Abstract
The prognosis for patients with newly diagnosed malignant gliomas remains poor; however there have been some recent advances in treatment that have generated optimism. Medical management usually includes administration of corticosteroids to control peritumoral edema. Anticonvulsants are indicated for patients with established tumor-related seizures; however, the prophylactic use of anticonvulsants remains controversial. Advances in neurosurgical techniques have improved the safety of tumor resection and most patients undergo the maximal safe surgical debulking of tumor. The tissue sample obtained provides conclusive pathologic diagnosis and tumor classification and extensive tumor resection may impact patient outcome. For glioblastoma, external beam radiation had been the conventional first line treatment; however a recent international phase III trial has provided level 1 evidence that a chemoradiation regimen using external beam radiation plus the oral chemotherapy agent temozolomide provides a survival advantage over radiation alone. Correlative studies were also performed that demonstrated better outcomes for patients with tumors demonstrating methylation (inactivation) of the promoter region of methyl guanine methyltransferase (MGMT) gene. Additional studies are in progress building on the clinical trial results using different dosing schedules of temozolomide and combination regimens. Studies are also underway to develop molecular markers, such as expression of MGMT that may help select the patients most likely to benefit from this treatment.
Keywords: glioblastoma, glioma, temozolomide, MGMT, patient management
Although the prognosis for patients with newly diagnosed malignant gliomas remains poor, recent advances in the treatment of these cancers have resulted in an incremental improvement in survival. More importantly, this progress has spawned renewed interest and enthusiasm for investigating new treatments, translating laboratory findings into new therapeutic approaches and optimizing the treatment of individual patients based on molecular characterization of the tumor. This article will review these recent advances.
The incidence of malignant gliomas in the United States has been estimated to be approximately 18,500 cases per year (CBTRUS 2005). The median age of these patients is in the mid-40s, although the more malignant tumors are more prevalent in older patients. The treatment of patients with malignant brain tumors has proven to be quite challenging. In addition to the typical patient management issues related to treatment toxicities such as myelotoxicity, nausea and vomiting, patients with brain tumors are at risk of seizures and have a high incidence of both deep venous thrombosis and pulmonary embolism (Marras et al 2000; Hildebrand et al 2005). In evaluating possible treatment regimens, patient clinical characteristics are important determinants of outcome and selection criteria may alter apparent treatment efficacy (Curran et al 1993). Many studies identify prognostic factors such as age and performance status, although tumor grade is the most important determinant of prognosis. Therefore, treatments that change the standard of care generally are large, randomized clinical trials or studies that carefully account for the potential of selection bias by performing appropriate comparisons with adjusted historical data.
Although the treatment of malignant gliomas is evolving, several fundamental principles of patient management remain. At time of presentation, most patients require treatment with corticosteroids because of peritumoral edema and resultant mass effect (Moots 1998). Because of the potential for long-term complications of corticosteroids, the lowest therapeutic dose should be used. Individual variation in the required dose will occur, but most often dexamethasone in doses ranging from 4–16 mg per day is required. Patients who present with seizures do require treatment with anticonvulsants. The choice of anticonvulsant can be complex and may be dictated by availability of a parenteral form, particularly if emergency treatment of the seizures is required. The first generation anticonvulsants such as phenytoin, phenobarbital and carbamazepine will augment hepatic cytochrome P450 enzyme activity (Fetell et al 1997). This enzyme enhancement has been shown to alter the pharmacokinetics of many cancer chemotherapy agents. For this reason, many patients with brain tumors are treated with the newer generation anticonvulsants such as levitiracetam, gabapentin, or lamotrigene. The prophylactic use of anticonvulsants in patients with brain tumors who have not had a seizure remains controversial. The American Academy of Neurology published a consensus paper in 2000, which after performing a meta-analysis of available phase III studies of prophylactic anticonvulsant use in brain tumor patients concluded that there was insufficient data to support use in patients who had not had a document seizure (Glantz et al 2000).
