Abstract
Over the past few decades, the average age at time of spinal cord injury (SCI) has increased. Here we examined locomotor recovery and myelin pathology in both young and aged adult rats following contusion SCI. Our assessment indicates that the rate of locomotor recovery following SCI is significantly delayed in aged rats as compared to young rats, and is associated with a greater degree of pathology and demyelination. Additionally, we examined the effect of voluntary exercise, pre- and post-injury, on locomotor recovery and myelin pathology following contusion SCI. Our data indicate that exercise improves the locomotor recovery of injured aged rats such that it is comparable to the recovery rate of injured young rats, and is associated with a decreased area of pathology and amount of demyelination. Interestingly, the rate of locomotor recovery and myelin pathology in the aged exercised rats was similar to that of the young sedentary rats after injury, indicating that exercise attenuates the delayed recovery of function and associated histopathology in aged rats. These data indicate that there is an age-related delay in locomotor recovery following SCI, and an age-related increase in histopathology following SCI. Importantly, our data indicate that exercise attenuates these age-related deficits following SCI.
Keywords: demyelination, remyelination, spinal cord injury, aging, voluntary exercise
Introduction
There are approximately 11,000 new spinal cord injury (SCI) cases reported every year in the United States. SCI primarily affects young adults, with most injuries occurring between the ages of 16 and 30. Currently, the average age of injury is 38, which is approximately 8 years higher than the average age of injury in the 1970’s. Additionally, the percentage of injuries in persons over the age of 60 has recently increased from 4.7% prior to 1980 to 10.9% since 2000 (NSCISC, 2004).
Behavioral and pathological outcomes following SCI may be influenced by age at time of injury. Gwak and colleagues (2004) demonstrated that aged rats exhibit slower locomotor and somatosensory recovery following hemisection SCI as compared to young rats. More recently, Genovese et alias (2006) demonstated that aged male rats have significantly greater locomotor deficits, pathology, and mortality following clip compression SCI. Several studies suggest that there are age-associated differences in myelin integrity and spontaneous remyelination that may contribute to these differences in behavioral recovery (Hinks and Franklin, 2000; Kovari et al., 2004; Peters and Sethares, 2003; Shields et al., 1999). Shields and colleagues (1999) demonstrated that aged rats remyelinate at a slower rate than young rats following toxin-induced demyelination of the spinal cord. The effect of age at time of injury on behavioral recovery and histopathology following contusion SCI remains to be elucidated.
Recently, exercise has been shown to influence the outcome of CNS injury. Physical activity has been shown to, 1) increase levels of IGF-1 and BDNF which are important in oligodendrocyte and neuron cell survival (Gomez-Pinilla et al., 2002; Schwarz et al., 1996; Trejo et al., 2001), 2) improve locomotor recovery following SCI (Edgerton et al., 2001; Engesser-Cesar et al., 2005; Engesser-Cesar et al., 2007; Multon et al., 2003), and 3) decrease susceptibility to free-radical damage, which has implications for neuroprotection (Ang et al., 2003; Nistico et al., 1992). Additionally, it has been demonstrated that physical activity is associated with a reduced risk of age-related dementia and cognitive decline (Laurin et al., 2001), the latter being associated with age-related demyelination (Brickman et al., 2006; Kovari et al., 2004).
Here we document an age-related delay in the rate of locomotor recovery following contusion SCI. Additionally, we demonstrate that aged rats have greater region of pathology and more demyelination following contusion SCI than young rats. Aged rats that engaged in voluntary wheel running prior to and following SCI had locomotor recovery rates comparable to that of young rats. Voluntary wheel running also decreased the region of pathology and amount of demyelination following contusion SCI in aged rats. Thus, we demonstrate for the first time that long term voluntary wheel running attenuates age-related locomotor and histopathological deficits following contusion SCI.
Materials and Methods
Experimental Groups
Young adult (n=12; 350–500 g; 6–8 weeks old) and aged adult (n=18; 600–800 g; 12 months old) male Sprague-Dawley rats were individually housed with ad libitum access to food and water in a 12:12 hour light/dark vivarium. Some aged adult animals (n=12) were given the opportunity to exercise, being housed in cages with freely accessible, stainless steel running wheels until they reached 12 months of age. These aged exercise animals were housed individually for one week post injury without access to a running wheel, and were given access to running wheels again starting the second week post injury. Running wheel rotations were monitored to keep track of running wheel activity. The experimental groups are summarized in Table 1.
Table 1. Experimental groups.
Young animals (2 months) were single housed prior to and post contusion SCI. Aged sedentary animals (12 months) were single housed prior to and post contusion SCI without access to a running wheel. Aged exercise animals (12 months) were single housed with ad libitum access to a running wheel prior to and post contusion SCI.
| Experimental group | Age at time of SCI | Housing | n |
|---|---|---|---|
| Young | 2 months | single | 12 |
| Aged sedentary | 12 months | single | 6 |
| Aged exercise | 12 months | single with running wheel | 12 |
Spinal Cord Injury
Spinal cord injuries were performed according to standard protocols (Keirstead et al., 2005). Animals were anesthetized with intraperitoneal injections of 80 mg/kg ketamine (Phoenix Pharmaceuticals, St. Joseph, MO) and 10 mg/kg xylazine (Phoenix Pharmaceuticals). The skin between the neck and hindlimbs, extending approximately 2 cm bilaterally from the spine, was shaved and disinfected with serial provodone and 70% ethanol scrubs. A midline incision exposed the spinal column at the level of T8–T11, and the paravertebral muscles were dissected bilaterally to visualize the transverse apophyses. A complete laminectomy was performed on the 10th thoracic vertebrae (T10). The spinal processes immediately adjacent to the laminectomy site were clamped and stabilized using a stereotactic device, and a contusion injury was induced by using the Infinite Horizon Impactor (Precision Systems and Instrumentation LLC, Fairfax, VA) with a force of 200 kDynes. Following impact, the deep and superficial muscle layers were sutured in layers over the intact dura, and the skin was closed with stainless steel wound clips. Immediately after surgery, animals were given subcutaneous saline and 2.5 mg/kg/day prophylactic enrofloxacin (Baytril; Bayer, Shawnee Mission, KS) and maintained on an isothermic pad until they were alert and mobile. Animals received manual bladder expression three times daily and were inspected for weight loss, dehydration, discomfort, and autophagia, with appropriate veterinary care as needed. All procedures were approved by the Institutional Animal Care and Use Committee at UC Irvine.
