Abstract
Tigecycline, a glycylcycline related to the tetracycline class of antibiotics, represents a new option for the treatment of complicated intra-abdominal and complicated skin and skin structure infections. It displays favorable activity in vitro against the most common causative Gram-positive, Gram-negative and anaerobic pathogens. In addition, tigecycline demonstrates activity against drug-resistant pathogens such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and organisms (such as Escherichia coli and Klebsiella pneumoniae) producing extended-spectrum beta-lactamases. Tigecycline lacks activity in vitro against Pseudomonas and Proteus spp. In randomized clinical trials, tigecycline administered intravenously twice daily has demonstrated efficacy similar to comparators for a variety of complicated skin and skin structure and complicated intra-abdominal infections. The potential for significant drug interactions with tigecycline appears to be minimal. Dosing adjustment is needed for patients with severe hepatic impairment. The predominant side effect associated with its use to date has been gastrointestinal intolerance (nausea and vomiting).
Keywords: tigecycline, intra-abdominal infections, complicated skin and skin structure infections
Introduction
Tigecycline, formerly GAR-936 (Tygacil®; Wyeth Pharmaceuticals, Inc., Philadelphia, PA, USA), is a glycylcycline antimicrobial currently approved by the Food and Drug Administration (FDA) for the treatment of complicated intra-abdominal infections (cIAIs) and complicated skin and skin structure infections (cSSSIs) (Wyeth Pharmaceuticals 2007b). In addition to its broad spectrum in vitro activity against Gram-positives, Gram-negatives and anerobes, tigecycline demonstrates activity in vitro against MRSA, VRE, and ESBL-producing organisms (Wyeth Pharmaceuticals 2007b). Therefore, it has potential applications in the management of polymicrobial infections or those due to resistant organisms.
Two of the most prevalent bacterial infections in clinical practice are cSSSIs and cIAIs. For example, surgical site infections are estimated to occur 500,000 times per year among the 27 million surgical procedures performed (CDCP 1997). Studies evaluating the impact of surgical site infections have demonstrated that these infections are consistently associated with an increase in healthcare costs, prolonged hospitalizations, and an increase in morbidity and mortality (Vegas et al 1993; Kirkland et al 1999). Specifically, one study, evaluating cSSSI following hip replacement surgeries, found a median increased length of stay of 32.5 days directly related to the cSSSI; additionally, the morbidity rate associated with the cSSSI was 14.3% (Monge et al 2006). Similarly, the incidence of cIAIs is also difficult to determine because of its inclusion of a broad range of diagnoses. Among these, complicated appendicitis may occur in up to 30% of appendicitis cases (Cueto et al 2006). Additionally, cIaIs account for considerable hospital cost (Solomkin, Mazuski et al 2003). Inappropriate treatment has been associated with both treatment failures as well as increased mortality (Solomkin, Mazuski et al 2003). For example, Sturkenboom et al (2005) found a clinical failure rate of 35.7% and a mortality rate of 10.7% among patients receiving initial inappropriate therapy with intraabdominal infections.
Effective management of both cIAIs and cSSSIs require the timely institution of appropriate antimicrobital therapy and, in select cases, surgical interventions (Solomkin, Mazuski et al 2003; Stevens et al 2005). However, increases in antibiotic resistance seen in bacteria commonly causing such infections has made selection of appropriate empiric therapy challenging (Bochicchio et al 2006; Moet et al 2007). Data recently published from a worldwide multi-center longitudinal antimicrobial resistance tracking program, the SENTRY Antimicrobial Surveillance Program, reported rates of methicillin-resistant Staphylococcus aureus (MRSA) causing skin and skin structure infections ranging from 22.8% in Europe to 35.9% in North America (Moet et al 2007). Isolation of vancomycin-resistant enterococci (VRE) ranged from 3.6% in Europe to 12.2% in North America. Furthermore, Gram-negative organisms have also demonstrated diminished susceptibility. For example, reported rates of multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase (ESBL) producing Klebsiella spp. were 3.2% and 11.3% in North America, and 24.7% and 48.0% in Latin America, respectively. ESBL-producing Escherichia coli rates ranged from 6.6% in North America to 15.1% in Latin America (Moet et al 2007).
Rates of resistant organisms isolated in patients with cIAIs are also increasing. In vitro susceptibilities for over 7,000 E. coli isolates from patients with intra-abdominal infections varied according to geographic region (Bochicchio et al 2006). The rate of ESBL-producing E. coli worldwide was 8.9% from 2002–2004, with the highest rates in the Asia/Pacific region (16.6%) (Bochicchio et al 2006).
The purpose of this article is to review the in vitro activity, pharmacokinetics, pharmacodynamics and clinical efficacy and safety of tigecycline for the treatment of cIAIs and cSSSIs. Tigecycline’s role in therapy will also be discussed.
Overview of tigecycline
Pharmacology
Tigecycline (C29H39N5O8) is the first of a new class of antimicrobials called glycylcyclines, which are related to the tetracycline class (Wyeth Pharmaceuticals 2007b). Although structurally similar to minocycline, it differs primarily by the presence of a side chain addition at position 9 (Figure 1). Tigecycline possesses a similar mechanism of action to tetracyclines in that it binds to the bacterial 30S ribosomal subunit, thereby inhibiting bacterial protein synthesis (Bergeron et al 1996). However, the binding affinity for tigecycline to this ribosomal site is approximately 5 times that of tetracyclines (Bergeron et al 1996). Tigecycline also demonstrates 70S ribosomal subunit binding, with up to 100-fold greater affinity as compared with tetracycline (Olson et al 2006).
Figure 1.
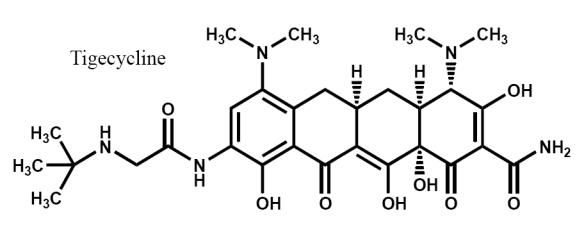
Chemical structure.
Resistance to the tetracycline class most frequently involves protection of the ribosome and/or efflux pumps (Chopra et al 1992; Speer et al 1992; Bergeron et al 1996) Binding to the 30S ribosomal subunit is thought to prevent ribosomal protection (Rasmussen et al 1994; Tally et al 1995; Projan 2000; Chopra et al 2001; Zhanel et al 2004). Efflux pumps are responsible for expelling drug from the intracellular to extracellular space, thus preventing action of the drug and therefore causing resistance (Li et al 1995; Poole et al 1996; Kohler et al 1997; Aires et al 1999; Mine et al 1999; Westbrock-Wadman et al 1999; Dean et al 2003). In contrast to tetracyclines, tigecycline is not usually affected by efflux pumps. However, tigecycline is susceptible to efflux pumps of the “resistance nodulation division” (RND) which are common among P. aeruginosa (Projan 2000); tigecycline is a know substrate for the pumps, described as MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM (Dean et al 2003). While Acinetobacter baumannii is generally sensitive to tigecycline, it can possess 2 of these RND pumps. Therefore, although further study is needed, emerging resistance to tigecycline while on therapy may be a concern for this organism (Rice 2006). Tigecycline appears to be unaffected by other mechanisms of resistance, including enzyme target changes and target site modifications. Production of beta-lactamases (including ESBLs) also do not influence tigecycline’s antimicrobial activity (Wyeth Pharmaceuticals 2007b).
Microbiology
The Clinical and Laboratory Standards Institute (CLSI) has set the tigecycline in vitro minimum inhibitory concentration (MIC) susceptibility breakpoints for Streptococcus spp. (excluding S. pneumoniae) and Enterococcus faecalis (vancomycin-susceptible organisms) at ≤0.25 μg/mL. MIC breakpoints to be considered susceptible to tigecycline for S. aureus (including both MSSA and MRSA) are ≤0.5 μg/mL, while Enterobacteriaceae and anaerobes are set at ≤2 μg/mL and ≤4 μg/mL, respectively (CLSI 2003a, 2003b, 2004, 2005; Wyeth Pharmaceuticals 2007b). The European Committee on Antimicrobial Susceptibility Testing (EUCAST) susceptibility breakpoint for Enterobacteriaceae however, is ≤1 μg/mL, and breakpoints have not been established for anaerobes (EUCAST Steering Committee 2006).
Tigecycline displays excellent in vitro activity against most Gram-positive organisms (Sader et al 2005). A recent study evaluating 26,474 bloodstream infection isolates from 6 different continents found 99.4% (n = 8765) of S. aureus isolates susceptible with an MIC90 = 0.5 μg/mL (range of ≤0.016–1 μg/mL) (Sader et al 2005). In this same study, 92.7% (n = 3258) of Enterococcus spp. were considered sensitive to tigecycline, with an MIC90 of 0.25 μg/mL (range of ≤0.016–2 μg/mL). Over 97 % (n = 605) of S. pneumoniae and viridans group streptococci (n = 378) were also considered susceptible, with MIC90 of ≤0.12 μg/mL (range ≤0.12–1 μg/mL) and ≤0.12 μg/mL (range ≤0.12–0.5 μg/mL), respectively (Sader et al 2005).
