Abstract
Non-alcoholic fatty liver disease, defined as the presence of macrovascular steatosis in the presence of less than 20 gm of alcohol ingestion per day, is the most common liver disease in the USA. It is most commonly associated with insulin resistance/type 2 diabetes mellitus and obesity. It is manifested by steatosis, steatohepatitis, cirrhosis, and, rarely, hepatocellular carcinoma.
Hepatic steatosis results from an imbalance between the uptake of fat and its oxidation and export. Insulin resistance, predisposing to lipolysis of peripheral fat with mobilization to and uptake of fatty acids by the liver, is the most consistent underlying pathogenic factor. It is not known why some patients progress to cirrhosis; however, the induction of CYP 2E1 with generation of reactive oxygen species appears to be important.
Treatment is directed at weight loss plus pharmacologic therapy targeted toward insulin resistance or dyslipidemia. Bariatric surgery has proved effective. While no pharmacologic therapy has been approved, emerging data on thiazolidinediones have demonstrated improvement in both liver enzymes and histology. There are fewer, but promising data, with statins which have been shown to be hepatoprotective in other liver diseases. The initial enthusiasm for ursodeoxycholic acid has not been supported by histologic studies.
Keywords: fatty liver, treatment
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of hepatic macrovesicular steatosis in the presence of less than 20 g of alcohol ingestion per day. It is the most common liver disease in the US (Clark et al 2002; Browning et al 2004; McCullough 2005) and refers to a broad spectrum of liver disease which varies from bland steatosis (NAFLD) to steatohepatitis (NASH) to progressive fibrosis and, ultimately, cirrhosis with portal hypertension (Silverman et al 1989; Powell et al 1990; Matteoni et al 1999; Marchesini et al 2003). Hepatocellular carcinoma has been reported in those with cirrhosis (Bugianesi et al 2002; Caldwell et al 2004).
Epidemiology and natural history
NAFLD was largely unknown prior to 1980 but is now recognized as the most common chronic liver disease in the US and many other parts of the world. The prevalence of NAFLD, as determined by population studies using ultrasound and serum enzymes, is estimated at 23%–30% (Clark et al 2002). The prevalence is expected to increase as the incidence of obesity and type 2 diabetes mellitus increases. While such studies do not distinguish NASH, the progressive form of the disease, from bland steatosis, it has been suggested that the prevalence of NASH is 5.7%–17% of the general population (McCullough 2005).
NAFLD may lead to NASH, cirrhosis and in some cases, hepatocellular carcinoma (Powell et al 1990; Matteoni et al 1999; Bugianesi et al 2002). Fifty percent of patients with NAFLD have NASH and 19% have cirrhosis at the time of diagnosis (Silverman et al 1989; Silverman et al 1990; Marchesini et al 2003). Once cirrhosis develops 30%–40% of patients will die of liver failure over a 10 year period (McCullough 2005), a rate that is at least equal to that seen with hepatitis C (Hui et al 2003). Hepatocellular carcinoma is an increasingly recognized outcome (Powell et al 1990; Bugianesi et al 2002). Why some patients develop progressive disease while most do not remains to be determined, although genetic factors may be involved (Struben et al 2000; Willner et al 2001).
Etiology
The precise etiology of NAFLD is unknown but there is a strong association with obesity, the metabolic/insulin resistance syndrome and dyslipidemia (Table 1). Most patients with NASH are obese and there is increasing evidence of an obesity epidemic in the US and elsewhere (Flegal et al 1998; Calle et al 1999; Livingston 2000; James et al 2004). It is estimated that 70%–80% of obese subjects have NAFLD with 15%–20% having NASH (Bugianesi et al 2002). A recent study demonstrated that 88% of patients with NASH have the insulin resistance syndrome (Marchesini et al 2003). Type 2 diabetes (DM2) is associated with NAFLD in 30%–80% of subjects (Silverman et al 1989, 1990; Marchesini et al 1999) and NAFLD is present in virtually 100% of patients with combined DM2 and obesity (Wanless and Lentz 1990).
Table 1.
Causes of non-alcoholic fatty liver disease
| Nutritional | Drugs |
| Starvation | Glucocorticoids |
| Obesity* | Tamoxifen |
| Bariatric surgery | Amiodarone |
| Parenteral nutrition | Valproic acid |
| Celiac disease | Zidovudine |
| Metabolic | Didanosine |
| Insulin resistance* | Other |
| Dyslipidemia* | Inflammatory bowel disease |
| Fatty liver of pregnancy | Halogenated hydrocarbons |
| Toxic mushrooms |
Most common causes.
In patients with diabetes, the standardized mortality ratio for cirrhosis (2.52) is greater than that for cardiovascular disease (1.34) (de Marco et al 1999).
Dyslipidemia is present in 50%–60% of individuals with NAFLD. Hypercholesterolemia alone is associated with a 33% prevalence (Assy et al 2000).
Pathogenesis
The pathologic sequence of events from steatosis (ie, the “first hit”), to steatohepatitis to cirrhosis is well established (Powell et al 1990; Bacon et al 1994) (Figure 1). However, fat, per se is not hepatotoxic (Teli et al 1995; Dam-Larsen et al 2004; Adams et al 2005). Why some patients progress and most do not is not known. It is now widely accepted that a “second hit” is necessary for NAFLD to progress to NASH and cirrhosis. What is common to virtually all patients with NAFLD is insulin resistance. It remains uncertain if this is a primary or secondary event to steatosis.
Figure 1.