Locomotor Testing
Before injury, each animal was acclimated and scored using the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB) (Basso et al., 1995). BBB scores were analyzed by chi-squared test at each time point using the values from the young group as the expected range when testing for significance with the aged sedentary group, and using the values from the aged sedentary group as the expected range when testing for significance with the aged exercise group.
Histology
Animals were sacrificed 2 months post-injury by intracardiac perfusion with 4% glutaraldehyde (Fisher Scientific, Pittsburgh, PA) in 0.1 M phosphate buffer, pH 7.4, under anesthesia. The spinal cord was removed, and the lesion-containing length was cut into 12 1-mm transverse blocks (6 blocks cranial and 6 blocks caudal to the injury site), which were then resin embedded according to standard protocols (Keirstead and Blakemore, 1997). Transverse semithin (1µm) sections were cut from the cranial face of each resin block, stained with alkaline toluidine blue (Sigma-Aldrich, St. Louis, MO), coverslipped, and examined by light microscopy on an Olympus AX-80 microscope using Olympus MicroSuite B3SV software (Olympus America, Melville, NY).
Area of Pathology Quantification
Area of pathology was defined as any region of the spinal cord with aberrant histology. Swelling, damaged axons, hypercellularity, and gross demyelination were determining features of aberrant histology within spinal cord sections. For each 1µm transverse semithin section, areas of pathology were located by viewing sections at 4× magnification (Fig. 1a, b), and traced using the Olympus MicroSuite B3SV software to calculate area. The accuracy of tracing was checked by viewing delineations at 200× magnification. When there was more than one area of pathology within a section, the areas of pathology were summed. The total area of pathology per section was averaged across animals within each group for each 1mm transverse tissue block, yielding the estimated average area of pathology for each 1mm segment of spinal cord.
Figure 1. Voluntary wheel running improves locomotor recovery in aged animals following contusion SCI.
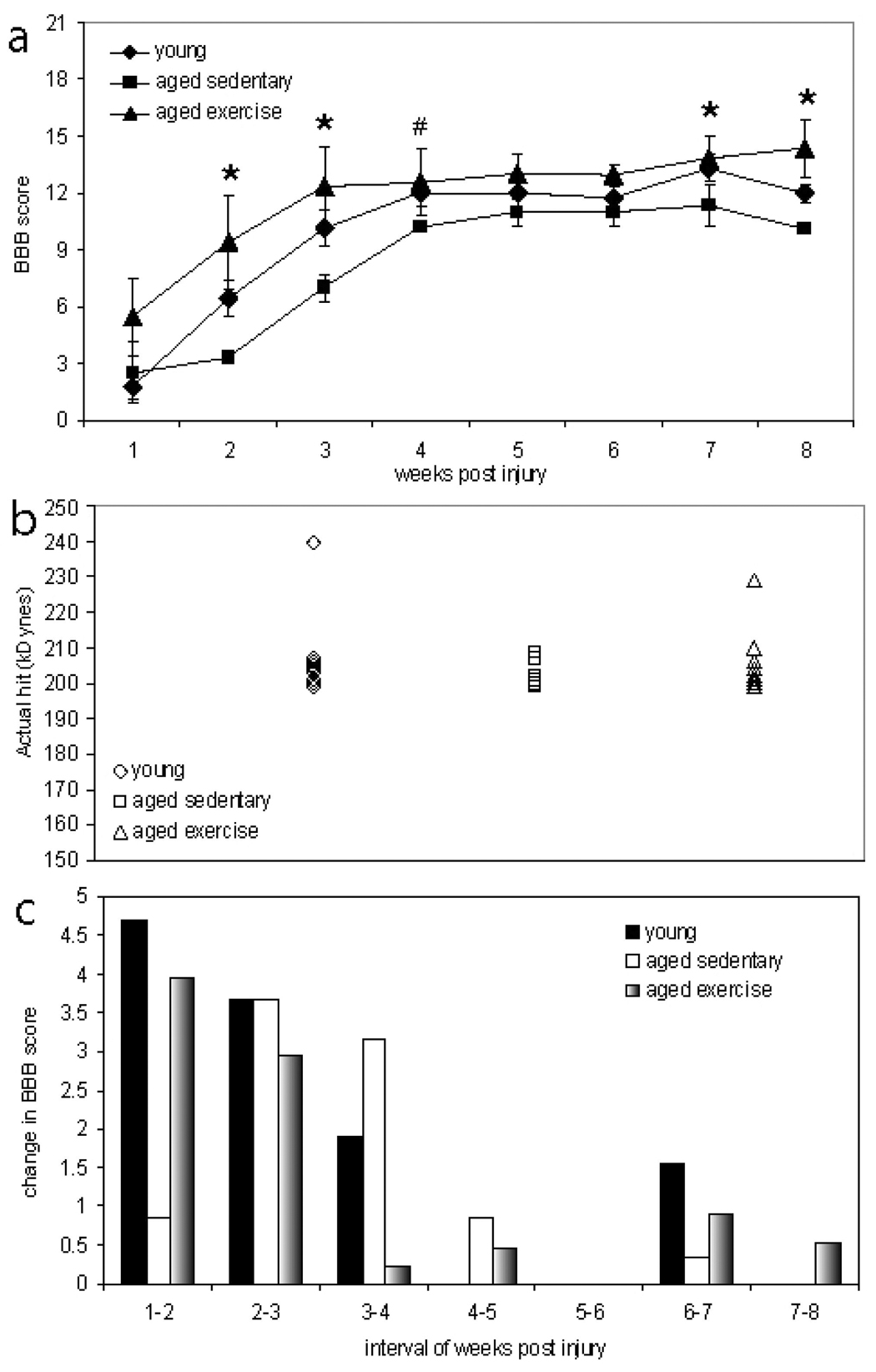
a, Aged exercise animals demonstrated significantly greater (p<0.05) locomotor capabilities at multiple times post-injury as compared to aged sedentary animals. Young animals demonstrated significantly greater (p<0.05) locomotor capabilities at 4 weeks post-injury (a). b, Actual contusion injury force for each animal produced by the IH device impactor. Group clustering demonstrates that all groups received similar force hits. c, The average change in BBB score during one week was greatest in young and aged exercise animals within the first week, gradually declining over the next couple of weeks. The greatest change in BBB score for aged sedentary animals, however, was delayed until the second week, after which the recovery decreases (c). # p<0.05 between young and aged sedentary groups; * p< 0.05 between aged sedentary and aged exercise groups.