In general, tigecycline demonstrated activity against Gram-positive bacteria resistant to other classes of antibiotics. Susceptibility of S. aureus to tigecycline appears to be independent of oxacillin susceptibility (Sader et al 2005). In addition, a vancomycin-resistant S. aureus strain (VRSA) isolated at Hershey Medical Center demonstrated an MIC of 0.125 μg/mL to tigecycline (Bogdanovich et al 2005). Tigecycline also has potent in vitro activity against quinolone-resistant S. pneumoniae, with a reported MIC of 0.12 μg/mL (Garrison et al 2007). For vancomycin-resistant E. faecium (n = 77) and vancomycin-resistant E. faecalis (n = 11), the MIC90 were 0.06 μg/mL and 0.12 μg/mL, respectively with 100% susceptibility in both species (Hoban et al 2005).
Tigecycline has shown potent in vitro activity against most Gram-negative organisms, with the exception of Proteus (n = 320) and Pseudomonas spp. (n = 1,338) with MIC90 (and ranges) of 4 μg/mL (0.25–16 μg/mL) and >32 mg/mL (0.008–≥32 μg/mL), respectively (Sader et al 2005). E. coli (n = 3217) and Klebsiella spp. (n = 1,503) are also susceptible to tigecycline. The MIC90 (and ranges) of 0.25 g/mL (0.03–4 μg/mL) and 1 μg/mL (0.06–8 μg/mL) have been reported for these organisms, respectively (Sader et al 2005). Tigecycline also demonstrates activity against ESBL-producing strains of these pathogens. MIC90 (range) values for 142 ESBL-producing E. coli isolates and 278 ESBL-producing K. pneumoniae isolates were 1 μg/mL (0.25–2 μg/mL) and 2 μg/mL (0.25–8 μg/mL), respectively (Bouchillon et al 2005b). MICs ranging 0.03–8 μg/mL and an MIC90 of 1 μg/mL was reported in the largest published study of Acinetobacter spp. isolates (n = 851) to date (Waites et al 2006). While several other in vitro studies have reported a high percentage of Acinetobacter spp. susceptible MICs according to CLSI criteria, many of these organisms would be considered resistant if utilizing EUCAST criteria (Bouchillon et al 2005a, 2005b; Sader et al 2005; Waites et al 2006). Tigecycline also remains active against carbapenem-resistant A. baumannii and pan-resistant A. baumannii according to 2 recent case reports, although tigecycline-resistant A. baumanii has emerged clinically (Bogaerts et al 2006; Taccone et al 2006; Peleg et al 2007).
Anaerobic activity of tigecycline has been studied in several clinical trials in which the results are summarized in a study by Bradford et al (Bradford et al 2005). Results from these studies demonstrate tigecycline’s potent anaerobic activity against Clostidium perfringens, Propionibacterium acnes, and Bacteroides fragilis. MICs for these organisms were below the CLSI susceptibility breakpoint of ≤4 μg/mL (Bradford et al 2005). Table 1 describes further the in vitro susceptibilities of tigecycline.
Table 1.
In vitro susceptibilities of select aerobic and anaerobic organisms to tigecyclinea
| Organism | No. of isolates | MIC90b | MIC rangeb | % Susceptiblec | References |
|---|---|---|---|---|---|
| Staphylococcus aureus | 8765 | 0.5 | ≤0.016–1 | 99.4 | (Sader et al 2005) |
| MSSA | 813 | 0.12 | 0.015–0.5 | 100 | (Waites et al 2006) |
| MRSA | 879 | 0.25 | 0.03–0.5 | 100 | (Waites et al 2006) |
| VISA | 19 | 0.5 | 0.06–1 | NAd | (Petersen et al 2002) |
| Staphylococci, coagulase-negative (CoNS) | 3570 | 0.5 | ≤0.016–2 | 97.5 | (Sader et al 2005) |
| CoNS, methicillin susceptible | 71 | 0.5 | 0.03–1 | NAd | (Fritsche, Sader et al 2005) |
| CoNS, methicillin resistant | 189 | 0.5 | ≤0.12–2 | NAd | (Fritsche, Sader et al 2005) |
| S. pneumoniae | 605 | ≤0.12 | ≤0.12–1 | --e | (Sader et al 2005) |
| S. pneumoniae, penicillin susceptible | 279 | 0.5 | NAd | NAd | (Hoban et al 2005) |
| S. pneumoniae, penicillin-resistant | 54 | 0.25 | NAd | NAd | (Hoban et al 2005) |
| Streptococci, β-hemolytic | 769 | ≤0.12 | ≤0.12–0.5 | 99.7 | (Sader et al 2005) |
| Streptococci, viridans group | 378 | ≤0.12 | ≤0.12–0.05 | 98.1 | (Sader et al 2005) |
| Enterococci spp. | 3258 | 0.25 | ≤0.016–2 | 92.7 | (Sader et al 2005) |
| Enterococcus spp.-vancomycin susceptible | 466 | 0.5 | ≤0.12–1 | NAd | (Fritsche, Sader et al 2005) |
| Enterococcus spp.-vancomycin resistant | 39 | 0.25 | 0.03–0.5 | NAd | (Fritsche, Sader et al 2005) |
| Nocardia spp. | 51 | 4 | ≤0.06–8 | NAd | (Cercenado et al 2007) |
| Escherichia coli | 3217 | 0.25 | 0.03–4 | >99.9 | (Sader et al 2005) |
| ESBL-producing E.coli | 115 | 0.38 | 0.047–0.75 | NAd | (Sorlozano et al 2006) |
| Klebsiella pneumonia | 1334 | 2 | ≤0.008–8 | 95 | (Waites et al 2006) |
| ESBL-producing | 126 | 2 | 0.12–8 | 92.1 | (Waites et al 2006) |
| K. pneumoniae | |||||
| Klebsiella oxytoca | 248 | 1 | 0.06–4 | 98.8 | (Waites et al 2006) |
| Enterobacter aerogenes | 419 | 1 | 0.06–8 | 95.7 | (Waites et al 2006) |
| Enterobacter cloacae | 1089 | 2 | ≤0.008–8 | 93 | (Waites et al 2006) |
| Haemophilus influenzae | 336 | 0.25 | NAd | --e | (Hoban et al 2005) |
| H. influenzae, β-lactamase positive | 93 | 0.25 | NAd | --e | (Hoban et al 2005) |
| Moraxella catarrhalis | 54 | 0.5 | NAd | --e | (Gales et al 2005) |
| Serratia marscens | 658 | 1 | 0.012–8 | 97 | (Waites et al 2006) |
| Citrobacter spp. | 252 | 0.5 | NAd | 86.9 | (Fritsche, Strabala et al 2005) |
| Acinetobacter baumannii | 851 | 1 | 0.03-8 | --e | (Waites et al 2006) |
| Pseudomonas aeruginosa | 1338 | ≥32 | ≤0.008–≥32 | --e | (Waites et al 2006) |
| Stenotrophomonas maltophilia | 203 | 2 | 0.12–8 | --e | (Sader et al 2005) |
| Burkholderia cepacia | 21 | 16 | 0.25–32 | --e | (Cheng et al 2005) |
| Campylobacter jejuni | 108 | 4 | 0.12–16 | --e | (Rodriguez-Avial et al 2006) |
| Campylobacter coli | 8 | 16 | 0.5–16 | --e | (Rodriguez-Avial et al 2006) |
| Proteus mirabilis | 320 | 4 | 0.25–16 | 46.9 | (Sader et al 2005) |
| Bacteroides fragilis | 2721 | 8 | 0.06–32 | 94.9 | (Snydman et al 2007) |
| Bacteroides fragilis group | 5225 | 8 | 0.06–64 | 95.7 | (Snydman et al 2007) |
| B. distasonis | 274 | 8 | 0.25–32 | 97.9 | (Snydman et al 2007) |
| B. ovatus | 545 | 8 | 0.125–16 | 96.7 | (Snydman et al 2007) |
| B. thetaiotaomicron | 978 | 8 | 0.25–32 | 96.4 | (Snydman et al 2007) |
| B. vulgatus | 306 | 4 | 0.25–16 | 98.4 | (Snydman et al 2007) |
| C. perfringens | 51 | 1.0 | ≤0.06–2 | NAd | (Bradford et al 2005) |
| Clostridium difficile | 12 | 0.06 | 0.06 | NAd | (Goldstein et al 2006) |
| Fusobacterium varium | 13 | 0.25 | 0.06–0.25 | NAd | (Goldstein et al 2006) |
| Lactobacillus spp. | 15 | 0.5 | 0.06–1 | NAd | (Goldstein et al 2006) |
Adapted with permission from (Townsend ML et al 2006. Tigecycline: a new glycylcycline antimicrobial. Int J Clin Pract, 60:1662–72. Blackwell Publishing.)