Patients may present with both slowly evolving signs and symptoms such as progressive memory loss or hemiparesis or abruptly with seizures or rapid loss of neurologic function (often as a consequence of intra-tumoral hemorrhage). Brain imaging with either magnetic resonance imaging (MRI) or computerized axial tomography (CAT) scans will reveal the tumor (Wen et al 2001). If no contraindication exists, MRI is the diagnostic test of choice, as this imaging modality provides better definition of tumor and neuroanatomy. At this point, a histopathologic diagnosis must be made. The decision to resect tumor or perform an open or stereotactic biopsy is usually based on tumor location, need for tumor decompression because of increased intracranial pressure or conversely comorbid conditions in the patient that increases the risk of an extensive surgical procedure. Only patients with infiltrating brainstem abnormalities are treated without histologic confirmation of tumor because of the risk of neurologic injury from the surgical procedure. In these situations, other processes such as multiple sclerosis, sarcoidosis or Lyme disease must be excluded before initiating brain tumor treatment.
The therapeutic role of surgery remains controversial. There have been no randomized studies comparing biopsy with tumor resection in patients with malignant gliomas. Statistical analyses of large clinical trials often uncover extent of tumor resection as a prognostic factor. However, other factors such as tumor location, concurrent medical illness and patient performance status may impact the decision for surgery and the resultant impact of surgical resection may reflect a selection bias. Some carefully evaluated retrospective series do provide some supportive evidence that surgical resection may improve outcomes. The study by LaCroix evaluated 417 consecutive patients with malignant gliomas who underwent tumor resection with the pre-operative intent to perform a complete resection (Lacroix et al 2001). After accounting for prognostic factors such as age and performance status, they found that patients who had undergone at least a 98 percent resection by volumetric analysis had a statistically significant improvement in overall survival (13 vs 8.8 months, p < 0.0001).
Most patients require treatment with radiation therapy. Studies from the late 1970s and early 1980s confirm the benefit of external beam radiation over surgery and supportive care with steroids alone (Walker et al 1978; Walker et al 1980). Early studies used either whole brain radiation or regional fields. Although proven effective, these methods did increase the incidence of late radiation-induced brain injury (Vick and Paleologos 1995). Newer technologies such as conformal fields with 3-dimentional planning and intensity-modulated radiation therapy (IMRT) delivers the desired dose of radiation to the target, but limits exposure to the surrounding normal brain parenchyma (reviewed in (Mehta and Silverberg 2005).
The role of chemotherapy for the treatment of malignant brain tumors has been less well defined. Despite a large number of clinical trials reported over the last 30 years, there has been very little evidence that the addition of chemotherapy significantly impacts survival in this patient population when combined with surgery and radiation therapy. The seminal studies performed by the Brain Tumor Study Group in the 1970s clearly demonstrated the survival benefit of radiation compared with supportive care.In these randomized trials, the addition of a nitrosourea, either carmustine (BCNU) or semustine (CCNU) did not significantly improve survival (Walker et al 1980; Green et al 1983). However, a meta-analysis performed in 1991 suggested that the addition of chemotherapy to radiation may have a modest impact, improving survival at 1 year by 10% (Fine et al 1993). A more extensive meta-analysis published in 2001 extended these findings (Stewart 2002). This study by Stewart and colleagues collected individual patient data from 12 randomized trials that compared radiation with a radiation and chemotherapy combination in patients with malignant gliomas. Their analysis revealed a statistically significant but modest improvement in survival at one year of 6 percent. The Medical Research Council (MRC) in the United Kingdom performed a large randomized trial comparing radiation therapy alone with radiation treatment followed by adjuvant treatment with PCV (procarbazine, CCNU and vincristine) (MRC 2001). Despite enrolling 673 patients, no difference was detected in survival between the two groups. Therefore, on the basis of these studies, the benefit of chemotherapy for treating malignant gliomas remained unproven.