Axon Quantification
For axon quantification, images of these regions were digitally captured at 2000× magnification, and a 25µm × 25µm (625µm2) digital grid was overlaid on the images using Olympus MicroSuite B3SV software. Demyelinated and oligodendrocyte cell remyelinated axons were counted on 5 × 625µm2 areas aligned on a radial oriented line, according to the line sampling technique outlined previously (Blight, 1993; Totoiu and Keirstead, 2005). The radial oriented line originated at the central canal and extended to the outermost edge of the spinal cord cross section through the middle of the area of pathology; in the absence of a central canal, the radial oriented line originated at the intersection of two digitally imposed lines, one line running from the outermost center point of the dorsal column to the outermost center point of the ventral column and the other line spanning the greatest mediolateral width of the spinal cord. The 625µm2 areas were then superimposed on this radial oriented line. Oligodendrocyte-remyelinated axons were identified by their characteristically thin myelin sheaths relative to the diameter of the axons (Gilson and Blakemore, 2002; Guy et al., 1989; Hildebrand and Hahn, 1978). The number of demyelinated and oligodendrocyte-remyelinated axons was determined by counting axons in each 625µm2 region, averaging the number of axons within the 625µm2 regions along the radial line. This number was then averaged across animals within each group for each 1mm transverse tissue block.
G-Ratio Analyses
G-ratios (myelin sheath thickness/axon diameter) were determined for randomly selected normally-myelinated or oligodendrocyte-remyelinated axons within the same 625µm2 areas of pathology used for axon quantification (described above). Random sampling was accomplished by measuring the G-ratio for only those axons bisected by horizontal grid lines digitally superimposed on 2000× digitally captured images. Measurements of myelin sheath thickness and axon diameter were made using Olympus MicroSuite B3SV software.
Results
General health following SCI
Two aged animals died immediately following SCI. A total of 5 animals died within the first week post-injury, four being aged sedentary and one being an aged exercise animal. One animal from each group died within the second week post injury. The majority of deaths were associated with severe bladder infections despite an extensive bladder expression routine. By the end of the study, the survival rates were 91.7%, 50%, and 75% for the young, aged sedentary and aged exercise groups, respectively (Table 2). Surviving animals were assessed for weight change at the end of 9 the study to compare with weights on the day of injury. Young animals gained an average of 49.55g ± 12.21. Both aged groups demonstrated weight loss, with the aged sedentary animals losing an average of 72.67g ± 14.17, and the aged exercise animals losing an average of 42.33g ± 15.68 (data reported as grams ± SEM). The weight change between young and aged animals (both sedentary and exercise) following contusion SCI was significantly different (p<0.01), but the change in weight between aged sedentary and aged exercise groups was not significantly different (Table 2). Additionally, there was no significant difference in average weight at time of injury between aged sedentary and aged exercise animals despite the fact that aged exercise rats had been participating in voluntary wheel running for nine months prior to injury, and continued to rotate the running wheel following injury. Running wheel data reveals that there was an average of 8,522 wheel rotations per day when rats were initially given access to the running wheel at 3 months of age. Prior to SCI, the average wheel rotations decreased to 941 per day. Interestingly, the approximate wheel rotations increased to 3,478 per day following SCI (1 rotation = 1 meter).
Table 2. Animal weights.
Young, aged sedentary, and aged exercise animals were weighed before and 8 weeks after SCI. Young animals gained an average of 49 g, whereas aged sedentary and aged exercise animals lost an average of 72 g and 42 g, respectively. Animal deaths are noted in the weight after SCI column.
| Group | Weight before SCI (g) | Weight after SCI (g) | Weight change (g) |
|---|---|---|---|
| Young | 428 | 470 | 42 |
| 430 | 420 | −10 | |
| 490 | 640 | 150 | |
| 430 | 490 | 60 | |
| 430 | 480 | 50 | |
| 360 | 410 | 50 | |
| 375 | 410 | 35 | |
| 440 | 485 | 45 | |
| 406 | 460 | 54 | |
| 395 | DIED | N/A | |
| 387 | 436 | 49 | |
| 410 | 430 | 20 | |
| Aged sedentary | 740 | DIED | N/A |
| 700 | 612 | −88 | |
| 700 | DIED | N/A | |
| 630 | 550 | −80 | |
| 670 | 620 | −50 | |
| 735 | DIED | N/A | |
| Aged exercise | 722 | 670 | −52 |
| 800 | DIED | N/A | |
| 590 | 565 | −25 | |
| 660 | 590 | −70 | |
| 690 | DIED | N/A | |
| 700 | 650 | −50 | |
| 585 | 555 | −30 | |
| 630 | 660 | 30 | |
| 570 | 500 | −70 | |
| 609 | 615 | 6 | |
| 800 | DIED | N/A | |
| 670 | 550 | −120 |
Locomotor recovery
To determine whether age at time of injury and chronic exercise affects recovery of function following contusion SCI, locomotion was assessed using the BBB locomotor scale. There was no significant difference (p>0.05) between the animal groups in the first day following injury, indicating that all groups had similar locomotor deficits; likewise, there was no significant difference (p>0.05) between the animal groups in the first week following injury (Fig. 1a). The aged exercise group had nearly a 3 point difference on the BBB scale in the first week following injury, which reflects a difference in the movement of hindlimb joints (Fig. 1a). Analysis of the displacement graphs produced by the IH device show similar impacts for all animals, demonstrating that the slightly greater BBB score for aged exercise animals, although not significant, is not a result of mechanical errors during injury (data not shown). Additionally, analysis of actual hit data indicated that all groups received similar force hits (Fig. 1b) There were significant differences between the aged sedentary animals and the young or aged exercise animals at 2, 3, 4, 7 and 8 weeks post injury, suggesting that there is an age-associated deficit in locomotor recovery that can be significantly improved with voluntary wheel running (Fig. 1a).