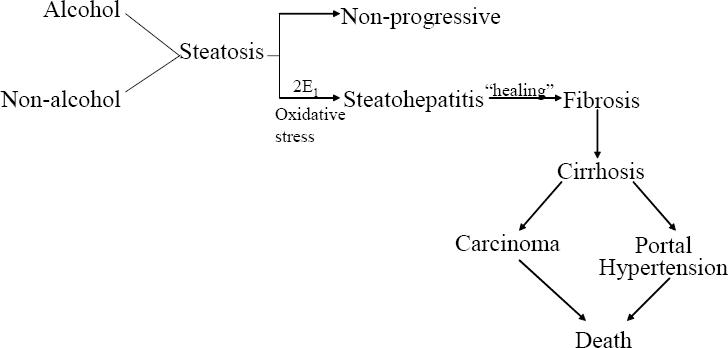
The natural history of non-alcoholic fatty liver disease.
The presence of insulin resistance and/or obesity permits/promotes disease progression (Clark et al 2002; Bellentani et al 2004). They also seem to promote susceptibility to additive injury from alcohol (You and Crabb 2004) and hepatitis C (Patel et al 2005). The clinical features which support progression are increasing age (>45 years), increasing BMI (>30), reversed ALT/AST ratio and elevated serum triglycerides (Angulo et al 1999). The mechanisms remain somewhat speculative but a unifying concept of the pathophysiologic events is now evolving and these are pertinent as therapeutic targets (Figure 2).
Figure 2.
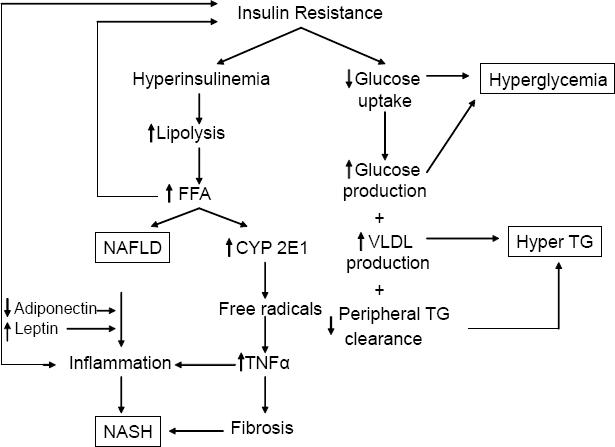
Unifying concept of the pathogenesis of NAFLD. ROS = reactive oxygen species, TG = triglycerides, VLDL = very low density lipoprotein, CYP = cytochrome P450.
Steatosis reflects a net retention of fat within hepatocytes and results from an imbalance between uptake of fat and its oxidation and export. The most consistent pathogenic factor is insulin resistance, leading to enhanced lipolysis which in turn increases circulating free fatty acids and their uptake by the liver (Marchesini et al 1999). Fat accumulating in the liver has several effects: (1) upregulation of apoptosis (Feldstein et al 2003a, 2003b), (2) indirect upregulation of TNFα which is pro-steatotic and pro-inflammatory (Diehl 2004; You and Crabb 2004), (3) mitochondrial dysfunction (Perez-Carreras et al 2003; Feldstein et al 2004; Kharroubi et al 2004; Begriche et al 2006) presumably increasing reactive oxygen species (ROS) and provoking lipid peroxidation of cell membranes, (4) induction of CYP 2E1 which generates ROS (Weltman et al 1998; Nieto et al 2002; Chalasani et al 2003), (5) induction of pro-inflammatory genes such as TNFα (Samuel et al 2004; Arkan et al 2005; Cai et al 2005), and COX2 which induce additional inflammatory mediators which are also pro-fibrotic (Nieto et al 2000). The net effect of the above is apoptosis, necroinflammation and fibrosis.
Hepatic fibrosis is promoted by steatosis even in the absence of liver cell injury (Reeves et al 1996). Adipokines, hormones secreted by adipocytes, appear to be important regulators of hepatic fibrosis. Leptin, which is increased in the metabolic syndrome, promotes fibrosis and induces pro-inflammatory cytokines (Saxena et al 2002, 2004; Aleffi et al 2005) while adiponectin, which is decreased in metabolic syndrome, inhibits stellate cell activation. The net affect of increased leptin and decreased adiponectin is pro-inflammatory and pro-fibrotic. Finally, activation of the renin-angiotensin system is a characteristic feature of the metabolic syndrome (Prasad and Quyyumi 2004). Angiotensin II, the most active mediator of this system, activates hepatic stellate cells and active collagen synthesis (Bataller, Gabele et al 2003; Bataller, Schwabe et al 2003; Bataller et al 2005).
Taken together these observations account for the characteristic histologic features of NASH (steatosis, apoptosis, inflammation and fibrosis) and provide important targets for therapy. A unifying concept of the pathophysiology is shown in Figure 2. In summary, insulin resistance leads to hyper-insulinemia which in turn leads to lipolysis of peripheral fat with mobilization of fatty acids to the liver. The fatty acids are substrate for ß-oxidation as an energy source. In some patients, ie, those with NASH, reactive species are formed leading to oxidative stress with cell damage, inflammation and fibrosis.