The introduction of temozolomide
In 1992, a research group at the MRC reported on the initial clinical testing of temozolomide, an imidazotetrazine derivative that is hydrolyzed at neutral pH to the active drug monomethyl 5-triazeno imidazole carboxamide (MTIC) (Newlands et al 1992). They reported excellent oral bioavailability and the initial phase I testing evaluated a single day regimen as well as a 5-consective day regimen. Experimental studies supported prolonged dosing, therefore, the 5 day regimen which was then established as the preferred treatment schedule and a maximum tolerated dose of 200 mg/m2 was determined. Pharmacokinetic studies demonstrated excellent oral bioavailability with nearly 100% absorption in fasting patients. Further testing revealed no significant alterations with hepatic cytochrome P450 enzyme inducers such as the early generation anticonvulsants. The early clinical testing demonstrated activity in glial malignancies, supporting the further development of this agent for primary central nervous system cancers (O’Reilly et al 1993). The toxicity profile from these early studies showed a low incidence of myelotoxicity that was not cumulative. Nausea and vomiting was generally easily controlled with antiemetics and constipation was the most frequently reported toxicity.
Subsequently, several clinical trials were performed. Two of the early studies markedly impacted the use of temozolomide. The first was a randomized phase II clinical trial comparing temozolomide with procarbazine in patients with recurrent glioblastoma (WHO Grade IV) (Yung et al 2000). Procarbazine, an oral agent, was considered an established treatment for recurrent malignant gliomas. The study randomized a total of 225 patients. The objective response rates were similar between the two treatments (5.4% for temozolomide and 5.3% for procarbazine). However, the 6-month progression free survival rate was significantly improved for patients on the temozolomide arm (21 vs 8%, p = 0.008) and additional quality of life testing using the EORTC QOL battery strongly favored temozolomide (Macdonald et al 2005). However, the primary endpoint of the study, objective response, did not demonstrate an advantage for temozolomide in this population and FDA approval for recurrent glioblastoma was denied.
At the same time, an open label, single arm phase II study was performed in patients with recurrent anaplastic glioma (WHO Grade III) (Yung et al 1999). Eligibility criteria included prior treatment with radiation therapy and some patients did under adjuvant treatment with a nitrosourea containing regimen, either PCV or a nitrosourea alone. This study accrued 162 patients, however on central neuropathology review only 111 patients were found to have a grade III glioma. Overall objective response rate (complete or partial response) for the entire patient group was 35%, the same as the response rate for the confirmed grade III tumors (111 of the 162 enrolled patients). The objective response rate for the subset of patients with grade III tumors and a prior history of nitrosourea use was 22%. On the basis of the response rate in previously treated patients, temozolomide received FDA approval for use in patients with grade III gliomas who had failed prior nitrosourea treatment.
Temozolomide use in newly diagnosed high-grade gliomas
Temozolomide has been tested in several clinical trials in patients with high grade gliomas after a surgical procedure to establish the diagnosis, but before the initiation of radiation therapy (often referred to as neoadjuvant treatment). The study by Gilbert et al (2002) treated 57 patients with either glioblastoma or anaplastic astrocytoma with up to 4 cycles of temozolomide (5 day out of 28 day cycle) prior to radiation(Gilbert et al 2002). The objective response rate to the chemotherapy component was 42% for glioblastoma and 34% for AA. However, the responses were often not durable and the overall median survival of GBM and AA at 13.2 and 23.5 months respectively did not suggest an improvement over other radiation and chemotherapy regimens. Similarly, Chibbaro and colleagues (2004) treated 42 patients with GBM, AA or anaplastic oligodendroglioma (AO) with pre-radiation chemotherapy. They report a 40% objective response rate, but an overall median survival of 14.1 months, despite including the Grade III tumors with an overall better prognosis. The study by Brada et al (2005) treated 187 patients with pre-radiation temozolomide. A maximum of 2 cycles were administered and a response rate of 20% was reported. Finally, Balana et al (2004) examined the efficacy of combining temozolomide with cisplatin as the initial treatment for patients with GBM. Forty patients underwent up to 3 cycles of the combination chemotherapy followed by conventional external beam radiation. Objective responses were reported in 45% of patients to the chemotherapy component, however the overall median survival was only 12.5 months. These studies confirm the activity of temozolomide in terms of response. However, neoadjuvant use did not demonstrate an overall survival benefit.