Examination of the rate of recovery, as indicated by the change in BBB score over one week, revealed that the greatest locomotor recovery occurred between 1- and 2- weeks post-injury for both the young group and the aged exercise group, whereas the peak locomotor recovery is delayed by one week in the aged sedentary group (Fig. 1c). The rate of recovery patterns suggests that locomotor recovery in aged sedentary animals is not only delayed, but also less than that of the young animals (Fig. 1c).
Area of pathology
Toluidine blue-stained sections were examined at low magnification for regions of pathology following contusion SCI. Contusion injury resulted in cavitation and loss of gross tissue structure within the central region of the spinal cord at the injury epicenter in all animal groups. Tissue swelling, demyelination, axonal death, and inflammation were abundant at the injury epicenter and extended several millimeters either side of the injury epicenter in all animal groups. The average cord area of a 1µm thin cross section was 25.15 mm2, 32.29 mm2, and 28.84 mm2 for young, aged sedentary, and aged exercise animals, respectively. Statistical analysis of the average cord area reveals that both aged groups (sedentary and exercise) have a greater cord area than that of the young group (p<0.05) (data not shown). There was no statistical difference in the cord area between the aged sedentary and aged exercise groups (p>0.05) (data not shown).
Quantification of the total area of pathology throughout 5 mm of spinal cord reveals that aged sedentary animals had an average area of pathology of 15.86 mm2, which is significantly larger than that of young animals, which had an average of 11.15 mm2 (p<0.01) (Fig. 2a). Aged exercise animals had an average area of pathology of 14.19 mm2, which is significantly less than that of aged sedentary animals (p<0.05) (Fig. 2a). These data reveal that there is an age-associated increase in the region of pathology following contusion SCI. Moreover, the age-associated increase in region of pathology can be significantly attenuated with voluntary wheel running. Figures 2b, c and d illustrate the qualitative differences in region of pathology between young, aged sedentary, and aged exercise animals 2 mm cranial to the injury site.
Figure 2. Voluntary wheel running lessens the region of pathology in aged animals following contusion SCI.
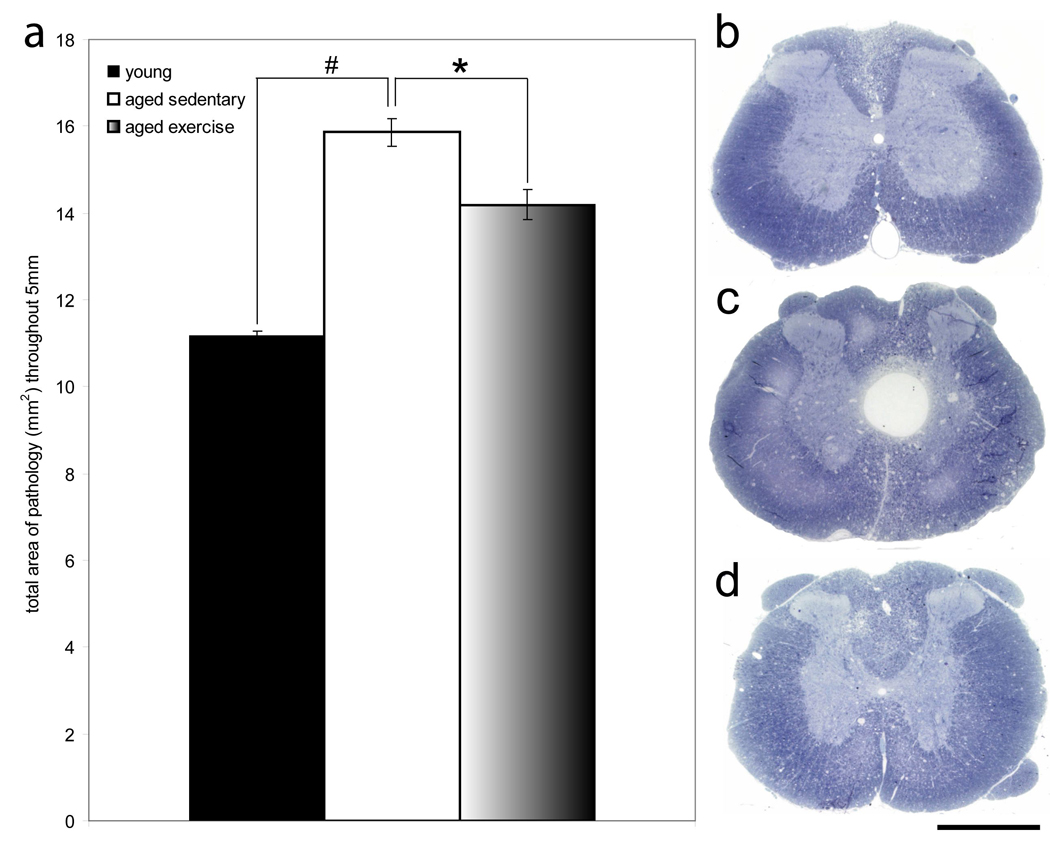
a, Aged sedentary animals had significantly greater area of pathology as compared to young animals (p<0.05). Aged exercise animals had significantly less pathology as compared to aged sedentary animals (p<0.05). b, c, d, Low magnification imaging of transverse spinal cord sections 2 mm cranial to injury epicenter of young (b), aged sedentary (c), and aged exercise (d) animals reveals qualitative differences in the area of pathology. Scale bar= 1mm.