Diagnosis
Most patients with NAFLD/NASH are asymptomatic or have only mild fatigue or slight right upper quadrant abdominal discomfort. The diagnosis should be suspected in anyone with the conditions listed in Table 1. The goals of evaluation are threefold: (1) establish a diagnosis of NAFLD, (2) determine the etiology, and (3) determine if there is progressive disease (ie, NASH). The gold standard for establishing a diagnosis of NAFLD and distinguishing it from NASH is the liver biopsy. A number of histologic scoring systems have been developed although none have been universally accepted (Kleiner et al 2005; Mendler et al 2005) (Table 2). The Pathology Committee of the NASH Clinical Research Network has devised a system derived from multiple logistic regressions and scored as the unweighted sum of scores of steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2). NASH is defined as a score of ≥5 (Kleiner et al 2005). Another scoring system includes scores for fatty change (1–4), portal fibrosis (0–6) and activity comprising lobular inflammation (0–3), Mallory bodies (0–3), hepatocyte ballooning (0–3) and perisinusoidal fibrosis (0–3) (Mendler et al 2005). The latter system has the advantage of scoring fibrosis. All scoring systems are compromised by sampling error with a discordance rate of 18% in one study (Ratziu et al 2005). Furthermore, it is not possible to distinguish alcoholic from non-alcoholic fatty liver disease on histologic grounds and the distinction continues to be made by clinical criteria ie, less than or greater than 20 gms of alcohol per day. Liver biopsy is costly, invasive, and subject to sampling error. The search for non-invasive techniques continues.
Table 2.
Types of NAFLD by histology and outcome
| Category | Histology | Outcome |
|---|---|---|
| Type 1 | steatosis only | non-progressive |
| Type 2 | steatosis plus lobular inflammation | benign course |
| Type 3 | steatosis, lobular inflammation and ballooning degeneration | NASH without fibrosis, may progress to cirrhosis |
| Type 4 | steatosis, ballooning degeneration with Mallory bodies and/or fibrosis | NASH, may progress to cirrhosis |
Ultrasound has an overall sensitivity of 89% with 93% specificity (Joseph et al 1991) but falls off greatly in those with mild disease or fibrotic disease when the amount of fat in the liver is less than 30% (Siegelman and Rosen 2001; Neuschwander-Tetri and Caldwell 2003). The characteristic ultrasonic feature is the “bright” liver with increased parenchymal echo texture and vascular blurring. Areas of focal sparring can give the appearance of metastases (Mitchell 1992). The positive and negative predictive value of ultrasound in patients with abnormal liver chemistries and other causes of liver disease ruled out is 96% and 19% respectively. The value of ultrasound as a screening test has not been established and, given its low negative predictive value, it seems unlikely that it will be. Ultrasound is further compromised by an inability to detect fibrosis (Saadeh et al 2002; Neuschwander-Tetri and Caldwell 2003). Non-invasive methods for detecting fibrosis have not yet surfaced for routine clinical use.
Factors which are predictive of fibrotic disease are reversed ALT/AST ratio, hypoalbuminemia, elevated pro-thrombin time and thrombocytopenia (Angulo et al 1999; Sorbi et al 1999). Clinical features such as ascites, esophageal varices, coagulopathy and encephalopathy are consistent with cirrhosis. Biopsy in such patients is not helpful and does not distinguish cirrhosis from other causes.
The search for etiology should include a serum lipid panel, a test for insulin resistance and a history of potential drug causes.
Treatment
Treatment falls into two categories: targeting either the steatosis or the pathogenesis of progression. There are no FDA approved pharmacologic agents and, in fact, no FDA guidelines for such drugs despite the fact that NAFLD is the most common liver disease in the US. The treatments used to date are outlined in Table 3. Virtually all are compromised by small numbers and lack of placebo control.
Table 3.
Summary of interventions in NAFLD
| Treatment | Ref | N | Study Design | ALT/AST | Histology | US/MRI | |
|---|---|---|---|---|---|---|---|
| Steatosis | Inflammation/fibrosis | ||||||
| Steatosis/Insulin Resistance | |||||||
| Weight loss | Huang et al 2005 | 23 | CS | U | U | U | ND |
| Weight loss | Suzuki et al 2005 | 348 | CS | I | ND | ND | ND |
| Weight loss | Petersen et al 2005 | 8 | C | ND | I | ND | I |
| Bariatric surgery | Barker et al 2006 | 19 | CS | ND | I | I | ND |
| Bariatric surgery | Mattar et al 2005 | 70 | CS | ND | I | I | ND |
| Bariatric surgery | Dixon et al 2004 | 36 | CS | ND | I | I | ND |
| Bariatric surgery | Clark et al 2005 | 16 | CS | ND | I | I | ND |
| Bariatric surgery | Klein et al 2006 | 7 | CS | U | I | U | U |
| Bariatric surgery | Mathurin et al 2006 | 185 | CS | I | I | I | ND |
| Orlistat | Harrison et al 2004 | 10 | CS | I | U | U | ND |
| Orlistat | Zelber-Sagi et al 2006 | 52 | R, DB, PC | ND | ND | ND | I |
| Orlistat | Sabuncu et al 2003 | 12 | CS | I | ND | ND | I |
| Sibutramine | Sabuncu et al 2003 | 13 | CS | I | ND | ND | I |
| Pioglitazone | Promrat et al 2004 | 18 | CS | I | I | I | I |
| Pioglitazone | Sanyal et al 2004 | 10 | R, C | U | U | I | ND |
| Pioglitazone | Belfort et al 2006 | 55 | R, B, PC | I | I | I | I |
| Rosiglitazone | Neuschwander-Tetri et al 2003 | 30 | CS | I | I | I | I |
| Rosiglitazone | Ratziu et al 2006 | 63 | R, DB, PC | I | ND | I | ND |