Phase II study of concurrent temozolomide and radiation followed by adjuvant temozolomide
A novel treatment regimen was published by Stupp and colleagues in 2002 (Stupp et al 2002). They performed a phase II study in patients with newly diagnosed glioblastoma. Patients received conventional external beam radiation therapy, a total dose of 60 Gy, with the concurrent administration of temozolomide at 75 mg/m2. The radiation treatment was administered 5 days per week, whereas the temozolomide was administered all 7 days. Therefore patients received a minimum of 42 consecutive days of temozolomide. One month after the completion of the chemoradiation, patients began adjuvant temozolomide using the conventional schedule of 150–200 mg/m2 days 1–5 of a 28 day cycle for a maximum of 6 cycles. A total of 62 patients were accrued and the median survival of 16.2 months looked promising. The possibility of inadvertent selection bias was addressed by comparing these results with the Radiation Therapy Oncology Group (RTOG) recursive partitioning database. In each class (III, IV, V), the patients on the new protocol showed a superior survival.
Subsequent studies have supported these phase II data. The trial performed by Combs et al treated 53 patients with GBM using a chemoradiation regimen consisting of 60 Gy of radiation delivered as 2 Gy fractions along with daily temozolomide at 50 mg/m2 (compared with the 75 mg/m2 dosing used by Stupp) (Combs et al 2005). No post-radiation chemotherapy was administered. Estimated median survival was reported to be 19 months. The chemoradiation regimen developed by Stupp et al described above was compared with conventional external beam radiation in a randomized phase II, reported by Athanassiou et al (Athanassiou et al 2005). A total of 130 patients with histologically confirmed GBM were randomized. Median survival was better in the experimental arm compared with the control (radiation only) group, (13.4 vs 7.7 months respectively).
Phase III trial comparing chemoradiation with radiation alone for newly diagnosed glioblastoma
The results reported by Stupp et al (2004), outlined above, supported the need for a phase III trial to confirm the benefit of chemoradiation over radiation alone for patients with newly diagnosed GBM. The EORTC and the NCI-Canada collaborated on this large trial. A total of 573 patients were accrued from August 2000 until March 2002. These patients were randomized to radiation only (286 patients) or the radiotherapy plus temozolomide regimen (287 patients), as shown in the Schema in Figure 1. The two groups were well balanced by demographic and prognostic characteristics at time of study entry. The majority of patients received the full dose of radiation (92% in radiation arm, 95% in chemoradiation arm); the full concurrent temozolomide course was administered to 87% of the patients. Adjuvant temozolomide was initiated in 78% of patients with a median of 3 cycles (out of 6 planned) administered. Disease progression was the most common reason for treatment discontinuation (39%).
Figure 1.
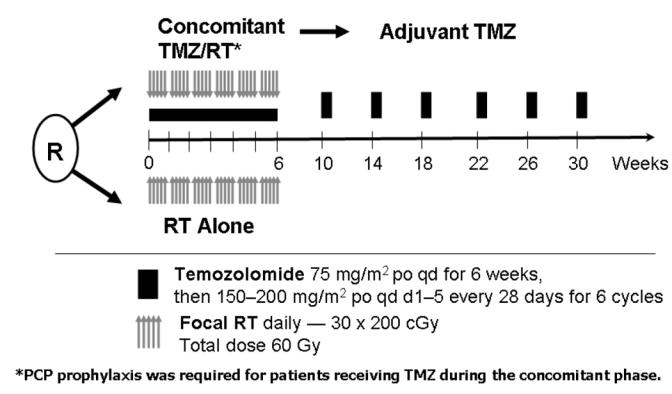
Schema for the phase III randomized trial.