Myelin pathology
High magnification imaging within the region of pathology revealed axonal and myelin pathology in all animal groups. Normally-myelinated (arrowheads), demyelinated (asterisk), and remyelinated axons (arrows) were visible in the high magnification images and were distinguishable based on the absence or thickness of the myelin sheath (Fig. 3a, b). Oligodendrocyte-remyelinated axons can be distinguished from normally myelinated axons due to the characteristically thin myelin sheaths. The mean G ratio of normally myelinated axons, 46 ± 3, is significantly greater (p<0.001) than the mean G ratio of oligodendrocyte remyelinated axons, 15 ± 1 (Fig. 3c). These data indicate that oligodendrocyte-remyelinated axons can accurately be distinguished from normally-myelinated axons.
Figure 3. Oligodendrocyte remyelinated axons can be distinguished from normally-myelinated axons.
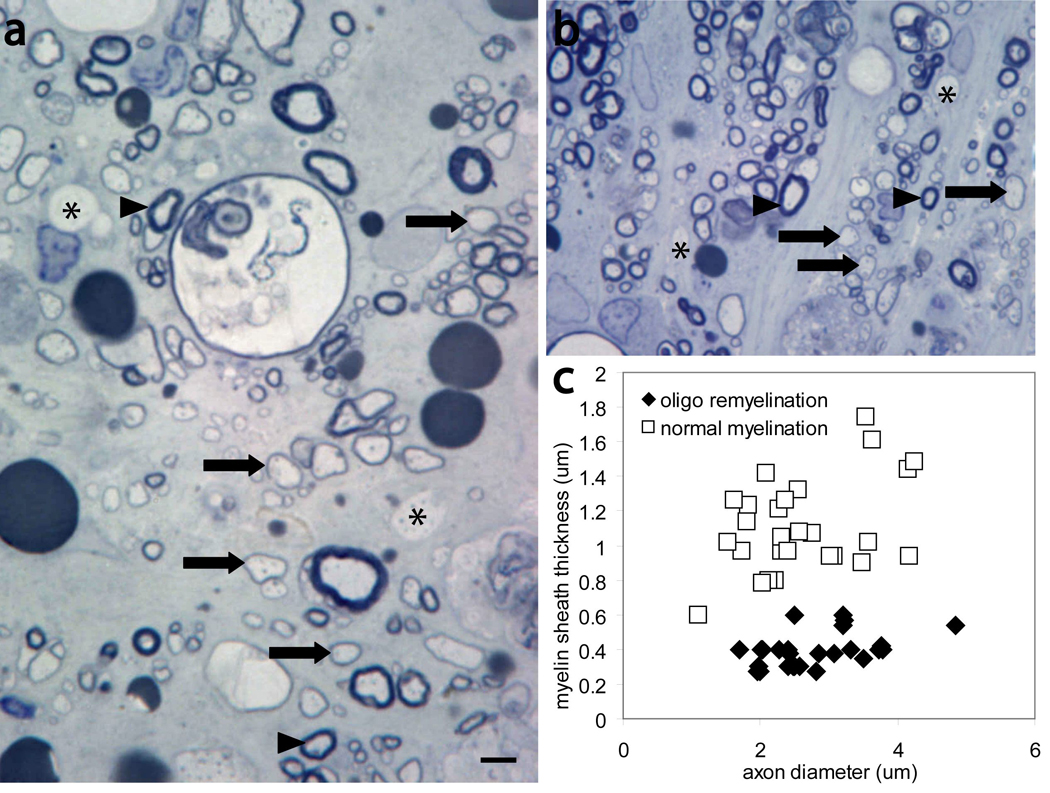
a, b, Toluidine blue-stained transverse sections of contused spinal cord at the magnification used for quantification and identification of normally-myelinated axons (arrowhead), oligodendrocyte remyelinated axons (arrows), demyelinated axons (asterisk). c, Myelin sheath thickness against axon diameter of normally-myelinated and oligodendrocyte remyelinated axons. The G ratio was 46 ± 3 (26)* for normally-myelinated axons and 15 ± 1 (26)* for oligodendrocyte remyelinated axons. *, Data are expressed as mean ± SEM; number in parentheses shows the number of axons scored. Scale bars= 5µm
High magnification imaging was used to quantify demyelinated and oligodendrocyte remyelinated axons within 625µm2 grid squares. The number of demyelinated and oligodendrocyte remyelinated axons per 625µm2 grid square was averaged in each spinal cord section and summed to obtain an estimate of demyelinated and remyelinated axons within a 625 µm2 area throughout 5 mm. This method of quantification was used rather than that of previous studies in which the number of demyelinated and remyelinated axons was multiplied by the area of pathology so as to determine if there are local differences in demyelination and remyelination without the compounding factor of differences in region of pathology. Figure 4a shows that aged sedentary animals had a significantly greater (p<0.05) amount of demyelination throughout the 5 mm spinal cord region as compared to young animals. In addition, aged sedentary animals had a significantly greater (p<0.05) amount of demyelination throughout the 5 mm spinal cord region as compared to aged exercise animals, suggesting that voluntary wheel running attenuates the age-associated increase in demyelination throughout the 5 mm spinal cord region analyzed (Fig. 4a).
Figure 4. Voluntary wheel running reduces demyelination and increases remyelination efficiency in aged animals following contusion SCI.
