has been generated[23], the authors noted defects in the development of membranous bones of the cranial skeleton causing perinatal death, but they did not analyze the hematopoietic system of Mn1 −/− mice. In collaboration with Dr. Zwarthoff we are currently addressing the effects of loss of Mn1 expression on hematopoiesis. Because overexpression of MN1 induces proliferation of myeloid cells[20], it is hardly a leap of faith to speculate that inactivation of Mn1 may have the opposite effect. Increased proliferation of MN1 overexpressing cells could be a combination of the observed increased cell cycle traverse[20] and inhibition of differentiation. Indication of the latter comes from data published by Heuser et al.[21] suggesting that MN1 overexpression inhibits ATRA (all-trans retinoic acid) induced differentiation of mouse myeloid cells. The combination of these 2 effects would keep GMP-derived cells longer in a proliferation-competent state in which they also cycle faster than normal GMP-derived cells.
Consequences of MN1 overexpression in human AML
As already mentioned, Heuser and coworkers[19] discovered that AML patients overexpressing MN1 without karyotypic abnormalities had a worse prognosis and a shorter survival rate. Our work strongly suggests that overexpression of MN1 in inv16 AML is important for the growth of CBFb-MYH11 expressing cells. In addition, elderly patients with AML (except those with M3-AML) who expressed low levels of MN1 and received ATRA fared significantly better than those who did not receive ATRA or patients who expressed high levels of MN1 and did or did not receive ATRA[21]. Together these data suggest a worsening of disease phenotype when MN1 is overexpressed, which would make this protein a worthwhile therapeutic target for the treatment of AML.
How does MN1 work?
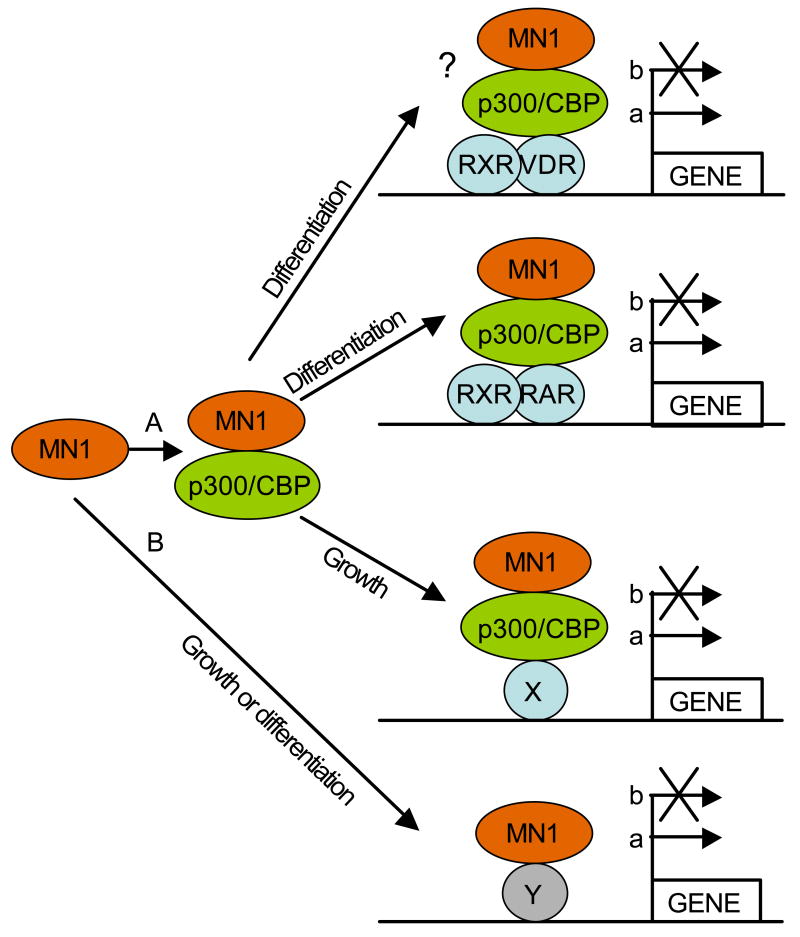
Several experiments have shown that MN1 functions as a transcriptional coactivator and appears not to bind DNA directly[3,6,9]. Thus, its output is relayed via other transcription factors, which may be contacted directly or indirectly. To date, only indirect interaction via p300/CBP and RAC3 to RAR/RXR has been well documented[3] and might elicit inhibition of differentiation of myeloid cells[21]. Given that MN1 can also regulate the activity of the VDR[6] and the fact that this nuclear receptor plays a role in monocyte/macrophage differentiation[24], it is possible that MN1 is also recruited to the VDR via p300/CBP in myeloid cells, although experimental evidence for that is currently lacking. MN1’s binding to p300/CBP makes it difficult to predict via which other transcription factor(s) MN1 relays its cell proliferative effects as many transcription factors that regulate myeloid cell growth and differentiation recruit p300/CBP including Aml1, Gata1, 2, and 3, cMyb, C/EBPα, and Pu1 (reviewed in Blobel, 2000, 2002)[25,26]. The observation that MN1 can also mediate transcriptional upregulation via CACCC-rich DNA sequences[9] is a clear indication that it also targets other transcription factors than nuclear receptors. The possible interactions of MN1 with transcription factors via p300/CBP or not are depicted in Figure 1.