The study demonstrated a clear survival benefit for patients treated with the chemoradiation regimen compared with radiation alone. The median overall survival was 14.6 months for patients treated with chemoradiation compared with 12.1 months for patients treated with radiation therapy alone (p < 0.001 by log rank test). Additionally, the 2-year survival rate was also significantly greater in the chemoradiation group compared with the radiation alone treatment arm (26% vs. 10%). The differences in survival persisted despite the fact that at time of disease progression, 161 of 282 patients (57%) in the radiation only arm received salvage treatment with temozolomide. Only 60 of 277 patients (22%) on the chemoradiation arm received additional temozolomide at disease progression. The chemoradiation treatment regimen was well-tolerated. Early discontinuation from severe myelotoxicity occurred in only 5% of patients. This study provided Level 1 evidence that the chemoradiation regimen is superior to radiation treatment alone and has established this treatment as the standard of care for patients with newly diagnosed GBM. Furthermore, the FDA analyzed these data and full approval was given for temozolomide in patients with newly diagnosed GBM (Cohen et al 2005). The current standard approach is radiation therapy with concurrent daily temozolomide at a dose of 75mg/m2. During the course of this therapy, prophylaxis against pneumocystis carinii pneumonia (PCP) is recommended. Adjuvant temozolomide is then started three to four weeks after the completion of the concurrent therapy. The daily dose of temozolomide is 150mg/m2 of days 1–5 of a 28-day cycle for the first course, and if tolerated the dose is escalated to 200mg/m2 daily for the five days every 28 days. This therapy is continued for at least six months in patients with no evidence of disease progression or severe treatment-related toxicity.
Methylguanine methyltransferase (MGMT) analysis
A laboratory correlative study was performed by Hegi and colleagues (2005) using tissue specimens from patients enrolled on the EORTC/NCI-C trial described above. These investigators hypothesized that tumors with hypermethylation (inactivation) of the methylguanine methyltransferase (MGMT) gene would have a better prognosis that those patients with only unmethylated, therefore active expression of the MGMT gene. Prior studies had identified MGMT expression as a potential mechanism of resistance to alkylating agent chemotherapy including temozolomide. Of the 573 patients enrolled, they were able to determine the methylation status of the MGMT gene in 203 samples using a methylation-specific polymerase chair reaction (PCR) technique. Correlation of methylation status of the MGMT gene promoter region with survival revealed that patients with tumors containing methylated MGMT promoter had a far better prognosis than those with only unmethylated promoter as measured by median survival (18.2 months vs 12.2 months). The two-year survival rate was also higher in the patients with tumors demonstrating methylated MGMT promoter (46% vs 10%). Amongst patients with methylated MGMT promoter, patients treated with the chemoradiation regimen fared better than those treated with radiation alone (median survival 21.7 months vs 15.3 months, respectively). These data support the potential prognostic and treatment predictive importance of the methylation status of the MGMT gene promoter region. However, these retrospective data require prospective validation.
Ongoing studies and future directions
The results of the collaborative efforts of the EORTC and NCI-C along with the compelling correlative data from the MGMT gene promoter methylation data has generated widespread interest in developing new treatment regimens building on this foundation. A clinical trial was recently launched that represents the collaborative efforts of colleagues from the RTOG and the EORTC that was designed to address the question whether increasing the dose-intensity of the temozolomide will impact efficacy. Preliminary data suggest that prolonged dosing (up to 21 days) of temozolomide will significantly reduce MGMT activity in peripheral blood mononuclear cells (Tolcher et al 2003). Furthermore, a phase II clinical trial using a 7 day on, 7 day off regimen of temozolomide in patients with recurrent GBM demonstrated a high response rate with 6 month PFS of 46% (Wick et al 2004).
RTOG/EORTC protocol 0525, as outlined in Figure 2, will randomize patients after standard concurrent chemoradiation to either standard dose adjuvant temozolomide or a dose-intense regimen of 21 out of 28 day dosing per cycle. Patients will be stratified for the randomization by clinical prognostic factors (using the RTOG Recursive Partitioning System) and MGMT gene promoter methylation status. A total accrual of 1154 patients is required, but the study will attempt to validate the prognostic and predictive ability of the MGMT analysis and determine if dose-intense treatment improves the outcome for patients with “resistant” tumors by modulating tumor MGMT activity using the prolonged dosing schedule. Additionally, net clinical benefit will be measured using validated instruments to measure symptom burden, quality of life, and neurocognitive function.
Figure 2.