MIC = minimum inhibitory concentration.
According to CLSI criteria.
NA = not available.
No CLSI criteria available.
Pharmacokinetics
Tigecycline exhibits linear kinetics following intravenous (IV) administration (Muralidharan, Micalizzi et al 2005). Data from 103 healthy adult volunteers who received tigecycline intravenously (100 mg followed by 50 mg every 12 hours over 60 minutes) produced steady state maximum plasma concentrations (Cmax) and minimum plasma concentrations (Cmin) of 0.63 μg/mL and 0.13 μg/mL, respectively. The area under the plasma concentration-time curve from 0 to 24 hours (AUC0–24) was 4.70 μg·h/mL (Muralidharan, Micalizzi, et al 2005; Wyeth Pharmaceuticals 2007b). Patients with cSSSIs (n = 81) participating in a phase II study demonstrated pharmacokinetic parameters similar to healthy adult volunteers, with a Cmax of 0.403 μg/ml and AUC0–12 of 2.24 μg·h/mL (Postier et al 2004).
Tigecycline is highly protein bound (71%–89%) at plasma drug concentrations achieved in clinical trials (0.1–1.0 μg/mL) (Wyeth Pharmaceuticals 2007b). The volume of distribution of tigecycline reported from healthy volunteer studies is 7–10 L/kg (Muralidharan, Micalizzi et al 2005). Based on animal and human studies, tigecycline can distribute into various bodily fluids and tissues, such as the lungs, skin, peritoneal fluid, gall bladder, colon, heart, liver, meninges and bone (Tombs 1999; Rodvold et al 2005; Conte et al 2005; Gotfried et al 2005; Sun et al 2005; Rodvold et al 2006; Scheetz et al 2006; Wyeth Pharmaceuticals 2007b). In adults undergoing medical or surgical procedures (n = 104), serum, tissue, and body fluid concentrations of tigecycline were evaluated following a single dose of 100 mg of tigecycline administered over 30 minutes (Rodvold et al 2006). The mean ratio of tigecycline in the tissue to serum (expressed as AUC0–24) was 537 in the bile, 23 for the gall bladder, 2.6 for the colon, and 2.0 for the lung (Rodvold et al 2006). The highest concentration of tigecycline was found in the bile, which is consistent with the drug’s known route of elimination. Additionally, lower tissue to serum concentrations were achieved in the bone, synovial fluid, and cerebrospinal fluid (CSF). The mean ratio of tigecycline in the tissue to serum (expressed as AUC0–24) was 0.41 for the bone, 0.31 for the synovial fluid, and 0.11 for the CSF. The highest CSF to serum ratios occurred approximately 24 hours after infusion. Of note, bone penetration of tigecycline in animal models was higher than what was achieved in this human study (Tombs 1999; Rodvold et al 2006). The inconsistency of bone penetration in this study versus previous animal studies may have been due to poor extraction techniques, tight binding of the drug to bone, or the single dose design of the study. Additionally, peritoneal fluid penetration of tigecycline has been reported in a critically ill patient. The extrapolated penetration into the peritoneal fluid was about 50% (Scheetz et al 2006). Tigecycline has also been shown to have a 74% (mean) penetration into cantharidin-induced blisters in healthy volunteers (n = 10) (Sun et al 2005).
Tigecycline is not extensively metabolized. The main metabolic pathway of tigecycline is glucuronidation. Non-active metabolites that were recovered in the urine and feces include a glucuronide, its epimer (M1 and M2), and N-acetyl-9-aminominocycline (M6) (Hoffmann et al 2004; Rello 2005; Wyeth Pharmaceuticals 2007b). The pharmacokinetic model of tigecycline follows a 2-compartment model with first-order elimination based on pooled data from Phase II and III studies involving patients with cSSSIs and cIAIs (Van Wart et al 2006). The primary route of elimination of tigecycline is through feces and the biliary tract (59%) as unchanged drug and metabolites. Secondary routes of elimination include glucuronidation and renal excretion (33%). Renal excretion only accounts for about 10%–15% of the systemic clearance of tigecycline (Hoffmann et al 2004; Muralidharan, Micalizzi et al 2005). The terminal half-life of tigecycline is 37–67 hours and the total systemic clearance is 0.2–0.3 L/h/kg (Muralidharan, Micalizzi et al 2005).
Pharmacodynamics
Tigecycline demonstrates time-dependent bacteriostatic activity in vitro (van Ogtrop et al 2000; Reese et al 2005). Its post-antibiotic effect against Gram-negative organisms ranges from 2 to 5 hours, and 8.9 hours for S. pneumoniae (van Ogtrop et al 2000; Reese et al 2005). Recent animal and clinical data suggests the area-under-the concentration-time curve (AUC) to MIC ratio (AUC/MIC) may be a reliable predictor for efficacy with tigecycline (Meagher et al 2005; Garrison et al 2007; Meagher et al 2007). The AUC/MIC ratios described in the literature for in vitro activity range from 79–158 when evaluating quinolone-resistant S. pneumoniae, MRSA and VRE (Garrison et al 2007). In a study by Meagher and colleagues (Meagher et al 2007), cSSSI patients with S.aureus and streptococci as the primary organisms were evaluated to determine the pharmacodynamic properties of tigecycline. Based on the results of this study, the AUC/MIC ratio of 17.9 or higher was a significant predictor of both microbiological and clinical response in cSSSI patients (Meagher et al 2007). Although the AUC/MIC ratios range in the literature depending on the organism and infection, no consensus to date has been reached to determine the ideal AUC/MIC ratio for particular disease states.
Special populations
Tigecycline’s pharmacokinetic profile appears to be independent of age, ethnic backgrounds (African-American, Hispanic, Asian, and Caucasian), and gender. (Meagher et al 2005; Muralidharan, Fruncillo et al 2005). Patients with renal impairment (creatinine clearance of <30 mL/min or hemodialysis-dependent) had a non-significant increase in Cmax and AUC in comparison to healthy volunteers (Troy et al 2003). Additionally, tigecycline was not found to be significantly removed via hemodialysis. Therefore, no dosing adjustments are necessary in patients with renal dysfunction or who are hemodialysis dependent (Troy et al 2003; Wyeth Pharmaceuticals 2007b). In contrast to patients with renal dysfunction, patients with severe hepatic impairment (ie, Child-Pugh Class C) had a 43% increase in tigecycline’s half-life and a 55% reduction in drug clearance (Saunders et al 2005). Thus, it is recommended in these patients that the maintenance dose of tigecycline be reduced to 25 mg every 12 hours in patients with severe hepatic insufficiency (Wyeth Pharmaceuticals 2007b). No adjustments are needed for patients with mild to moderate hepatic impairment (Child-Pugh Class A or B) (Wyeth Pharmaceuticals 2007b). Pharmacokinetic studies are currently lacking in obese/low-body-weight individuals, the pediatric population, and patients who are lactating or pregnant.
Drug interactions
To date, no significant drug-drug interactions have been reported with tigecycline. Tigecycline is not metabolized by the cytochrome P450 system and as a result, it does not alter the metabolism of drugs that go through this system nor do these drugs affect the concentration of tigecycline (Wyeth Pharmaceuticals 2007b). Studies evaluating the concurrent administration of tigecycline with either digoxin or warfarin in healthy adults have not demonstrated a significant drug-drug interaction between tigecycline and either of these drugs (Zimmerman et al 2004; Raible et al 2005; Wyeth Pharmaceuticals 2007b; Zimmerman et al 2007). However, the manufacturer of tigecycline does recommend that the international normalized ratio (INR) as well as signs and symptoms of bleeding be routinely assessed when tigecycline is administered with warfarin (Wyeth Pharmaceuticals 2007b).
Safety and tolerability
Overall, tigecycline was well-tolerated in phase III clinical studies with only 5% of patients discontinuing therapy due to adverse events in comparison to 4.7% in the comparator arms (vancomycin-aztreonam 5.3% and imipenem-cilastatin 4.4%) (Babinchak et al 2005; Ellis-Grosse, Babinchak et al 2005; Wyeth Pharmaceuticals 2007b).