| Metformin | Marchesini et al 2001 | 14 | C | I | ND | ND | ND |
| Metformin | Uygun et al 2004 | 17 | R, C | I | U | U | I |
| Metformin | Schwimmer et al 2005 | 10 | CS | I | ND | ND | ND |
| Atorvastatin | Horlander et al 2001 | 7 | CS | U | U | I | ND |
| Atorvastatin | Kiyici et al 2003 | 27 | CS | I | I | ND | I |
| Pravastatin | Rallidis et al 2004 | 5 | CS | I | ND | I | ND |
| Rosuvastatin | Antonopoulos et al 2006 | 23 | CS | I | ND | ND | ND |
| Gemfibrozil | Basaranoglu, Acbay et al 1999 | 23 | R, C | I | ND | ND | ND |
| Apoptosis | |||||||
| Ursodiol | Laurin et al 1996 | 24 | R, C | I | I | U | ND |
| Ursodiol | Kiyici et al 2003 | 27 | CS | I | I | U | U |
| Ursodiol | Lindor et al 2004 | 80 | R, B, PC | U | U | U | ND |
| Oxidative Stress/Inflammation: Antioxidants | |||||||
| Vitamin E | Lavine 2000 | 11 | CS | I | ND | ND | ND |
| Vitamin E & C | Harrison et al 2003 | 23 | R, DB, PC | U | ND | U | ND |
| Vitamin E | Bugianesi et al 2005 | 28 | R, C | I | ND | ND | ND |
| Oxidative Stress/Inflammation: Pro-biotics/Pre-biotics | |||||||
| VSL#3 | Loguercio et al 2005 | 22 | CS | I | ND | ND | ND |
| Oxidative Stress/Inflammation: Anti-cytokines | |||||||
| Pentoxifylline | Adams et al 2004 | 20 | CS | I | ND | ND | ND |
| Pentoxifylline | Satapathy et al 2004 | 18 | CS | I | ND | ND | ND |
| Pentoxifylline | Lee et al 2006 | 11 | R, DB, PC | U | ND | ND | ND |
| Oxidative Stress/Inflammation: Glutathione recursors | |||||||
| Betaine | Abdelmalek et al 2001 | 10 | CS | I | U | I | ND |
| Betaine | Abdelmalek et al 2006 | 55 | R, PC | U | ND | U | ND |
| Fibrosis | |||||||
| Losartan | Yokohama et al 2004, 2006 | 7 | CS | I | U | I | ND |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; B, blinded; C, controlled; CS, case series; DB, double-blinded; I, improved; MRI, magnetic resonance imaging; ND, no data; PC, placebo-controlled; R, randomized; U, unchanged; US, ultrasound.
Treatment of steatosis/insulin resistance
The treatment of steatosis is inexorably linked to obesity, insulin resistance and dyslipidemia. In general, factors that decrease steatosis consist of weight loss or pharmacologic therapy directed at insulin resistance or dyslipidemia. The treatment of steatohepatitis is directed at oxidative stress, inflammation and fibrosis. Factors that decrease oxidative stress and inflammation include antioxidants, probiotics, anti-cytokines and glutathione precursors. Anti-fibrotic therapy is in its infancy.
Weight loss
Weight loss improves liver chemistries, steatosis, necroinflammatory changes and fibrosis (Huang et al 2005; Petersen et al 2005; Suzuki et al 2005). Furthermore, gradual weight reduction has been shown to lower insulin levels and improve quality of life (Petersen et al 2005). Weight loss may be achieved through diet and exercise or bariatric surgery.
Diet
The ideal diet and rate of weight loss is yet to be determined although it is known that rapid weight loss may exacerbate disease (Andersen et al 1991). A number of studies, both controlled and uncontrolled, indicate that weight loss decreases hepatic steatosis (Huang et al 2005; Petersen et al 2005; Suzuki et al 2005). The durability of weight loss on hepatic steatosis remains to be determined. Low fat diets should be avoided (Solga et al 2004; Kang et al 2006). Some have suggested that a Mediterranean diet (ie, high consumption of complex carbohydrates and monounsaturated fat, low amounts of red meat, and low/moderate amounts of wine) is preferred (Musso et al 2003; Esposito et al 2004). A low glycemic, low calorie diet with a weight loss of 1–2 kg/wk seems reasonable.
Bariatric surgery
Bariatric surgery, recently reviewed by Angulo (2006) has proved successful in a number of studies (Dixon et al 2004; Clark et al 2005; Mattar et al 2005; Barker et al 2006; Klein et al 2006; Mathurin et al 2006) The formerly used ileal bypass surgery was, however, associated with fatty liver and even hepatic failure (Marubbio et al 1976). The durability of bariatric surgery has yet to be determined but it seems likely to be the only therapy that will change the natural history of NASH (Angulo 2006).
Orlistat
Orlistat is a lipase inhibitor that promotes weight loss by reduction of fat absorption. A trial by Harrison et al (2004) in 10 patients reported a mean weight loss of 10 kg with 6 months of treatment. Aminotransferases improved during treatment. No change in histology was reported. Another double blind, placebo-controlled trial by Zelber-Sagi et al (2006) randomized 52 patients with NAFLD (diagnosed by ultrasound and confirmed with biopsy) to orlistat or placebo for 6 months. Orlistat decreased aminotransferase levels and reversed fatty liver as determined by ultrasound. Similar results were seen in another open label trial of 12 nonrandomized, obese patients with NASH (Sabuncu et al 2003), although alkaline phosphatase levels increased during therapy. Orlistat has recently become available over the counter in the US. The side effects of gas, bloating and steatorrhea are problematic.
Sibutramine
Sibutramine, an appetite suppressant, is a serotonin reuptake antagonist approved for weight loss. It also has been studied in patients with NAFLD. It significantly improved aminotransferases in 13 of 13 patients and decreased evidence of hepatic steatosis on ultrasound in 11 of 13 patients in an open label, nonrandomized study (Sabuncu et al 2003). These patients were all obese and were diagnosed with NASH. Alkaline phosphatase levels increased during therapy.