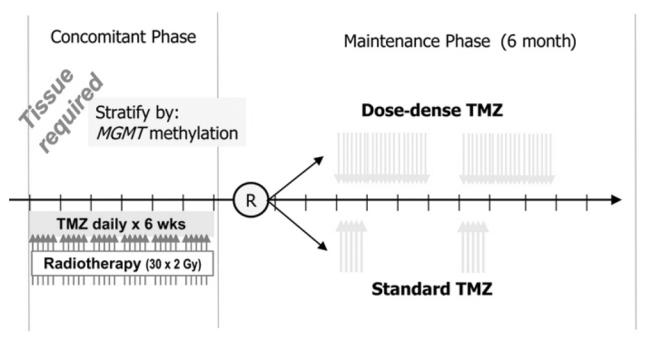
Design of the Radiation Therapy Oncology Group (RTOG) 0525/European Organization for Research and Treatment of Cancer(EORTC) 26052 Intergroup trial.
Conclusion
The treatment of malignant primary brain tumors, particularly glioblastoma, remains challenging. In addition to the inherent refractory nature of these cancers, issues such as loss of neurologic function complicate care. The introduction of temozolomide represents a significant advance in treatment. Temozolomide has both excellent oral bioavailability and lack of significant cumulative myelotoxicity which permits testing of novel and prolonged dosing schedules. These characteristics allowed the development of the concurrent use of temozolomide with external beam radiation followed by adjuvant temozolomide treatment. This regimen has proven to be better than radiation alone and is now the standard of care for patients with newly diagnosed GBM. A recently launched trial is evaluating a new, more intensive schedule to determine if this benefit of temozolomide can be enhanced.
References
- Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–7. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- CBTRUS. 2005. Statistical report: primary brain tumors in the United States, 1998–2002. Published by the Central Brain Tumor Registry of the United States. [Google Scholar]
- Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767–71. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- Combs SE, Gutwein S, Schulz-Ertner D, et al. Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Phase I/II study. Strahlenther Onkol. 2005;181:372–7. doi: 10.1007/s00066-005-1359-x. [DOI] [PubMed] [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Fetell MR, Grossman SA, Fisher JD, et al. Preirradiation paclitaxel in glioblastoma multiforme: efficacy, pharmacology, and drug interactions. New Approaches to Brain Tumor Therapy Central Nervous System Consortium. J Clin Oncol. 1997;15:3121–8. doi: 10.1200/JCO.1997.15.9.3121. [DOI] [PubMed] [Google Scholar]
- Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–97. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro-oncol. 2002;4:261–7. doi: 10.1093/neuonc/4.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–93. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121–32. [PubMed] [Google Scholar]
- Hildebrand J, Lecaille C, Perennes J, et al. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–15. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- Macdonald DR, Kiebert G, Prados M, et al. Benefit of temozolomide compared to procarbazine in treatment of glioblastoma multiforme at first relapse: effect on neurological functioning, performance status, and health related quality of life. Cancer Investigation. 2005;23:138–44. [PubMed] [Google Scholar]
- Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer. 2000;89:640–6. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mehta N, Silverberg W. Role of radiotherapy in the treatment of gliomas. Expert Rev Neurother. 2005;5(6 Suppl):S51–61. doi: 10.1586/14737175.5.6.S51. [DOI] [PubMed] [Google Scholar]
- Moots PL. Pitfalls in the management of patients with malignant gliomas. Semin Neurol. 1998;18:257–65. doi: 10.1055/s-2008-1040878. [DOI] [PubMed] [Google Scholar]
- [MRC] Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–18. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: [MRC] and B 39831: NSC 362856) Br J Cancer. 1992;65:287–91. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly SM, Newlands ES, Glaser MG, et al. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A:940–2. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–18. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–82. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–11. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick NA, Paleologos NA. External beam radiotherapy: hard facts and painful realities. J Neurooncol. 1995;24:93–5. doi: 10.1007/BF01052665. [DOI] [PubMed] [Google Scholar]
- Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–9. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wen P, Toon TS, Black P. Clinical imaging, and laboratory diagnosis of brain tumors. In: Laws E, Kaye A, editors. Brain tumors. New York: Jones and Bartlett; 2001. pp. 24–50. [Google Scholar]
- Wick W, Steinbach JP, Kuker WM, et al. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113–15. doi: 10.1212/01.wnl.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–93. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]