The most common adverse events associated with the administration of tigecycline in phase II and III studies was mild to moderate nausea and vomiting. This occurred most often during the first 2 days of drug therapy, and was the most common reason for discontinuing drug therapy (Postier et al 2004; Oliva et al 2005; Babinchak et al 2005; Breedt et al 2005; Fomin et al 2005; Muralidharan, Fruncillo et al 2005; Muralidharan, Micalizzi et al 2005; Sacchidanand et al 2005; Wyeth Pharmaceuticals 2007b). The incidence of nausea was 34.5% (versus 8.2% in vancomycin-aztreonam; p < 0.001) in the cSSSIs studies and 24.4% (versus 19% in imipenem-cilastatin; p = 0.01) in the cIAIs studies. The incidence of vomiting was 19.6% (versus 3.6% in vancomycin-aztreonam; p < 0.001) and 19.2% (versus 14.3% in imipenem-cilastatin; p = 0.008) in cSSSIs and cIAIs studies, respectively (Oliva et al 2005; Babinchak et al 2005; Fomin et al 2005; Breedt et al 2005; Ellis-Grosse, Babinchak et al 2005). The exact mechanism of tigecycline-induced nausea and vomiting remains unknown, but it is not related to the release of serotonin in the gastrointestinal tract (Muralidharan, Micalizzi et al 2005). Nausea and vomiting has occurred more frequently at higher doses and in patients <50 years of age, female, and non-European descent (Muralidharan, Fruncillo et al 2005; Wyeth Pharmaceuticals 2007b). While coadministration of food may potentially improve the tolerability of tigecycline, altering the rate of infusion has not been successful in deceasing the incidence of nausea and vomiting (Muralidharan, Micalizzi et al 2005). Likewise, administration of antiemetics (such as prochlorperazine, ondansetron, or metoclopramide) does not significantly alter the incidence of nausea and vomiting (Muralidharan, Micalizzi et al 2005; Wyeth Pharmaceuticals 2007b). During phase III clinical studies, diarrhea was reported in 12.7% of patients receiving tigecycline (Wyeth Pharmaceuticals 2007b). However, there were no published cases of Clostridium difficile associated diarrhea in these clinical studies (Babinchak et al 2005; Ellis-Grosse, Babinchak et al 2005; Wyeth Pharmaceuticals 2007b).
Tigecycline’s ability to induce C. difficile infections has also been evaluated (Baines et al 2006). In a human gut model involving 2 epidemic strains of C. difficile, the gut flora was significantly decreased although the C. difficile spores did not “proliferate”; in addition, cytotoxin was not produced (Baines et al 2006). This seems to correlate clinically, as only limited cases of C. difficile infections have been reported with tigecycline to date (Wyeth Pharmaceuticals 2007a). However, as with all antimicrobials, tigecycline can theoretically predispose a patient to a C. difficile infection.
Due to the structural similarities between tetracyclines and tigecycline, cross-reactivity may occur between these two classes of drugs, and caution should be used in patients with known hypersensitivity reactions to tetracyclines (Zhanel et al 2004; Wyeth Pharmaceuticals 2007b). Furthermore, similar side effects may exist between tetracyclines and tigecycline such as photosensitivity reactions, pancreatitis, and tooth discoloration in children under 8 years old (Zhanel et al 2004; Wyeth Pharmaceuticals 2007b). Long-term safety has not been published with the use of tigecycline to date.
Clinical efficacy
Complicated intra-abdominal infections
As with any type of infection, the objectives for the treatment of cIAI are to minimize the time to clinical improvement, prevent recurrence, and eradicate the causative microorganisms. As with most infections, healthcare-associated infectious diseases generally require broader antibacterial coverage for such resistant organisms as P. aerunginosa, Enterobacter spp. and MRSA as compared with community-associated infections (Solomkin, Mazuski et al 2003).
Guidelines published by the Infectious Diseases Society of America for the treatment of cIAIs describe the use of a single-agent, broad-spectrum antimicrobial agent or the use of a combination of antibiotics with activity against common enteric flora (Solomkin, Mazuski et al 2003). They summarize data from numerous trials. For example, monotherapy for the treatment of cIAIs studied in randomized, prospective clinical trials include the β-lactam/β-lactamase inhibitors such as ampicillin/sulbactam, piperacillin/tazobactam, and ticarcillin/clavulanic acid (Eklund et al 1993; Walker et al 1993; Dougherty et al 1995; Jaccard et al 1998; Allo et al 1999; Ohlin et al 1999; Cohn et al 2000). The carbapenems (ertapenem, imipenem/cilastatin and meropenem) as well as certain cephalosporins (ceftotetan and cefoxitin) have also been studied (Poenaru et al 1990; Brismar et al 1992; Eklund et al 1993; Brismar et al 1995; Condon et al 1995; Geroulanos 1995; Huizinga et al 1995; Angeras et al 1996; Berne et al 1996; Christou et al 1996; Colardyn et al 1996; Solomkin et al 1996; Barie et al 1997; Basoli et al 1997; Donahue et al 1998; Allo et al 1999; Solomkin et al 2001; Solomkin, Mazuski et al 2003; Solomkin, Yellin et al 2003). As for combination regimens, aminoglycosides, quinolones or certain cephalosporin agents in addition to anti-anaerobic medications (clindamycin or metronidazole) also have data to support their use (Solomkin, Mazuski et al 2003).
Tigecycline has been studied specifically in adult patients with cIAIs in 2 phase III, noninferiority, multicenter, randomized, double-blind trials (Oliva et al 2005; Fomin et al 2005) and are presented together in a pooled analysis (Babinchak et al 2005). Patients 18 years old and older who also required surgical intervention for treatment of cIAIs were included. The cIAIs were defined as perforated intestines, intra-abdominal abscesses, appendicitis, diverticulitis, or cholecysitis with perforation and/or abscess with fecal contamination, or perforated gastric/duodenal ulcers, and complicated peritonitis (Babinchak et al 2005). Patients were stratified by randomization according to their APACHE II scores and received either intravenous tigecycline 100 mg followed by 50 mg every 12 hours or intravenous imipenem-cilastatin 500 mg every 6 hours (adjusted based on the patient’s weight and renal function). Patients were generally treated for 5–14 days.
The primary endpoint for these studies was “the clinical response at the test-of-cure visit (12–42 days after therapy) in the co-primary end point microbiologically evaluable [ME] and microbiological modified intent-to-treat [mm-ITT] populations” (Babinchak et al 2005).
A total of 1658 patients were randomized in these 2 trials; the mm-ITT population included 1262 patients, and the ME population was composed of 1025 patients. The mean of subject age was 47 years, and the most commonly reported intra-abdominal infection was complicated appendicitis (50.6%, tigecycline and 48.7%, imipenem-cilastatin) followed by complicated cholecystitis (12.8%, tigecycline and 15.1%, imipenem-cilastatin). The average APACHE II score was 6.3 (tigecycline group) and 6 (imipenem-cilastatin) with only 35 patients having an APACHE II score >15. The mean duration of therapy with either agent was approximately 8 days (Babinchak et al 2005). Clinical cures were reported in 80.2% (506/631) and 81.5% (514/631) of tigecycline and imipenem m-mITT groups, respectively (% difference (95%CI): −1.3% (−5.8% to 3.2%)). The ME population had similar response rates, with 86.1% (441/512) and 86.2% (442/513) clinical cure rate in the tigecycline and imipenem-cilastatin groups, respectively (Babinchak et al 2005). Although many organisms were identified, the most commonly isolated organisms included E. coli (n = 665), S. anginosus (n = 198), K. pneumonia (n = 112) and B. fragilis (n = 160) (Babinchak et al 2005). The most commonly reported adverse events reported in these studies included gastrointestinal complaints with a statistically higher rate in the tigecycline group compared with those receiving imipenem-cilastatin. There were a total of 44.4% and 39.4% of reported adverse events with the digestive system in the tigecycline and imipenem-cilastatin patients, respectively (p = 0.04) (Babinchak et al 2005).
Based on the results of this analysis, tigecycline appears to be as safe and effective as imipenem in cIAIs. One limitation in this trial was the relatively few resistant organisms isolated. Thus, these studies may not apply to the patient population in which resistance is a concern. Additional clinical trials examining tigecycline’s use in cIAIs including resistant organisms will further the utility of tigecycline in this type of infection.
Complicated skin and skin structure infections
Complicated skin and skin structure infections (cSSSIs) either involve deep soft tissues or require surgical debridement or interventions. These infections often require parenteral antimicrobial treatment and frequently occur in patients with other comorbid disease states (such as diabetes or peripheral vascular disease) in which their response to antimicrobial treatment can be suboptimal. Examples of cSSSIs include major abscesses, burns, surgical site infections, diabetic foot, and infected ulcers (CDER 1998; Nichols 1999, 2001; Dinubile et al 2004; Lee et al 2005).