Pharmacologic therapy
Thiazolidinediones
Thiazolidinediones (TZDs) are PPARγ agonists which increase insulin sensitivity and increase the number and activation of adipocytes (Shulman 2000). This leads to a redistribution of lipids from liver and muscle cells to adipocytes which, in turn, restores insulin sensitivity (Shulman 2000; Bajaj et al 2004). They also increase adiponectin expression, decrease TNFα expression, (Iwata et al 2001; Hernandez et al 2004) and reduce collagen synthesis (Galli et al 2002). The net effect of PPARγ agonists is an increase in insulin sensitivity, a redistribution of fat from liver to adipocytes and a reduction in hepatic fibrosis. Animal studies have confirmed these observations (Jia et al 2000; Galli et al 2002) and human trials are beginning to confirm the beneficial effects (Caldwell et al 2001). In the initial study using troglitazone, 7 of 10 patients showed improvement in ALT after 6 months. There was, however, no histologic improvement and troglitazone was removed from the market because of hepatotoxicity. Subsequent trials with pioglitazone and rosiglitazone have not shown evidence of hepatotoxicity.
Pioglitazone
Three small trials and a large controlled trial have evaluated pioglitazone in the treatment of NAFLD (Shadid and Jensen 2003; Promrat et al 2004; Sanyal et al 2004; Belfort et al 2006). A 48 week trial of pioglitazone, 30 mg daily, in 18 patients by Promrat et al (2004) showed improvement in ALT and histology. Fibrosis decreased in 61% and remained stable in 22%. A 6 month controlled trial by Sanyal et al (2004) of 20 patients compared vitamin E (400 I.U./d) to pioglitazone (30mg/day) plus vitamin E. The combination showed improvement in insulin sensitivity and histology.
In the largest controlled trial to date with pioglitazone, Belfort et al compared diet plus pioglitazone to diet plus placebo in 55 patients (Belfort et al 2006). The pioglitazone group showed significant improvement in ALT (by 58%), hepatic fat content (by 54%) and insulin sensitivity (by 48%). There was significant histologic improvement in steatosis, ballooning necrosis and inflammation but not fibrosis.
Rosiglitazone
There have been 3 trials, including a placebo controlled trial, with rosiglitazone (Neuschwander-Tetri et al 2003; Tiikkainen et al 2004; Ratziu et al 2006). In the first of these studies (Neuschwander-Tetri et al 2003), 30 patients were treated with rosiglitazone 8mg daily for 48 weeks. There was significant improvement in ALT, AST, GGT and insulin sensitivity. Of the 22 patients who had histologic evaluation, steatosis improved in 13 and worsened in one; fibrosis score improved in 8 and worsened in 3. This study was confounded by the use of statins. The results of the 63 patient, French multicenter placebo controlled trial known as FLIRT have just been presented (Ratziu et al 2006). There was improvement in histology (47%) compared to placebo (16%) and ALT (38%) versus placebo (7%). Interestingly, the non-diabetic patients did better than the diabetic patients in this study.
In summary, several trials have shown a beneficial effect of TZDs in patients with insulin resistance syndrome/DM2. Issues about hepatotoxicity have been dispelled (Tolman and Chandramouli 2003) and there is evidence that the use of TZDs in patients with elevated baseline liver chemistries is safe (Chalasani et al 2005). It is yet to be determined if there are long-term benefits. However, there has been consistent short-term benefit in surrogate (serum ALT) and histologic markers. TZDs, despite their shortcomings, are emerging as the drugs of choice for treating diabetic patients with NASH. However, as recently stated by McCullough (2006), the best description of TZDs for NASH may be, “Promising but not ready for prime time”.
Metformin
Metformin is a biguanide that stimulates ß-oxidation in the mitochondria (DeFronzo et al 1991). It also suppresses lipogenic enzymes. In so doing it bypasses insulin resistance by utilizing fatty acids as an energy source. It is in this way that it reduces hyperinsulinemia (Stumvoll et al 1995; Cusi et al 1996). Animal studies in ob/ob mice with fatty liver disease have shown improvement in steatosis and aminotransferase abnormalities (Lin et al 2000). Human trials have been less convincing. Three small trails resulted in a significant reduction in aminotransferase levels (Marchesini et al 2001; Uygun et al 2004; Schwimmer et al 2005). One of the trials showed enhanced insulin sensitivity and a transient improvement in serum aminotransferase levels (Marchesini et al 2001). In another trial, metformin and diet were compared to diet alone over 6 months. A statistically significant reduction in ALT, insulin and C-peptide was detected. There was also an improvement in necroinflammation that did not reach statistical significance (Uygun et al 2004). The increase in anaerobic respiration and potential for lactic acidosis is more a theoretical than actual concern except in patients with alcoholism and underlying renal insufficiency. Long term benefits and histologic benefit have not been demonstrated. At the present time, metformin cannot be recommended for non-diabetic patients with NASH.
Statins
Statins are currently used to treat NAFLD (Horlander et al 2001; Kiyici et al 2003; Rallidis et al 2004; Antonopoulos et al 2006). Recent studies suggest that statins are hepatoprotective in patients with other forms of liver disease including hepatitis C (Chalasani et al 2004). Statins may reduce hepatic fat content in patients with hyperlipidemia and NASH (Horlander et al 2001; Kiyici et al 2003). To date, atorvastatin, pravastatin, and rosuvastatin have been studied. An important point about these studies is that the statin doses were not equipotent nor comparable in their lipid lowering effect.
