Abstract
Invasive fungal infections are a major cause of morbidity and mortality in immunocompromised patients, such as subjects with hematological malignancies and patients who underwent to hematopoietic stem cell transplantation (HSCT) or solid organ transplantation (SOT). Fusarium spp. cause a broad spectrum of infections in humans. Immunologically competent hosts show mainly localized skin infections, whereas disseminated fusariosis occurs almost exclusively in immunocompromised patients. Fusarium spp. are resistant to many antifungal agents with equivocal in vitro and in vivo susceptibility to amphotericin B. Voriconazole (VRC) is a triazole shown to be safe, well tolerated, and in vitro efficacious against Fusarium spp. Although clinical experience is limited, many case reports have shown the efficacy of VRC in the treatment of fusariosis.
Keywords: fusariosis, voriconazole, immunocompromised patient, cancer, fungal infections, aspergillus
Introduction
Invasive fungal infections are a major cause of mortality from infection in immunocompromised patients with hematological malignancies, hematopoietic stem cell transplantation (HSCT), and solid organ transplantation (SOT) (De Pauw et al 1999; Nucci 2003; Nucci et al 2003). The introduction of fluconazole prophylaxis in such patients has led to a shift in the epidemiology of fungal infections with a dramatic reduction of the incidence of candidiasis (Anaissie et al 1986; Boutati and Anaissie 1997; Marr et al 2002). By contrast, the incidence of mould infections such as aspergillosis and other non-aspergillar fungal infections has increased significantly (Marr et al 2002; Walsh et al 2004). Among immunocompromised patients, invasive fusariosis is the second most common cause of mould infections after aspergillosis with an increasing incidence (Boutati and Anaissie 1997; Nucci 2003; Nucci et al 2004).
Fusarium spp. are plant pathogens and soil saprophytes that cause a broad spectrum of infections in humans, including superficial (keratitis, onychomycosis), locally invasive, and disseminated infection. Disseminated fusariosis occurs almost exclusively in immunocompromised individuals (Nucci et al 2002; Dignani et al 2004). Recently, Nucci et al reported the clinical characteristics and prognostic factors of 61 patients with fusariosis after HSCT (54 allogeneic HSCT, 7 autologous HSCT). The reported incidence of fusariosis ranged from 5 infections per 1000 HSCTs in human leucocyte antigen (HLA) matched related transplantations to 20 infections per 1000 HSCTs in HLA mismatched transplantations. The survival rate was 13%, with a median onset of 13 days from the diagnosis, and the single prognostic factor for death by multivariate analysis was persistent neutropenia (Nucci et al 2004). The incidence of invasive fungal infections is also increasing in SOT ranging between 5% and 20% (Lodato et al 2006), probably due to the use of more intense immunosuppression regimens to reduce acute allograft rejection. Between 1996 and 2007, 10 cases of fusariosis in patients who underwent SOT were reported in literature. Nine of them were localized whereas one patient experienced disseminated fusariosis. The infection resolved in 9 of these patients. None died from fusariosis (Young and Meyers 1979; Heinz et al 1996; Arney et al 1997; Girardi et al 1999; Linden et al 2000; Sampathkumar et al 2001; Cocuroccia et al 2003; Garbino et al 2004; Lodato et al 2005). Part of these data are presented in Table 1.
Table 1.