Numerous pathogens have been associated with cSSSIs and are often dependent upon the patient and clinical scenario. In general though, S. aureus and Streptococcus spp. tend to be the predominant pathogens with Gram-negatives, anaerobes, and resistant pathogens such as MRSA becoming more of a factor in immunocompromised patients, injection drug users, and nosocomially-acquired infections (Rennie et al 2003; Dinubile et al 2004). Additionally, some infections (such as lower extremity infections in diabetic patients) tend to be more polymicrobial in nature (Doern et al 1999; Rennie et al 2003; Dinubile et al 2004).
Besides surgical debridement, there are multiple antimicrobial options that are available for the treatment of cSSSIs. Empiric antimicrobial therapy should include coverage for Gram-positive cocci such as staphlococci and streptococci. Additional coverage for Gram-negative organisms, anaerobes (such as B. fragilis group), or resistant pathogens is dependent upon patient risk factors for such organisms (Nichols 1999; Dinubile et al 2004; Stevens et al 2005; Lee et al 2005). Local resistance patterns should also play an important role in deciding appropriate empiric treatment. According to the skin and soft tissue infections guidelines set forth by the Infectious Diseases Society of America (Fass et al 1985; Tan et al 1993; Talan et al 2000; Grayson et al 2002; Graham, Lucasti et al 2002; Graham, Talan et al 2002; Stevens et al 2005; Fabian et al 2005; Giordano et al 2005), treatment options include broad-spectrum antibiotics such as carbapenems (eg, imipenem/cilastin, meropenem, ertapenem), beta-lactam/beta-lactamase inhibitor combinations (eg, piperacillin-tazobactam, ticarcillin-clavulanate, ampicillin-sulbactam), cephalosporins (eg, cefazolin, cefoxitin), and fluoroquinolones (eg, levofloxacin, moxifloxacin) used alone or in combination with clindamycin or metronidazole for anerobic coverage. The addition of vancomycin or other newer antimicrobial agents (eg, daptomycin, linezolid, quinupristin/dalfopristin, tigecycline) with activity against resistant organisms such as MRSA, VRE, and ESBL-producing gram negative organisms is dependent upon the clinical circumstances of the patient (Nichols et al 1999; Stevens et al 2000; Stevens et al 2005; Lipsky et al 2005).
Tigecycline has been evaluated for the treatment of cSSSIs in two randomized, multi-centered, double-blind phase 3 studies (Breedt et al 2005; Ellis-Grosse, Babinchak et al 2005; Sacchidanand et al 2005). In both studies, hospitalized adult patients with cSSSIs (defined as deep soft tissue infections, soft tissue infections requiring surgical debridement, or soft tissue infections in patients with underlying disease such as diabetes or peripheral vascular disease) were randomized (1:1) to receive either tigecycline (100 mg loading dose followed by 50 mg every 12 hours over 60 minutes) or vancomycin (1 g every 12 hours over 60 minutes with adjustments based on renal function) plus aztreonam (2 g every 12 hours over 60 minutes) intravenously for up to 14 days. At the discretion of the investigators, aztreonam therapy could be discontinued after 48 hours of treatment. The clinical response at the test-of-cure-visit (12–92 days after the last dose) in the clinically evaluable (CE) and the clinical modified intention-to-treat (c-mITT) was the primary endpoint of these studies (Ellis-Grosse, Babinchak et al 2005).
Pooled analysis of the data (N = 1129) demonstrated that baseline demographics between each group was similar in terms of type of infection and incidence of other comorbid disease states (Ellis-Grosse, Babinchak et al 2005). Caucasian (68.2%) men (62.1%) with a mean age of 48 made up the majority of the patients enrolled in the studies. Patients were on antibiotic treatment for a mean of 8 days in each group. The most common type of cSSSIs was cellulitis (59%). In the c-mITT analysis (comprised of patients who received at least 1 dose of the study drug and had clinical evidence of a cSSSI; ) (N = 1057), 79.7% in the tigecycline arm (429/538) versus 81.9% in the vancomycin-aztreonam arm (425/519) [95% CI for the difference −2.1 (−7.1% to 2.8%)] had a clinical cure, defined as resolution of the signs and symptoms of cSSSI and completion of antibiotic therapy. Clinical cure rates for the CE population (defined as c-mITT population without P. aeruginosa as sole isolate, no other concurrent antibiotic therapy, and assessed for failure or cure at the TOC visit) (N = 833) were 86.5% for patients receiving tigecycline (365/422) versus 88.6% in the comparator arm (364/411) [95% CI for the difference, −2.1 (−6.8 to 2.7)]. The most common organism isolated was MSSA (N = 254). Cure rates for MSSA were 88.8% (N = 119/134) versus 90.8% (N = 109/120), respectively for tigecycline and vancomycin-aztreonam arms. Sixty-five patients had MRSA isolates, of which 32% (N = 21/65) were considered to be community-acquired strains. Overall cure rates for MRSA were 78.1% (N = 25/32) in the tigecycline arm and 75.8% (N = 25/33) for the vancomycin-aztreonam arm (Ellis-Grosse, Babinchak et al 2005). ESBL-producing organisms treated with tigecycline had clinical cure rates of 77.8% (N = 9) for E. coli, 85.7% (N = 7) for K. pneumoniae, and 100% (N = 3) for P. mirabilis (Ellis-Grosse, Bradford et al 2005). The authors concluded from these pooled analysis, that tigecycline was noninferior to the combination of vancomycin-aztreonam in the treatment of cSSSIs. The incident of adverse events was similar between the groups (67.7% tigecycline versus 61.1% vancomycin-aztreonam) with the most common adverse events being related to gastrointestinal complaints (46% tigecycline versus 21% vancomycin-aztreonam; p < 0.001) (Ellis-Grosse, Babinchak et al 2005).
Conclusions
Tigecycline represents a new treatment option for both cSSSIs and cIAIs due, in part, to its favorable in vitro activity against a wide variety of aerobic Gram-positive, Gram-negative and anaerobic organisms (including multidrug-resistant pathogens such as MRSA, VRE, and ESBL-producing strains of E. coli and Klebsiella). In contrast, tigecycline lacks activity in vitro against P. aeruginosa and P. mirabilis. Following twice daily intravenous administration, it is extensively distributed to various body tissues and fluids. Dose modification is required in patients with significant hepatic impairment. Because of the metabolic profile of tigecycline, the potential for drug interactions appears to be minimal.
Based on existing clinical efficacy and safety data, tigecycline has been FDA-approved for use as monotherapy for the treatment of cSSSIs and cIAIs. Published clinical efficacy data in humans reports tigecycline as noninferior to comparators for such indications. Tigecycline might be particularly useful in suspected or documented polymicrobial infections, including those patients otherwise requiring combination therapies due to the presence of drug-resistant pathogens such as MRSA, VRE, or ESBL-producing strains of E. coli and K. pneumoniae. In contrast, its role as part of combination therapy with other antimicrobials is uncertain. Gastrointestinal side effects (mainly nausea) may be problematic in some patients.