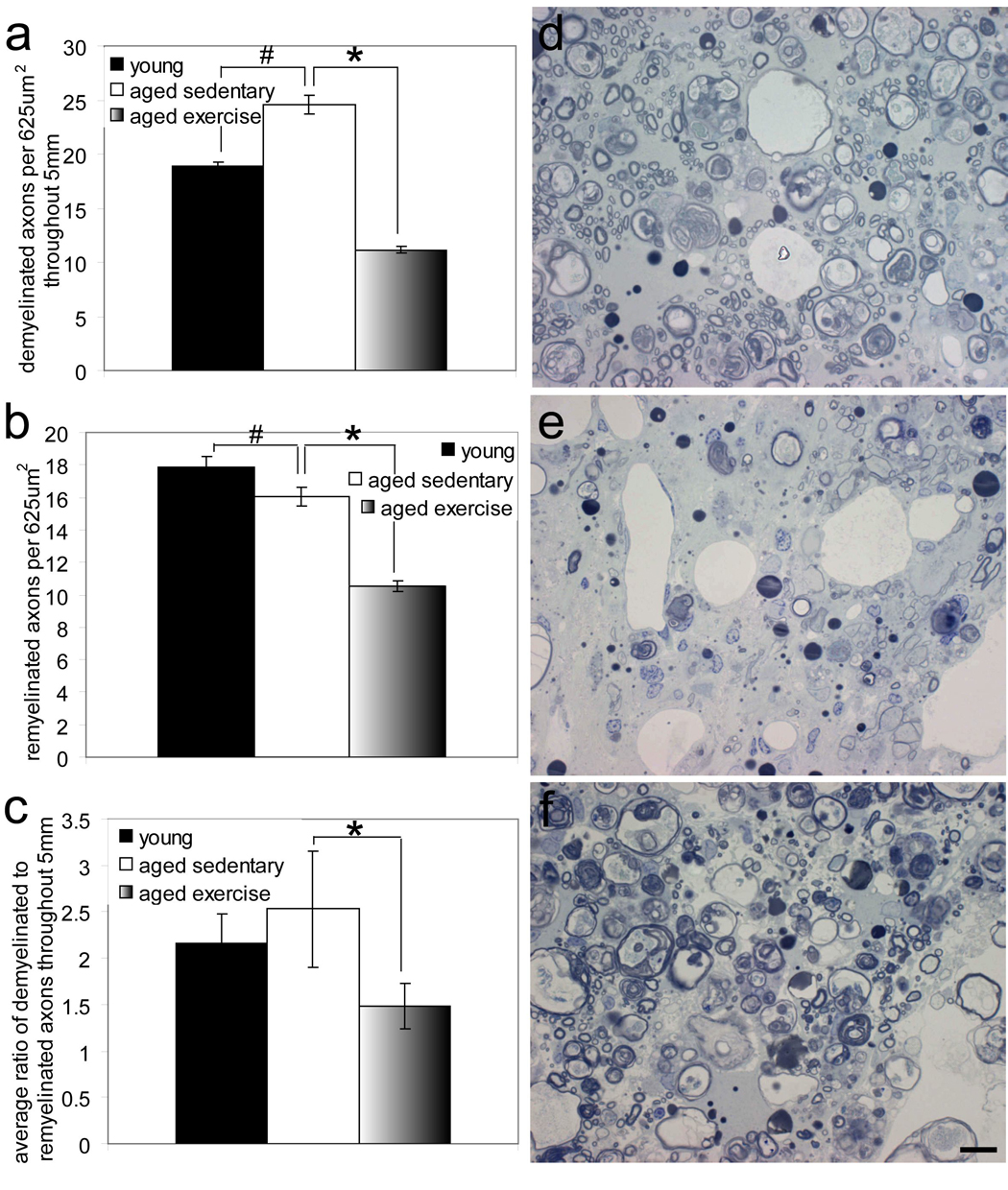
a, Aged sedentary animals had significantly more demyelinated axons as compared to young animals (p<0.05), and significantly more demyelinated axons as compared to aged exercised animals (p<0.05). b, Aged sedentary animals had significantly fewer remyelinated axons as compared to young animals (p<0.05), and significantly more remyelinated axons as compared to aged exercised animals (p<0.05). c, Aged sedentary animals had a significantly greater ratio of demyelinated to remyelinated axons as compared to aged exercised animals (p<0.05), indicating less efficient remyelination. d, e, f, High magnification imaging of toluidine-blue stained transverse spinal cord sections 2 mm cranial to injury epicenter of young (d), aged sedentary (e), and aged exercise (f) animals reveals qualitative differences in the myelin pathology. Scale bar= 10µm
Figure 4b illustrates that aged sedentary animals had significantly less remyelination (p<0.05) throughout the 5 mm spinal cord region as compared to young animals. Aged sedentary animals had significantly greater remyelination (p<0.05) throughout the 5 mm spinal cord region as compared to aged exercise animals (Fig. 4b). Because the number of remyelinated axons is limited by the number of demyelinated axons, the ratio of demyelinated to remyelinated axons was examined in order to assess the efficiency of remyelination. Figure 4c shows the average ratio of demyelinated to remyelinated axons throughout the 5 mm spinal cord region. Aged sedentary animals had the least efficient remyelination, with a ratio of 2.52 (Fig. 4c). Young animals had a remyelination efficiency ratio of 2.15 (Fig. 4c). Figure 4c reveals that aged exercise animals, with a ratio of 1.48, had a significantly better remyelination efficiency (p<0.05) throughout the 5 mm spinal cord section as compared to aged sedentary animals. These quantitative differences are qualitatively visible in spinal cord sections from each group, as is illustrated in Figures 4d, e and f. These data demonstrate that aging increases the amount of demyelination and decreases the amount of remyelination that occurs following contusion SCI, suggesting that pathologic mechanisms are greater and reparative mechanisms are lessened. Exercise, however, decreases the age-associated increase in demyelination, and increases the efficiency of remyelination as indicated by the significantly lower ratio of demyelinated to remyelinated axons than that of sedentary aged animals.
Discussion
Age-associated deficits
This study reveals age-associated deficits following contusion SCI. We demonstrate that SCI in aged rats results in less locomotor recovery, and a greater amount of pathology with more demyelinated and less remyelinated axons as compared to SCI in young rats. Additionally, we reveal that chronic exercise is sufficient in attenuating the 14 age-associated deficits following contusion SCI to the extent that aged exercise rats are more comparable to young rats than sedentary aged rats. Voluntary wheel running in aged rats improves locomotor recovery, lessens the amount of pathology, and decreases demyelination of axons following contusion SCI.