Atorvastatin was compared with ursodeoxycholic acid in a small trial of 44 obese adults with NASH, including 10 patients with diabetes (Kiyici et al 2003). In the statin arm of the study, hyperlipidemic patients received atorvastatin 10 mg daily for 6 months. Liver chemistries improved and an increase in liver density, suggesting a decrease in fat content, occurred in the atorvastatin group.
Two other statins have been evaluated in patients with NAFLD. Pravastatin, at a dose of 20 mg daily for 6 months, normalized liver enzymes and improved hepatic inflammation in 5 of 5 patients (Rallidis et al 2004). Rosuvastatin was studied in 23 patients with hyperlipidemia and biochemical and ultrasound evidence of NAFLD (Antonopoulos et al 2006). After 8 months of rosuvastatin 10 mg daily, all patients had normal ALT and AST and all achieved LDL goals. Histology was not evaluated. In summary, further studies are needed but statins are promising agents for the treatment of NASH as well as other liver diseases.
Fibric acid derivatives
Gemfibrozil, 600 mg daily for 1 month, has been studied in a randomized, controlled study of 46 patients with NAFLD. The ALT normalized over this period, but histologic changes were not evaluated (Basaranoglu, Acbay et al 1999). The dose of gemfibrozil in this trial was less than the labeled dose of 1200 mg per day.
Treatment of pathophysiologic mechanisms
There is increasing interest in treating NASH by targeting the pathophysiologic mechanisms.
Apoptosis
Apoptosis is an important mechanism of cell death in NAFLD.
Ursodeoxycholic acid
Ursodiol (ursodeoxycholic acid) is an anti-apoptotic, cyto-protective, immune-modulating, anti-inflammatory agent that is widely used in liver disease. The results of initial studies varied, but some showed promising results in improving ALT (Laurin et al 1996; Kiyici et al 2003) while others reported no significant difference in ALT (Vajro et al 2000; Lindor et al 2004). In one of the studies, ursodiol did show improvement in steatosis, but no improvement in inflammation or fibrosis (Laurin et al 1996). All of these studies were small (17 to 24 patients) and had no comparator group. To further study ursodiol, a randomized, placebo controlled trial involving 166 patients with NASH compared ursodiol to placebo for 2 years (Lindor et al 2004). Liver function tests improved in both groups; however, significant differences were not detected between placebo and ursodiol. Histologic changes (steatosis, necroinflammation, or fibrosis) also were not significantly different between the ursodiol and placebo groups. At the present time, ursodiol cannot be recommended. Studies are underway using it as add-on therapy.
Oxidative stress/inflammation
Antioxidants
Oxidative stress is important in the pathogenesis of NASH, and antioxidants (vitamin E) may decrease levels of profibrinogenic TGF-β, improve histology, and inhibit hepatic stellate cell activation.
Vitamin E/vitamin C
Pilot studies with vitamin E have been conducted (Lavine 2000; Hasegawa et al 2001; Harrison et al 2003; Sanyal et al 2004; Bugianesi et al 2005) with promising results in reducing aminotransferases. One randomized placebo controlled trial looked at the combination of vitamin E and vitamin C (Harrison et al 2003). Improvement in hepatic inflammation and fibrosis was detected. However, these differences were not significantly different from the placebo arm. A recent open label study compared vitamin E to metformin and weight loss (Bugianesi et al 2005). Vitamin E was inferior to metformin and/or weight loss in improving aminotransferases.
A recent meta-analysis of high dose vitamin E in the general population revealed an increase in overall mortality (Miller et al 2005). Due to the possible increase in mortality with general use of antioxidants and the mixed results from clinical trials in NASH, the use of antioxidants is not recommended.
Pro-biotics/pre-biotics
Probiotics may reduce hepatic injury in animal models where intestinal derived bacterial endotoxin sensitizes fatty livers to the effects of TNF-α. A 3-month treatment period of a commercially available probiotic, VSL #3, given to 22 patients with NAFLD did improve ALT levels and markers of lipid peroxidation (Loguercio et al 2005). Histology was not evaluated in this trial.
Anti-cytokines
Pentoxifylline
TNF-α is a pro-inflammatory cytokine that triggers the production of additional cytokines that recruit inflammatory cells and leads to the destruction of hepatocytes and induction of fibrogenesis. This cytokine is increased in NASH. Pentoxifyl-line is a methylxanthine compound that inhibits TNF-α and is a promising agent in the treatment of alcoholic hepatitis. A pilot study in 20 cpatients detected improvement in liver enzymes in patients with NASH (Adams et al 2004). However, the high incidence of gastrointestinal side effects led to early withdrawal in many patients. Satapathy et al (2004) found that pentoxifylline reduced mean transaminase levels, reduced serum TNF-α levels, and improved insulin resistance in 18 patients over a 6 month period. Another trial by Lee et al (2006) studied 20 patients with NASH and randomized them to 3 months of pentoxifylline or placebo. Both groups had significant decreases in BMI and aminotransferase levels, but there were no significant differences between groups. More patients who received pentoxifylline achieved normal AST. Both groups reported a significant decrease in TNF-α, Il-6, Il-8, and serum hyaluronic acid.
Glutathione precursors
Betaine
Betaine is a component of the metabolic cycle of thionine and may increase S-adenosylmethionine levels. This process may protect against steatosis in alcoholic liver disease animal models. A small, 1-year trial showed that betaine significantly improved aminotransferase levels versus baseline (Abdelmalek et al 2001). In addition, a marked improvement in the degree of steatosis, necroinflammatory grade, and stage of fibrosis was observed. Abdelmalek et al (2006) then conducted a placebo-controlled, 12-month trial of 55 patients and reported that betaine did not significantly improve aminotransferases or liver histology.