References
- Aires JR, Kohler T, Nikaido H, et al. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–8. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allo MD, Bennion RS, Kathir K, et al. Ticarcillin/clavulanate versus imipenem/cilistatin for the treatment of infections associated with gangrenous and perforated appendicitis. Am Surg. 1999;65:99–104. [PubMed] [Google Scholar]
- Angeras MH, Darle N, Hamnstrom K, et al. A comparison of imipenem/cilastatin with the combination of cefuroxime and metronidazole in the treatment of intra-abdominal infections. Scand J Infect Dis. 1996;28:513–18. doi: 10.3109/00365549609037950. [DOI] [PubMed] [Google Scholar]
- Babinchak T, Ellis-Grosse E, Dartois N, et al. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005;41(Suppl 5):S354–67. doi: 10.1086/431676. [DOI] [PubMed] [Google Scholar]
- Baines SD, Saxton K, Freeman J, et al. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother. 2006;58:1062–5. doi: 10.1093/jac/dkl364. [DOI] [PubMed] [Google Scholar]
- Barie PS, Vogel SB, Dellinger EP, et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Cefepime Intra-abdominal Infection Study Group. Arch Surg. 1997;132:1294–302. doi: 10.1001/archsurg.1997.01430360040008. [DOI] [PubMed] [Google Scholar]
- Basoli A, Meli EZ, Mazzocchi P, et al. Imipenem/cilastatin (1.5 g daily) versus meropenem (3.0 g daily) in patients with intra-abdominal infections: results of a prospective, randomized, multicentre trial. Scand J Infect Dis. 1997;29:503–8. doi: 10.3109/00365549709011863. [DOI] [PubMed] [Google Scholar]
- Bergeron J, Ammirati M, Danley D, et al. Glycylcyclines bind to the high-affinity tetracycline ribosomal binding site and evade Tet(M)- and Tet(O)-mediated ribosomal protection. Antimicrob Agents Chemother. 1996;40:2226–8. doi: 10.1128/aac.40.9.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne TV, Yellin AE, Appleman MD, et al. Meropenem versus tobramycin with clindamycin in the antibiotic management of patients with advanced appendicitis. J Am Coll Surg. 1996;182:403–7. [PubMed] [Google Scholar]
- Bochicchio GV, Baquero F, Hsueh PR, et al. In vitro susceptibilities of Escherichia coli isolated from patients with intra-abdominal infections worldwide in 2002-2004: results from SMART (Study for Monitoring Antimicrobial Resistance Trends) Surg Infect (Larchmt) 2006;7:537–45. doi: 10.1089/sur.2006.7.537. [DOI] [PubMed] [Google Scholar]
- Bogaerts P, Naas T, Wybo I, et al. Outbreak of infection by carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-58 in Belgium. J Clin Microbiol. 2006;44:4189–92. doi: 10.1128/JCM.00796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovich T, Esel D, Kelly LM, et al. Antistaphylococcal activity of DX-619, a new des-F(6)-quinolone, compared to those of other agents. Antimicrob Agents Chemother. 2005;49:3325–33. doi: 10.1128/AAC.49.8.3325-3333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchillon SK, Hoban DJ, Johnson BM, et al. In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (TEST Program; 2004) Diagn Microbiol Infect Dis. 2005a;52:173–9. doi: 10.1016/j.diagmicrobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bouchillon SK, Hoban DJ, Johnson BM, et al. In vitro evaluation of tigecycline and comparative agents in 3049 clinical isolates: 2001 to 2002. Diagn Microbiol Infect Dis. 2005b;51:291–5. doi: 10.1016/j.diagmicrobio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Bradford PA, Weaver-Sands DT, Petersen PJ. In vitro activity of tigecycline against isolates from patients enrolled in phase 3 clinical trials of treatment for complicated skin and skin-structure infections and complicated intra-abdominal infections. Clin Infect Dis. 2005;41(Suppl 5):S315–32. doi: 10.1086/431673. [DOI] [PubMed] [Google Scholar]
- Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49:4658–66. doi: 10.1128/AAC.49.11.4658-4666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar B, Malmborg AS, Tunevall G, et al. Meropenem versus imipenem/cilastatin in the treatment of intra-abdominal infections. J Antimicrob Chemother. 1995;35:139–48. doi: 10.1093/jac/35.1.139. [DOI] [PubMed] [Google Scholar]
- Brismar B, Malmborg AS, Tunevall G, et al. Piperacillin-tazobactam versus imipenem-cilastatin for treatment of intra-abdominal infections. Antimicrob Agents Chemother. 1992;36:2766–73. doi: 10.1128/aac.36.12.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CDCP] Centers for Disease Control and Prevention, National Health Center for Statistics. Vital and Health Statistics: Detailed Diagnoses and Procedures. Hyattsville, MD: U.S. Department of Health and Human Services; 1997. National Hospital Discharge Survey, 1994. Vol. 127. [Google Scholar]
- [CDER] Centers for Drug Evaluation and Research. Uncomplicated and complicated skin and skin structure infections – Developing antimicrobial drugs for treatement. Guidance for Industry (online) 1998 Accessed 5 May 2007. URL: http://www.fda.gov/cder/guidance/2566dft.pdf.
- Cercenado E, Marin M, Sanchez-Martinez M, et al. In Vitro Activities of Tigecycline and Eight Other Antimicrobials against Different Nocardia Species Identified by Molecular Methods. Antimicrob Agents Chemother. 2007;51:1102–4. doi: 10.1128/AAC.01102-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NC, Hsueh PR, Liu YC, et al. In vitro activities of tigecycline, ertapenem, isepamicin, and other antimicrobial agents against clinically isolated organisms in Taiwan. Microb Drug Resist. 2005;11:330–41. doi: 10.1089/mdr.2005.11.330. [DOI] [PubMed] [Google Scholar]
- Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29:245–77. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–60. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou NV, Turgeon P, Wassef R, et al. Management of intra-abdominal infections. The case for intraoperative cultures and comprehensive broad-spectrum antibiotic coverage. The Canadian Intra-abdominal Infection Study Group. Arch Surg. 1996;131:1193–201. doi: 10.1001/archsurg.1996.01430230075014. [DOI] [PubMed] [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute [formerly National Committee for Clinical Laboratory Standards (NCCLS)] Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 6. Wayne, PA: 2003a. [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute [formerly National Committee for Clinical Laboratory Standards (NCCLS)] Performance standards for antimicrobial disk diffusion susceptibility tests. 8. Wayne, PA: 2003b. [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute [formerly National Committee for Clinical Laboratory Standards (NCCLS)] Methods for antimicrobial susceptibility testing of anaerobic bacteria. 6. Wayne, PA: 2004. [Google Scholar]
- [CLSI] Clinical and Laboratory Standards Institute [formerly National Committee for Clinical Laboratory Standards (NCCLS)] Performance standards for antimicrobial susceptibility testing – 15th informational supplement. Wayne, PA: 2005. [Google Scholar]
- Cohn SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg. 2000;232:254–62. doi: 10.1097/00000658-200008000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colardyn F, Faulkner KL. Intravenous meropenem versus imipenem/cilastatin in the treatment of serious bacterial infections in hospitalized patients. Meropenem Serious Infection Study Group. J Antimicrob Chemother. 1996;38:523–37. doi: 10.1093/jac/38.3.523. [DOI] [PubMed] [Google Scholar]
- Condon RE, Walker AP, Sirinek KR, et al. Meropenem versus tobramycin plus clindamycin for treatment of intraabdominal infections: results of a prospective, randomized, double-blind clinical trial. Clin Infect Dis. 1995;21:544–50. doi: 10.1093/clinids/21.3.544. [DOI] [PubMed] [Google Scholar]
- Conte JE, Jr, Golden JA, Kelly MG, et al. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int J Antimicrob Agents. 2005;25:523–9. doi: 10.1016/j.ijantimicag.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Cueto J, D’Allemagne B, Vazquez-Frias JA, et al. Morbidity of laparoscopic surgery for complicated appendicitis: an international study. Surg Endosc. 2006;20:717–20. doi: 10.1007/s00464-005-0402-4. [DOI] [PubMed] [Google Scholar]
- Dean CR, Visalli MA, Projan SJ, et al. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob Agents Chemother. 2003;47:972–8. doi: 10.1128/AAC.47.3.972-978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinubile MJ, Lipsky BA. Complicated infections of skin and skin structures: When the infection is more than skin deep. J Antimicrob Chemother. 2004;53(Suppl S2):ii37–50. doi: 10.1093/jac/dkh202. [DOI] [PubMed] [Google Scholar]
- Doern GV, Jones RN, Pfaller MA, et al. Bacterial pathogens isolated from patients with skin and soft tissue infections: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). SENTRY Study Group (North America) Diagn Microbiol Infect Dis. 1999;34:65–72. doi: 10.1016/s0732-8893(98)00162-x. [DOI] [PubMed] [Google Scholar]
- Donahue PE, Smith DL, Yellin AE, et al. Trovafloxacin in the treatment of intra-abdominal infections: results of a double-blind, multi-center comparison with imipenem/cilastatin. Trovafloxacin Surgical Group. Am J Surg. 1998;176:53S–61S. doi: 10.1016/s0002-9610(98)00221-9. [DOI] [PubMed] [Google Scholar]
- Dougherty SH, Sirinek KR, Schauer PR, et al. Ticarcillin/clavulanate compared with clindamycin/gentamicin (with or without ampicillin) for the treatment of intra-abdominal infections in pediatric and adult patients. Am Surg. 1995;61:297–303. [PubMed] [Google Scholar]
- Eklund AE, Nord CE. A randomized multicenter trial of piperacillin/tazobactam versus imipenem/cilastatin in the treatment of severe intra-abdominal infections. Swedish Study Group. J Antimicrob Chemother. 1993;31(Suppl A):79–85. doi: 10.1093/jac/31.suppl_a.79. [DOI] [PubMed] [Google Scholar]