These data extend and complement a cohort of studies that demonstrate age-associated decline in neural maintenance, protection, repair, and recovery in the CNS. An aged CNS differs from a young CNS with respect to the amount of oxidative stress, mitochondrial damage, and glial activation (Keller et al., 2000; Kyrkanides et al., 2001; Navarro and Boveris, 2007a; Navarro and Boveris, 2007b). These conditions greatly affect the integrity and functionality of the aged neural systems, which often manifests in age-related dementia and cognitive decline (Laurin et al., 2001), the latter being associated with age-related demyelination of axons (Kovari et al., 2004). The loss of myelin integrity with age has been documented both in the brain and in the spinal cord (Kullberg et al., 1998; Peters and Sethares, 2003; Raz and Rodrigue, 2006).
The loss of neural integrity with age due to greater oxidative stress, mitochondrial damage, and glial activation increases the susceptibility of the CNS to injury with age (Kyrkanides et al., 2001). With respect to the spinal cord, aged rats remyelinate at a slower rate than young rats following toxin-induced demyelination (Shields et al., 1999), suggesting a slower recovery from injury with age. Moreover, aged rats exhibit slower locomotor and somatosensory behavioral recovery following transection and clip compression SCI as compared to young rats (Genovese et al., 2006; Gwak et al., 2004).
Here we demonstrate not only that aged rats recover from contusion SCI at a slower rate than young rats, but also that the amount of pathology is greater in aged rats as compared to young rats. These data indicate for the first time that age at time of contusion SCI influences the extent of pathology in the spinal cord. Similar age-related differences have been documented in pathological extent and outcome following both traumatic brain injury and experimental stroke (Davis et al., 1995; Erdincler et al., 2002; Gong et al., 2004; Luerssen et al., 1988; Sutherland et al., 1996; Teasdale et al., 1979; Vollmer and Dacey, 1991). These differences have been attributed to a loss of neuroprotection with age (Davis et al., 1995; Veiga et al., 2004). Age-related decreases in neurotrophins, such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1), within the brain and spinal cord may play a role in the loss of neuroprotection with age, making the CNS more susceptible to injury (Croll et al., 1998; Florini et al., 1981; Hayashi et al., 1997; Lommatzsch et al., 2005; Sonntag et al., 1999a; Sonntag et al., 1999b; Velasco et al., 1998). BDNF and IGF-1 both promote cell survival of existing neurons and myelin-producing oligodendrocytes in addition to promoting progenitor cell proliferation and remyelination following demyelination (Arsenijevic and Weiss, 1998; Cheng and Mattson, 1994; Du et al., 2003; Kokaia et al., 1994; Larsson et al., 1999; Lindvall et al., 1994; Mason et al., 2000; Mason et al., 2003; McTigue et al., 1998; Nakao et al., 1995). Therefore, decreases in such growth factors with age may also lessen the capacity of the natural repair mechanisms following injury. Additionally, greater pro-inflammatory mRNA levels, such as that of GFAP, IL-1β, TNF-α, iNOS, and MCP-1, following traumatic brain injury in aged rats indicate that the age-associated loss in neuroprotection is accompanied by a more severe inflammatory response (Kyrkanides et al., 2001). The inflammatory response is a major component of secondary degeneration that follows the acute stages of SCI (Hausmann, 2003). Several studies support the notion that the inflammatory response is detrimental following injury, causing further loss of tissue that was spared by the initial mechanical insult (Glaser et al., 2004; Glaser et al., 2006; Gonzalez et al., 2003; Popovich et al., 1997; Popovich et al., 1999; Popovich et al., 2002; Taoka et al., 1997). This age-associated loss in neuroprotection and regulation of inflammation may drive the age-associated increases in pathology and demyelination that we observed.
Exercise attenuates age-associated deficits
Beneficial effects of exercise are well documented in both young and aged animals. Physical activity improves recovery of function following SCI in young rodents, perhaps due to the ability of exercise to alter the levels of neurotrophins, influencing growth and synaptic plasticity following SCI (Edgerton et al., 2001; Engesser-Cesar et al., 2005; Engesser-Cesar et al., 2007; Erschbamer et al., 2006; Multon et al., 2003; Van Meeteren et al., 2003; Ying et al., 2005). We report here for the first time the beneficial effects of long term exercise on recovery from contusion SCI in aged rats. Our data indicates that chronic voluntary wheel running improves locomotor recovery, lessens the amount of pathology and demyelination, and improves the efficiency of remyelination following contusion SCI in aged rats. Our study examined the effects of chronic exercise prior to injury along with exercise post-injury. Therefore, we are unable to determine whether pre- or post- injury exercise alone is sufficient in attenuating the age-associated deficits following contusion SCI.
Vaynman and Gomez-Pinilla (2005) discuss several effects of exercise on the intact and injured CNS, including the ability of exercise to lessen the degree of damage by limiting the secondary degenerative response. Exercise prior to CNS injury has been shown to be neuroprotective (Ang et al., 2003; Ding et al., 2006b; Stummer et al., 1994). The exercise-induced neuroprotection may be linked to the exercise-induced increase in neurotrophins and growth factors such as BDNF and IGF-1 (Ding et al., 2006a; Heinemeier et al., 2003; Schwarz et al., 1996; Trejo et al., 2001). Additionally, exercise attenuates the effects of oxidative stress, as indicated by a reduction in the level of membrane lipid peroxidation and oxidative damage to DNA and proteins (Radak et al., 2001a; Radak et al., 2001b; Vaynman and Gomez-Pinilla, 2005).
Together, these studies suggest that exercise decreases injury and increases recovery. Our data are consistent with and extend these findings, indicating that aged rats with a physically active lifestyle had greater neuroprotection and recovery from injury.