Fibrosis
Animal studies have shown that angiotensin II promotes insulin resistance and hepatic fibrosis.
Angiotensin II receptor antagonists
Lorsartan
Losartan, an angiotensin II receptor antagonist, has been used in two studies (Yokohama et al 2004, 2006). In an historically controlled study (Yokohama et al 2004, 2006), losartan 50 mg daily was associated with improved aminotransferases, serum markers of fibrosis, and plasma TGF-β1. Histological improvements were detected in several of the patients: necroinflammation (5 patients), reduction of hepatic fibrosis (4 patients), and reduced iron disposition (2 patients).
Summary
There is increasing understanding of the risk factors for NAFLD and its underlying pathophysiology. New therapies are evolving but weight loss remains the mainstay of therapy. Targeted pharmacologic therapy is evolving in the treatment of the underlying pathophysiologic events of steatosis, apoptosis, oxidative stress and fibrosis. The thiazolidinediones, have shown promising results in the reduction of steatosis and, perhaps, the progression to cirrhosis. However, further controlled clinical trials are needed before any specific therapy other than weight loss and exercise can be recommended without reservation. Patients with insulin resistance can be treated with thiazolidinediones while patients with dyslipidemia can be treated with lipid lowering agents.
Conflicts of interest
Dr. Tolman serves on the ACTOS Drug Safety Monitoring Board for Takeda Pharmaceuticals and is on the Speakers Bureau of Eli Lilly and Company.
References
- Abdelmalek MF, Angulo P, et al. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711–17. doi: 10.1111/j.1572-0241.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- Abdelmalek MF, Sanderson SO, et al. Betaine for treatment of nonalcoholic steatohepatitis. AASLD, October, 2006. Final ID:33. Hepatology. 2006;44(Suppl 1):200A. [Google Scholar]
- Adams LA, Lymp JF, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Adams LA, Zein CO, et al. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–8. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- Aleffi S, Petrai I, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–48. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- Andersen T, Gluud C, et al. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224–9. doi: 10.1016/0168-8278(91)90942-5. [DOI] [PubMed] [Google Scholar]
- Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006;130:1848–52. doi: 10.1053/j.gastro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Angulo P, Keach JC, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- Antonopoulos S, Mikros S, et al. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis. 2006;184:233–4. doi: 10.1016/j.atherosclerosis.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Assy N, Kaita K, et al. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45:1929–34. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- Bacon BR, Farahvash MJ, et al. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–9. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–6. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- Barker KB, Palekar NA, et al. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368–73. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Basaranoglu M, Acbay O, et al. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31 doi: 10.1016/s0168-8278(99)80243-8. [DOI] [PubMed] [Google Scholar]
- Bataller R, Gabele E, et al. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G642–51. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- Bataller R, Sancho-Bru P, et al. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal. 2005;7:1346–55. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- Bataller R, Schwabe RF, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–94. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K, Igoudjil A, et al. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Bedogni G, et al. The epidemiology of fatty liver. Eur J Gastroenterol Hepatol. 2004;16:1087–93. doi: 10.1097/00042737-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Gentilcore E, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Leone N, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SH, Crespo DM, et al. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, Hespenheide EE, et al. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–25. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- Calle EE, Thun MJ, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Aljadhey H, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Gorski JC, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–50. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Teal E, et al. Effect of rosiglitazone on serum liver biochemistries in diabetic patients with normal and elevated baseline liver enzymes. Am J Gastroenterol. 2005;100:1317–21. doi: 10.1111/j.1572-0241.2005.41690.x. [DOI] [PubMed] [Google Scholar]
- Clark JM, Alkhuraishi AR, et al. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13:1180–6. doi: 10.1038/oby.2005.140. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, et al. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- Cusi K, Consoli A, et al. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–67. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- Dam-Larsen S, Franzmann M, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–5. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco R, Locatelli F, et al. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756–61. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Barzilai N, et al. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294–301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Tumor necrosis factor and its potential role in insulin resistance and nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:619–38. x. doi: 10.1016/j.cld.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Bhathal PS, et al. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003a;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, et al. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003b;39:978–83. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Werneburg NW, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb DW, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–40. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Fincke C, et al. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20:623–8. doi: 10.1111/j.1365-2036.2004.02153.x. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Torgerson S, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Yoneda M, et al. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–72. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- Hernandez R, Teruel T, et al. Rosiglitazone ameliorates insulin resistance in brown adipocytes of Wistar rats by impairing TNF-alpha induction of p38 and p42/p44 mitogen-activated protein kinases. Diabetologia. 2004;47:1615–24. doi: 10.1007/s00125-004-1503-7. [DOI] [PubMed] [Google Scholar]
- Horlander J, Kwo P, et al. Atorvastatin for the treatment of NASH. Gastroenterology. 2001;5:A-544–2767. abstract. [Google Scholar]
- Huang MA, Greenson JK, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–81. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- Hui JM, Kench JG, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420–7. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- Iwata M, Haruta T, et al. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator – activated receptor-gamma. Diabetes. 2001;50:1083–92. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- James PT, Rigby N, et al. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- Jia DM, Tabaru A, et al. Troglitazone prevents fatty changes of the liver in obese diabetic rats. J Gastroenterol Hepatol. 2000;15:1183–91. doi: 10.1046/j.1440-1746.2000.02316.x. [DOI] [PubMed] [Google Scholar]