- Ellis-Grosse EJ, Babinchak T, Dartois N, et al. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis. 2005;41(Suppl 5):S341–53. doi: 10.1086/431675. [DOI] [PubMed] [Google Scholar]
- Ellis-Grosse E, Bradford PA, Loh E. Tigecycline in patients with extended-spectrum beta lactamases (ESBLs) - producing bacteria: experience from Phase III clinical trials. 43rd Infectious Diseases Society of America; October 6–9; San Francisco, CA. 2005. meeting abstract. [Google Scholar]
- Fabian TC, File TM, Embil JM, et al. Meropenem versus imipenem-cilastatin for the treatment of hospitalized patients with complicated skin and skin structure infections: results of a multicenter, randomized, double-blind comparative study. Surg Infect (Larchmt) 2005;6:269–82. doi: 10.1089/sur.2005.6.269. [DOI] [PubMed] [Google Scholar]
- Fass RJ, Freimer EH, McCloskey RV. Treatment of skin and soft tissue infections with imipenem/cilastatin. Am J Med. 1985;78:110–12. doi: 10.1016/0002-9343(85)90111-1. [DOI] [PubMed] [Google Scholar]
- Fomin P, Beuran M, Gradauskas Aeal. Tigecycline is efficacious in the treatment of complicated intra-abdominal infections. Int J Surg. 2005;3:35–47. doi: 10.1016/j.ijsu.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Fritsche TR, Sader HS, Stilwell MG, et al. Potency and spectrum of tigecycline tested against an international collection of bacterial pathogens associated with skin and soft tissue infections (2000–2004) Diagn Microbiol Infect Dis. 2005;52:195–201. doi: 10.1016/j.diagmicrobio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Fritsche TR, Strabala PA, Sader HS, et al. Activity of tigecycline tested against a global collection of Enterobacteriaceae, including tetracycline-resistant isolates. Diagn Microbiol Infect Dis. 2005;52:209–13. doi: 10.1016/j.diagmicrobio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Andrade SS, et al. In vitro activity of tigecycline, a new glycylcycline, tested against 1,326 clinical bacterial strains isolated from Latin America. Braz J Infect Dis. 2005;9:348–56. doi: 10.1590/s1413-86702005000500001. [DOI] [PubMed] [Google Scholar]
- Garrison MW, Nuemiller JJ. In vitro activity of tigecycline against quinolone-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Int J Antimicrob Agents. 2007;29:191–6. doi: 10.1016/j.ijantimicag.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Geroulanos SJ. Meropenem versus imipenem/cilastatin in intra-abdominal infections requiring surgery. Meropenem Study Group. J Antimicrob Chemother. 1995;36(Suppl A):191–205. doi: 10.1093/jac/36.suppl_a.191. [DOI] [PubMed] [Google Scholar]
- Giordano P, Song J, Pertel P, et al. Sequential intravenous/oral moxifloxacin versus intravenous piperacillin-tazobactam followed by oral amoxicillin-clavulanate for the treatment of complicated skin and skin structure infection. Int J Antimicrob Agents. 2005;26:357–65. doi: 10.1016/j.ijantimicag.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Goldstein EJ, Citron DM, Merriam CV, et al. Comparative in vitro susceptibilities of 396 unusual anaerobic strains to tigecycline and eight other antimicrobial agents. Antimicrob Agents Chemother. 2006;50:3507–13. doi: 10.1128/AAC.00499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfried MH, Rodvold KA, Cwik M, et al. An open-label clinical evaluation of tigecycline concentrations in selected tissues and fluids. Clin Pharmacol Ther. 2005;77:98. [Google Scholar]
- Graham DR, Lucasti C, Malafaia O, et al. Ertapenem once daily versus piperacillin-tazobactam 4 times per day for treatment of complicated skin and skin-structure infections in adults: results of a prospective, randomized, double-blind multicenter study. Clin Infect Dis. 2002;34:1460–8. doi: 10.1086/340348. [DOI] [PubMed] [Google Scholar]
- Graham DR, Talan DA, Nichols RL, et al. Once-daily, high-dose levofloxacin versus ticarcillin-clavulanate alone or followed by amoxicillin-clavulanate for complicated skin and skin-structure infections: a randomized, open-label trial. Clin Infect Dis. 2002;35:381–9. doi: 10.1086/341026. [DOI] [PubMed] [Google Scholar]
- Grayson ML, McDonald M, Gibson K, et al. Once-daily intravenous cefazolin plus oral probenecid is equivalent to once-daily intravenous ceftriaxone plus oral placebo for the treatment of moderate-to-severe cellulitis in adults. Clin Infect Dis. 2002;34:1440–8. doi: 10.1086/340056. [DOI] [PubMed] [Google Scholar]
- Hoban DJ, Bouchillon SK, Johnson BM, et al. In vitro activity of tigecycline against 6792 Gram-negative and Gram-positive clinical isolates from the global Tigecycline Evaluation and Surveillance Trial (TEST Program, 2004) Diagn Microbiol Infect Dis. 2005;52:215–27. doi: 10.1016/j.diagmicrobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, DeMaio W, Jordan RA, et al. Metabolic disposition (14C) tigecycline in human volunteers following intravenous infusion. American Association of Pharmaceutical Scientists (AAPS) Annual Meeting; November 7–11; Baltimore, MD. 2004. meeting abstract. [Google Scholar]
- Huizinga WK, Warren BL, Baker LW, et al. Antibiotic monotherapy with meropenem in the surgical management of intra-abdominal infections. J Antimicrob Chemother. 1995;36(Suppl A):179–89. doi: 10.1093/jac/36.suppl_a.179. [DOI] [PubMed] [Google Scholar]
- Jaccard C, Troillet N, Harbarth S, et al. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob Agents Chemother. 1998;42:2966–72. doi: 10.1128/aac.42.11.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- Kohler T, Michea-Hamzehpour M, Henze U, et al. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–54. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kuti JL, Nicolau DP. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg Infect. 2005;6:283–95. doi: 10.1089/sur.2005.6.283. [DOI] [PubMed] [Google Scholar]
- Li XZ, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–53. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J Antimicrob Chemother. 2005;55:240–5. doi: 10.1093/jac/dkh531. [DOI] [PubMed] [Google Scholar]
- Meagher AK, Ambrose PG, Grasela TH, et al. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis. 2005;52:165–71. doi: 10.1016/j.diagmicrobio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Meagher AK, Passarell JA, Cirincione BB, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother. 2007;51:1939–45. doi: 10.1128/AAC.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine T, Morita Y, Kataoka A, et al. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–17. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: Report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagn Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Monge JV, de Los Terreros L, Soler L, Diaz-Agero Perez C, et al. Excess length of stay attributable to surgical site infection following hip replacement: a nested case-control study. Infect Control Hosp Epidemiol. 2006;27:1299–303. doi: 10.1086/509828. [DOI] [PubMed] [Google Scholar]
- Muralidharan G, Fruncillo RJ, Micalizzi M, et al. Effects of age and sex on single-dose pharmacokinetics of tigecycline in healthy subjects. Antimicrob Agents Chemother. 2005;49:1656–9. doi: 10.1128/AAC.49.4.1656-1659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan G, Micalizzi M, Speth J, et al. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother. 2005;49:220–9. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RL. Optimal treatment of complicated skin and skin structure infections. J Antimicrob Chemother. 1999;44(Suppl A):19–23. doi: 10.1093/jac/44.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(Suppl 2):S84–93. doi: 10.1086/321862. [DOI] [PubMed] [Google Scholar]
- Nichols RL, Graham DR, Barriere SL, et al. Treatment of hospitalized patients with complicated gram-positive skin and skin structure infections: two randomized, multicentre studies of quinupristin/dalfopristin versus cefazolin, oxacillin or vancomycin. Synercid Skin and Skin Structure Infection Group. J Antimicrob Chemother. 1999;44:263–73. doi: 10.1093/jac/44.2.263. [DOI] [PubMed] [Google Scholar]
- Ohlin B, Cederberg A, Forssell H, et al. Piperacillin/tazobactam compared with cefuroxime/metronidazole in the treatment of intra-abdominal infections. Eur J Surg. 1999;165:875–84. doi: 10.1080/11024159950189393. [DOI] [PubMed] [Google Scholar]
- Oliva ME, Rekha A, Yellin A, et al. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744] BMC Infect Dis. 2005;5:88. doi: 10.1186/1471-2334-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MW, Ruzin A, Feyfant E, et al. Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob Agents Chemother. 2006;50:2156–66. doi: 10.1128/AAC.01499-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–31. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- Petersen PJ, Bradford PA, Weiss WJ, et al. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediat Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob Agents Chemother. 2002;46:2595–601. doi: 10.1128/AAC.46.8.2595-2601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenaru D, De SM, Christou NV. Imipenem versus tobramycin – antianaerobe antibiotic therapy in intra-abdominal infections. Can J Surg. 1990;33:415–22. [PubMed] [Google Scholar]
- Poole K, Gotoh N, Tsujimoto H, et al. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–24. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- Postier RG, Green SL, Klein SR, et al. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin Ther. 2004;26:704–14. doi: 10.1016/s0149-2918(04)90070-7. [DOI] [PubMed] [Google Scholar]