Figure 1.
Model for MN1 function in myeloid cells. A) Via p300/CBP MN1 is recruited to, and regulates the transcriptional activity of RAR/RXR and VDR/RXR heterodimers affecting the differentiation of myeloid progenitors via up- (a), or downregulation (b) of target genes. MN1 can also be recruited to other transcription factors (X) that bind p300/CBP, again affecting transcription of target genes. B) MN1 might also bind directly to transcription factors (Y), affecting the transcription of target genes. The interaction with Y might also be indirect, i.e. via another interacting protein than p300/CBP (not indicated).
A proteomics approach involving isolation and identification of MN1-containing transcription factor complexes in myeloid cells overexpressing MN1 might be one of the experimental approaches that will shed light on the identity of the crucial transcription factor targets of MN1 in myeloid cells. Extensive array analysis of myeloid cells overexpressing MN1 compared with wild type myeloid cells might be helpful in determining the crucial target genes involved in the growth phenotype. Together with the analysis of the hematopoietic phenotype of Mn1 knockout mice, these studies should provide extensive insight into the effects of Mn1 on myelopoiesis and into the mechanisms with which Mn1 promotes the proliferation of myeloid progenitors. This knowledge might eventually be used to design specific inhibitors for MN1, which should be beneficial for the treatment of leukemias that overexpress this protein.
Acknowledgments
This paper is based on a presentation at the Seventh International Workshop on Molecular Aspects of Myeloid Stem Cell Development and Leukemia in Annapolis, Maryland May 13–16, 2007, sponsored by The Leukemia & Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lekanne Deprez RH, Riegman PH, Groen NA, et al. Cloning and characterization of MN1, a gene from chromosome 22q11, which is disrupted by a balanced translocation in a meningioma 8. Oncogene. 1995;10:1521–1528. [PubMed] [Google Scholar]
- 2.Buijs A, van Rompaey L, Molijn AC, et al. The MN1-TEL fusion protein, encoded by the translocation (12;22)(p13;q11) in myeloid leukemia, is a transcription factor with transforming activity 24. Mol Cell Biol. 2000;20:9281–9293. doi: 10.1128/mcb.20.24.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Wely KH, Molijn AC, Buijs A, et al. The MN1 oncoprotein synergizes with coactivators RAC3 and p300 in RAR-RXR-mediated transcription 5. Oncogene. 2003;22:699–709. doi: 10.1038/sj.onc.1206124. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Fisher RJ, Riggs CW, et al. Inhibition of vascular endothelial growth factor-induced endothelial cell migration by ETS1 antisense oligonucleotides 10. Cancer Res. 1997;57:2013–2019. [PubMed] [Google Scholar]
- 5.Eckner R, Ewen ME, Newsome D, et al. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 6.Sutton AL, Zhang X, Ellison TI, Macdonald PN. The 1,25(OH)2D3-regulated transcription factor MN1 stimulates vitamin D receptor-mediated transcription and inhibits osteoblastic cell proliferation 9. Mol Endocrinol. 2005;19:2234–2244. doi: 10.1210/me.2005-0081. [DOI] [PubMed] [Google Scholar]
- 7.Chen CR, Kang Y, Massague J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor beta growth arrest program 3. Proc Natl Acad Sci U S A. 2001;98:992–999. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells 4. Mol Cell. 2003;11:915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 9.Meester-Smoor MA, Molijn AC, Zhao Y, et al. The MN1 oncoprotein activates transcription of the IGFBP5 promoter through a CACCC-rich consensus sequence. J Mol Endocrinol. 2007;38:113–125. doi: 10.1677/jme.1.02110. [DOI] [PubMed] [Google Scholar]