- Joseph AE, Saverymuttu SH, et al. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- Kang H, Greenson JK, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–53. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, et al. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–96. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- Kiyici M, Gulten M, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17:713–18. doi: 10.1155/2003/857869. [DOI] [PubMed] [Google Scholar]
- Klein S, Mittendorfer B, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–72. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Laurin J, Lindor KD, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–7. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–8. [PubMed] [Google Scholar]
- Lee Y, Sutedja D, et al. A randomized controlled double blind study of pentoxifylline in patients with non-alcoholic steatohepatitis (NASH) Hepatology. 2006;44(Suppl 1):654A. doi: 10.1007/s12072-008-9058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Kowdley KV, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- Livingston B. Epidemiology of childhood obesity in Europe. Eur J Pediatr. 2000;159(Suppl 1):S14–34. doi: 10.1007/pl00014363. [DOI] [PubMed] [Google Scholar]
- Loguercio C, Federico A, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, et al. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–4. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Marubbio AT, Jr, Buchwald H, et al. Hepatic lesions of central peri-cellular fibrosis in morbid obesity, and after jejunoileal bypass. Am J Clin Pathol. 1976;66:684–91. doi: 10.1093/ajcp/66.4.684. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Gonzalez F, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617–24. doi: 10.1053/j.gastro.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Mattar SG, Velcu LM, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–17. doi: 10.1097/01.sla.0000179652.07502.3f. discussion 618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–19. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- McCullough AJ. The epidemiology and risk factors of NASH. In: Farrell GC, George J, Hall P, et al., editors. Fatty liver disease: NASH and related disorders. Oxford: Blackwell; 2005. pp. 23–37. [Google Scholar]
- McCullough AJ. Thiazolidinediones for nonalcoholic steatohepatitis – promising but not ready for prime time. N Engl J Med. 2006;355:2361–3. doi: 10.1056/NEJMe068232. [DOI] [PubMed] [Google Scholar]
- Mendler MH, Kanel G, et al. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver Int. 2005;25:294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- Miller ER, 3rd, Pastor-Barriuso R, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Mitchell DG. Focal manifestations of diffuse liver disease at MR imaging. Radiology. 1992;185:1–11. doi: 10.1148/radiology.185.1.1523289. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37(4):909–16. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Brunt EM, et al. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Nieto N, Friedman SL, et al. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–64. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- Nieto N, Greenwel P, et al. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–45. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- Patel K, Zekry A, et al. Steatosis and chronic hepatitis C virus infection: mechanisms and significance. Clin Liver Dis. 2005;9:399–410. vi. doi: 10.1016/j.cld.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Perez-Carreras M, Del Hoyo P, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EE, Cooksley WG, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- Prasad A, Quyyumi AA. Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation. 2004;110:1507–12. doi: 10.1161/01.CIR.0000141736.76561.78. [DOI] [PubMed] [Google Scholar]
- Promrat K, Lutchman G, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- Rallidis LS, Drakoulis CK, et al. Pravastatin in patients with non-alcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193–6. doi: 10.1016/j.atherosclerosis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, et al. A one year randomized, placebo-controlled, double-blind trial of rosiglitazone in non alcoholic steatohepatitis: results of the FLIRT pilot trial. Hepatology. 2006;44(4 Suppl 1):201A. [Google Scholar]
- Reeves HL, Burt AD, et al. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677–83. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Younossi ZM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- Sabuncu T, Nazligul Y, et al. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12:189–92. [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Mofrad PS, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- Satapathy SK, Garg S, et al. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–52. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- Saxena NK, Ikeda K, et al. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Titus MA, et al. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–14. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer JB, Middleton MS, et al. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–9. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- Shadid S, Jensen MD. Effect of pioglitazone on biochemical indices of non-alcoholic fatty liver disease in upper body obesity. Clin Gastroenterol Hepatol. 2003;1:384–7. doi: 10.1053/s1542-3565(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman ES, Rosen MA. Imaging of hepatic steatosis. Semin Liver Dis. 2001;21:71–80. doi: 10.1055/s-2001-12930. [DOI] [PubMed] [Google Scholar]
- Silverman JF, O’Brien KF, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–55. [PubMed] [Google Scholar]
- Silverman JF, Pories WJ, et al. Liver pathology in diabetes mellitus and morbid obesity. Clinical, pathological, and biochemical considerations. Pathol Annu. 1989;24(Pt 1):275–302. [PubMed] [Google Scholar]
- Solga S, Alkhuraishe AR, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004;49:1578–83. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- Sorbi D, Boynton J, et al. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–22. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- Struben VM, Hespenheide EE, et al. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, et al. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–4. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Lindor K, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–6. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Teli MR, James OF, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–19. [PubMed] [Google Scholar]
- Tiikkainen M, Hakkinen AM, et al. Effects of rosiglitazone and met-formin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–76. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- Tolman KG, Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis. 2003;7:369–79. vi. doi: 10.1016/s1089-3261(03)00020-5. [DOI] [PubMed] [Google Scholar]
- Uygun A, Kadayifci A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–44. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- Vajro P, Franzese A, et al. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–43. [PubMed] [Google Scholar]
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–10. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- Weltman MD, Farrell GC, et al. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–33. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- Willner IR, Waters B, et al. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–61. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- Yokohama S, Tokusashi Y, et al. Inhibitory effect of angiotensin II receptor antagonist on hepatic stellate cell activation in non-alcoholic steatohepatitis. World J Gastroenterol. 2006;12:322–6. doi: 10.3748/wjg.v12.i2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokohama S, Yoneda M, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222–5. doi: 10.1002/hep.20420. [DOI] [PubMed] [Google Scholar]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S, Kessler A, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:639–44. doi: 10.1016/j.cgh.2006.02.004. [DOI] [PubMed] [Google Scholar]