- Projan SJ. Preclinical pharmacology of GAR-936, a novel glycylcycline antibacterial agent. Pharmacotherapy. 2000;20:219S–23. doi: 10.1592/phco.20.14.219s.35046. [DOI] [PubMed] [Google Scholar]
- Raible D, Zimmerman JJ, Harper D, et al. Pharmacokinetics and Pharmacodynamics of Tigecycline and Warfarin Coadministered to Healthy Subjects. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19; Washington, DC. 2005. meeting abstract. [Google Scholar]
- Rasmussen BA, Gluzman Y, Tally FP. Inhibition of protein synthesis occurring on tetracycline-resistant, TetM-protected ribosomes by a novel class of tetracyclines, the glycylcyclines. Antimicrob Agents Chemother. 1994;38:1658–60. doi: 10.1128/aac.38.7.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A, Burgess D. In vitro pharmacodynamics and post-antibiotic effcts (PAE) of tigecycline against gram-negative bacteria. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19; Washington, DC. 2005. meeting abstract. [Google Scholar]
- Rello j. Pharmacokinetics, pharmacodynamics, safety and tolerability of tigecycline. J Chemother. 2005;17(Suppl 1):12–22. doi: 10.1179/joc.2005.17.Supplement-1.12. [DOI] [PubMed] [Google Scholar]
- Rennie RP, Jones RN, Mutnick AH, et al. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: Report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000) Diagn Microbiol Infect Dis. 2003;45:287–93. doi: 10.1016/s0732-8893(02)00543-6. [DOI] [PubMed] [Google Scholar]
- Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S100–5. doi: 10.1086/504487. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Avial I, Rodriguez-Avial C, Lopez O, et al. In vitro activity of tigecycline (GAR-936) and other antimicrobials against tetracycline- and ciprofloxacin-resistant Campylobacter clinical isolates. Int J Antimicrob Agents. 2006;27:303–6. doi: 10.1016/j.ijantimicag.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Rodvold KA, Gotfried MH, Cwik M, et al. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58:1221–9. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- Rodvold K, Gotfried M, Cwik M, et al. Tigeycline (TGC) concentration (Cp) in lung tissue, cerebrospinal fluid (CSF), and bile of human subjects. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19; Washington, DC. 2005. meeting abstract. [Google Scholar]
- Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: Results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9:251–61. doi: 10.1016/j.ijid.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sader HS, Jones RN, Stilwell MG, et al. Tigecycline activity tested against 26,474 bloodstream infection isolates: a collection from 6 continents. Diagn Microbiol Infect Dis. 2005;52:181–6. doi: 10.1016/j.diagmicrobio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Saunders S, Baird-Bellaire SJ, Patat AA, et al. Pharmacokinetics of tigecycline (TGC) in patients with hepatic impairment. European Association for Clinical Pharmacology and Therapeutics; June 24–29; Poznan, Poland. 2005. meeting abstract. [Google Scholar]
- Scheetz MH, Reddy P, Nicolau DP, et al. Peritoneal fluid penetration of tigecycline. Ann Pharmacother. 2006;40:2064–7. doi: 10.1345/aph.1H229. [DOI] [PubMed] [Google Scholar]
- Snydman DR, Jacobus NV, McDermott LA, et al. National Survey on the Susceptibility of B. fragilis Group: Report and Analysis of Trends for 1997–2004: A US Survey. Antimicrob Agents Chemother. 2007;51:1649–55. doi: 10.1128/AAC.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin JS, Mazuski JE, Baron EJ, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37:997–1005. doi: 10.1086/378702. [DOI] [PubMed] [Google Scholar]
- Solomkin JS, Reinhart HH, Dellinger EP, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. The Intra-Abdominal Infection Study Group. Ann Surg. 1996;223:303–15. doi: 10.1097/00000658-199603000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin JS, Wilson SE, Christou NV, et al. Results of a clinical trial of clinafloxacin versus imipenem/cilastatin for intraabdominal infections. Ann Surg. 2001;233:79–87. doi: 10.1097/00000658-200101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin JS, Yellin AE, Rotstein OD, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intra-abdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003;237:235–45. doi: 10.1097/01.SLA.0000048551.32606.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlozano A, Gutierrez J, Salmeron A, et al. Activity of tigecycline against clinical isolates of Staphylococcus aureus and extended-spectrum beta-lactamase-producing Escherichia coli in Granada, Spain. Int J Antimicrob Agents. 2006;28:532–6. doi: 10.1016/j.ijantimicag.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Speer BS, Shoemaker NB, Salyers AA. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992;5:387–99. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- Stevens DL, Smith LG, Bruss JB, et al. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2000;44:3408–13. doi: 10.1128/aac.44.12.3408-3413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturkenboom MC, Goettsch WG, Picelli G, et al. Inappropriate initial treatment of secondary intra-abdominal infections leads to increased risk of clinical failure and costs. Br J Clin Pharmacol. 2005;60:438–43. doi: 10.1111/j.1365-2125.2005.02443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HK, Ong CT, Umer A, et al. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob Agents Chemother. 2005;49:1629–32. doi: 10.1128/AAC.49.4.1629-1632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccone FS, Rodriguez-Villalobos H, De BD, et al. Successful treatment of septic shock due to pan-resistant Acinetobacter baumannii using combined antimicrobial therapy including tigecycline. Eur J Clin Microbiol Infect Dis. 2006;25:257–60. doi: 10.1007/s10096-006-0123-1. [DOI] [PubMed] [Google Scholar]
- Talan DA, Summanen PH, Finegold SM. Ampicillin/sulbactam and cefoxitin in the treatment of cutaneous and other soft-tissue abscesses in patients with or without histories of injection drug abuse. Clin Infect Dis. 2000;31:464–71. doi: 10.1086/313971. [DOI] [PubMed] [Google Scholar]
- Tally FT, Ellestad GA, Testa RT. Glycylcyclines: a new generation of tetracyclines. J Antimicrob Chemother. 1995;35:449–52. doi: 10.1093/jac/35.4.449. [DOI] [PubMed] [Google Scholar]
- Tan JS, Wishnow RM, Talan DA, et al. Treatment of hospitalized patients with complicated skin and skin structure infections: double-blind, randomized, multicenter study of piperacillin-tazobactam versus ticarcillin-clavulanate. The Piperacillin/Tazobactam Skin and Skin Structure Study Group. Antimicrob Agents Chemother. 1993;37:1580–6. doi: 10.1128/aac.37.8.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [EUCAST Steering Committee] The European Committee on Antimicrobial Susceptility Testing (EUCAST) Steering Committee. EUCAST technical note on tigecycline. Clin Microbiol Infect. 2006;12:1147–9. doi: 10.1111/j.1469-0691.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- Tombs NL. Tissue distribution of GAR-936, a broad spectrum antibiotic in male rats. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 26–29; San Francisco, CA. 1999. meeting abstract. [Google Scholar]
- Townsend ML, Pound MW, Drew RH. Tigecycline: a new glycylcycline antimicrobial. Int J Clin Pract. 2006;60:1662–72. doi: 10.1111/j.1742-1241.2006.01188.x. [DOI] [PubMed] [Google Scholar]
- Troy SM, Muralidharan G, Micalizzi M, et al. The effects of renal disease on the pharmacokinetics of tigecycline (GAR-936). 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17; Chicago, IL. 2003. meeting abstract. [Google Scholar]
- van Ogtrop ML, Andes D, Stamstad TJ, et al. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:943–9. doi: 10.1128/aac.44.4.943-949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart SA, Owen JS, Ludwig EA, et al. Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections. Antimicrob Agents Chemother. 2006;50:3701–7. doi: 10.1128/AAC.01636-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas AA, Jodra VM, Garcia ML. Nosocomial infection in surgery wards: a controlled study of increased duration of hospital stays and direct cost of hospitalization. Eur J Epidemiol. 1993;9:504–10. [PubMed] [Google Scholar]
- Waites KB, Duffy LB, Dowzicky MJ. Antimicrobial susceptibility among pathogens collected from hospitalized patients in the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Antimicrob Agents Chemother. 2006;50:3479–84. doi: 10.1128/AAC.00210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AP, Nichols RL, Wilson RF, et al. Efficacy of a beta-lactamase inhibitor combination for serious intra-abdominal infections. Ann Surg. 1993;217:115–21. doi: 10.1097/00000658-199302000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrock-Wadman S, Sherman DR, Hickey MJ, et al. Characterization of Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–83. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth Pharmaceuticals. Data on File (CSR-44355) Philadelphia, PA: 2007a. [Google Scholar]
- Wyeth Pharmaceuticals. Tygacil (package insert) Philadelphia, PA: 2007b. [Google Scholar]
- Zhanel GG, Homenuik K, Nichol K, et al. The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64:63–88. doi: 10.2165/00003495-200464010-00005. [DOI] [PubMed] [Google Scholar]
- Zimmerman JJ, Harper D, Matschke K, et al. Tigecycline and digoxin co-administered to healthy men. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; October 30–November 2; Washington, DC. 2004. meeting abstract. [Google Scholar]
- Zimmerman JJ, Harper DM, Matschke K, et al. Absence of an interaction between tigecycline and digoxin in healthy men. Pharmacotherapy. 2007;27:835–44. doi: 10.1592/phco.27.6.835. [DOI] [PubMed] [Google Scholar]