- 10.Buijs A, Sherr S, van Baal S, et al. Translocation (12;22)(p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11. Oncogene. 1995;10:1511–1519. [PubMed] [Google Scholar]
- 11.Kawagoe H, Grosveld GC. Conditional MN1-TEL knock-in mice develop acute myeloid leukemia in conjunction with overexpression of HOXA9 13. Blood. 2005;106:4269–4277. doi: 10.1182/blood-2005-04-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawagoe H, Grosveld GC. MN1-TEL myeloid oncoprotein expressed in multipotent progenitors perturbs both myeloid and lymphoid growth and causes T-lymphoid tumors in mice 13. Blood. 2005;106:4278–4286. doi: 10.1182/blood-2005-04-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carella C, Potter M, Bonten J, et al. The ETS factor TEL2 is a hematopoietic oncoprotein 3. Blood. 2006;107:1124–1132. doi: 10.1182/blood-2005-03-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross ME, Mahfouz R, Onciu M, et al. Gene expression profiling of pediatric acute myelogenous leukemia 12. Blood. 2004;104:3679–3687. doi: 10.1182/blood-2004-03-1154. [DOI] [PubMed] [Google Scholar]
- 15.Valk PJ, Verhaak RG, Beijen MA, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia 16. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 16.Liu PP, Hajra A, Wijmenga C, Collins FS. Molecular pathogenesis of the chromosome 16 inversion in the M4Eo subtype of acute myeloid leukemia 9. Blood. 1995;85:2289–2302. [PubMed] [Google Scholar]
- 17.Du Y, Jenkins NA, Copeland NG. Insertional mutagenesis identifies genes that promote the immortalization of primary bone marrow progenitor cells. Blood. 2005 doi: 10.1182/blood-2005-03-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, van Putten WL, et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients 3. Blood. 2003;101:837–845. doi: 10.1182/blood-2002-05-1459. [DOI] [PubMed] [Google Scholar]
- 19.Heuser M, Beutel G, Krauter J, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006 doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 20.Carella C, Bonten J, Sirma S, et al. MN1 overexpression is an important step in the development of inv(16) AML. Leukemia. 2007 doi: 10.1038/sj.leu.2404778. [DOI] [PubMed] [Google Scholar]
- 21.Heuser M, Argiropoulos B, Kuchenbauer F, et al. MN1 overexpression induces acute myeloid leukemia in mice and predicts ATRA resistance in AML patients. Blood. 2007 doi: 10.1182/blood-2007-03-080523. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YH, Landrette SF, Heilman SA, et al. Cbf beta-SMMHC induces distinct abnormal myeloid progenitors able to develop acute myeloid leukemia 1. Cancer Cell. 2006;9:57–68. doi: 10.1016/j.ccr.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Meester-Smoor MA, Vermeij M, van Helmond MJ, et al. Targeted disruption of the Mn1 oncogene results in severe defects in development of membranous bones of the cranial skeleton 10. Mol Cell Biol. 2005;25:4229–4236. doi: 10.1128/MCB.25.10.4229-4236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Kelly J, Hisatake J, Hisatake Y, et al. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091–1099. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blobel GA. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood. 2000;95:745–755. [PubMed] [Google Scholar]
- 26.Blobel GA. CBP and p300: versatile coregulators with important roles in hematopoietic gene expression. J Leukoc Biol. 2002;71:545–556. [PubMed] [Google Scholar]