Acknowledgements
We thank Charles Mendoza and Julio Espinosa for assistance with animal care, Kevin Bordonaro and Brandon Shepard for assistance with tissue processing, and Ellika Sadr for assistance with locomotor testing. This project was supported by Geron Corporation, UC Discovery, Research for Cure, and individual donations to the Reeve-Irvine Research Center. Monica Siegenthaler was supported by a pre-doctoral NIH training grant (#AG00096-22) and the Bill and Joan Jackson Scholars Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang ET, Wong PT, Moochhala S, Ng YK. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118:335–345. doi: 10.1016/s0306-4522(02)00989-2. [DOI] [PubMed] [Google Scholar]
- Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Blight AR. Remyelination, revascularization, and recovery of function in experimental spinal cord injury. Adv Neurol. 1993;59:91–104. [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006a;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ding YH, Mrizek M, Lai Q, Wu Y, Reyes R, Jr, Li J, Davis WW, Ding Y. Exercise preconditioning reduces brain damage and inhibits TNF-alpha receptor expression after hypoxia/reoxygenation: an in vivo and in vitro study. Curr Neurovasc Res. 2006b;3:263–271. doi: 10.2174/156720206778792911. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- Erdincler P, Tuzgen S, Erdincler UD, Oguz E, Korpinar A, Ciplak N, Kuday C. Influence of aging on blood-brain barrier permeability and free radical formation following experimental brain cold injury. Acta Neurochir (Wien) 2002;144:195–199. doi: 10.1007/s007010200024. discussion 199–200. [DOI] [PubMed] [Google Scholar]
- Erschbamer MK, Pham TM, Zwart MC, Baumans V, Olson L. Neither environmental enrichment nor voluntary wheel running enhances recovery from incomplete spinal cord injury in rats. Exp Neurol. 2006;201:154–164. doi: 10.1016/j.expneurol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Florini JR, Harned JA, Richman RA, Weiss JP. Effect of rat age on serum levels of growth hormone and somatomedins. Mech Ageing Dev. 1981;15:165–176. doi: 10.1016/0047-6374(81)90072-5. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Crisafulli C, Muia C, Bramanti P, Cuzzocrea S. Increased oxidative-related mechanisms in the spinal cord injury in old rats. Neurosci Lett. 2006;393:141–146. doi: 10.1016/j.neulet.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Gilson JM, Blakemore WF. Schwann cell remyelination is not replaced by oligodendrocyte remyelination following ethidium bromide induced demyelination. Neuroreport. 2002;13:1205–1208. doi: 10.1097/00001756-200207020-00027. [DOI] [PubMed] [Google Scholar]
- Glaser J, Gonzalez R, Perreau VM, Cotman CW, Keirstead HS. Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J Neurosci Res. 2004;77:701–708. doi: 10.1002/jnr.20204. [DOI] [PubMed] [Google Scholar]
- Glaser J, Gonzalez R, Sadr E, Keirstead HS. Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J Neurosci Res. 2006;84:724–734. doi: 10.1002/jnr.20982. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Guy J, Ellis EA, Kelley K, Hope GM. Spectra of G ratio, myelin sheath thickness, and axon and fiber diameter in the guinea pig optic nerve. J Comp Neurol. 1989;287:446–454. doi: 10.1002/cne.902870404. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Effect of age at time of spinal cord injury on behavioral outcomes in rat. J Neurotrauma. 2004;21:983–993. doi: 10.1089/0897715041650999. [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2004;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 1997;749:283–289. doi: 10.1016/S0006-8993(96)01317-0. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Kjaer M. Exercise-induced changes in circulating levels of transforming growth factor-beta-1 in humans: methodological considerations. Eur J Appl Physiol. 2003;90:171–177. doi: 10.1007/s00421-003-0881-8. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Hahn R. Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. J Neurol Sci. 1978;38:421–434. doi: 10.1016/0022-510x(78)90147-8. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Othberg A, Kokaia M, Lindvall O. BDNF makes cultured dentate granule cells more resistant to hypoglycaemic damage. Neuroreport. 1994;5:1241–1244. doi: 10.1097/00001756-199406020-00021. [DOI] [PubMed] [Google Scholar]
- Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- Kullberg S, Ramirez-Leon V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998;53:B369–379. doi: 10.1093/gerona/53a.5.b369. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, O'Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Larsson E, Nanobashvili A, Kokaia Z, Lindvall O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1220–1228. doi: 10.1097/00004647-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient's age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multon S, Franzen R, Poirrier AL, Scholtes F, Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- Nakao N, Kokaia Z, Odin P, Lindvall O. Protective effects of BDNF and NT-3 but not PDGF against hypoglycemic injury to cultured striatal neurons. Exp Neurol. 1995;131:1–10. doi: 10.1016/0014-4886(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007a;292:C670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction in aging: conditions that improve survival, neurological performance and mitochondrial function. Front Biosci. 2007b;12:1154–1163. doi: 10.2741/2133. [DOI] [PubMed] [Google Scholar]
- Nistico G, Ciriolo MR, Fiskin K, Iannone M, De Martino A, Rotilio G. NGF restores decrease in catalase activity and increases superoxide dismutase and glutathione peroxidase activity in the brain of aged rats. Free Radic Biol Med. 1992;12:177–181. doi: 10.1016/0891-5849(92)90024-b. [DOI] [PubMed] [Google Scholar]
- NSCISC. Facts and Figures at a Glance. 2004. [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460:238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001a;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001b;7:90–107. [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999b;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann A, Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 25:1862–1869. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667. doi: 10.1161/01.str.27.9.1663. discussion 1668. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Skene A, Parker L, Jennett B. Age and outcome of severe head injury. Acta Neurochir Suppl (Wien) 1979;28:140–143. doi: 10.1007/978-3-7091-4088-8_33. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Veiga S, Melcangi RC, Doncarlos LL, Garcia-Segura LM, Azcoitia I. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Velasco B, Cacicedo L, Escalada J, Lopez-Fernandez J, Sanchez-Franco F. Growth hormone gene expression and secretion in aging rats is age dependent and not age-associated weight increase related. Endocrinology. 1998;139:1314–1320. doi: 10.1210/endo.139.3.5779. [DOI] [PubMed] [Google Scholar]
- Vollmer DG, Dacey RG., Jr The management of mild and moderate head injuries. Neurosurg Clin N Am. 1991;2:437–455. [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]