Cases of fusariosis found by a computerized search of MEDLINE and published from January 2000 to April 2007
| Article, year (Reference) | No. | Type | Pattern | Drug | Disease | Setting | Outcome with respect to the last drug |
|---|---|---|---|---|---|---|---|
| Linden et al 2000 | 1 | not spec. | - | ABLC | - | SOT | resolved |
| Musa et al 2000 | 11 | not spec. | 1 cutaneous | - | Diabetes | - | - |
| 1 cutaneous | - | Diabetes | - | - | |||
| 1 cutaneous | - | Diabetes | - | - | |||
| 1 cutaneous | AmB-L | NHL | CTH | resolved | |||
| 1 pneumonia | - | NHL | CTH | - | |||
| 1 pneumonia | AMBD | ALL | CTH | resolved | |||
| 1 disseminated | AMBD | HM | CTH | died | |||
| 1 disseminated | AMBD | HM | CTH | died | |||
| 1 disseminated | AmB-L | HM | CTH | died | |||
| 1 disseminated | AMBD, AmB-L | HM | CTH | died | |||
| 1 disseminated | AMBD, AmB-L | HM | CTH | died | |||
| Reis et al 2000 | 1 | solani | keratitis | Fluco,Itra,Vorico(iv, oral) | - | - | resolved |
| Austen et al 2001 | 1 | dimerum | disseminated | AmB-L | ALL | CHT | died |
| Pereiro et al 2001 | 1 | oxysporum | cutaneous | Itra, Fluco | - | - | improvement |
| Sampathkumar and Paya 2001 | 1 | not spec. | soft tissue | ABLC | Amyl | SOT | resolved |
| Bodey et al 2002 | 35 | solani, | 20 disseminated | Fluco 8 | Cancer | 8 BMT | - |
| moniliforme, | 15 localized | Itra 1 | |||||
| oxysporum, | AMBD, AmB-L 8 | ||||||
| proliferatum, | Other 18 | ||||||
| dimerum | |||||||
| Sponsel et al 2002 | 1 | solani | endophthalmitis | AMBD + Keto, Posa | - | CL | resolved |
| Apostolidis et al 2003 | 1 | not spec. | fungemia | AmB-L, Caspo | ALL | CHT | resolved |
| Cocuroccia et al 2003 | 1 | solani | cutaneous | Itra | AS | SOT | improvement |
| Consigny et al 2003 | 1 | not spec. | disseminated | AMBD, AmB-L, Vorico(iv, oral) | AML | CHT | resolved |
| Khoury and Ball 2003 | 1 | not spec. | disseminated | AMBD | HM | BMT | resolved |
| Perfect et al 2003 | 11 | not spec. | disseminated | Vorico(iv, oral) | HM | CTH+BMT | 45% resp. |
| Rodriguez et al 2003 | 1 | oxysporum | disseminated | AmB-L + Vorico(iv, oral) | SAA | - | resolved |
| Vincent et al 2003 | 1 | solani | disseminated | ABCL, Itra, AmB-L, | AML | CTH | resolved |
| Vorico(iv, oral) | |||||||
| Garbino et al 2004 | 1 | not spec. | peritonitis | Vorico(iv) | Diabetes | SOT | resolved |
| Bigley et al 2004 | 1 | dimerum | disseminated | AmB-L, Vorico(iv, oral) | SAA | BMT | resolved |
| Guimerá-MartÍn-Neda et al 2004 | 1 | not spec. | cutaneous | AmB-L, Vorico | FES | PDN | resolved |
| Guzman-Cotrilli et al 2004 | 1 | solani | disseminated | AmB-L + Vorico(iv, oral) | AML | CTH | improved |
| Hamaki et al 2004 | 1 | solanii | disseminated | AMBD | NHL | BMT | died |
| Herbrecht et al 2004 | 1 | proliferatum | pneumonia | Posa | - | SOT | resolved |
| Jensen et al 2004 | 4 | not spec. | disseminated | AmB-L | AML | CTH | resolved |
| verticillioides | disseminated | AMBD, AmB-L | CLL | CTH | resolved | ||
| verticillioides | disseminated | AmB-L | AML | CTH | resolved | ||
| solani | disseminated | AmB-L | NHL | CTH | died | ||
| Kivivouri et al 2004 | 2 | solani | disseminated | AmB-L | ALL | BMT | died |
| not spec. | disseminated | - | AML | BMT | died | ||
| Polizzi et al 2004 | 1 | solani | corneal abscess | AMBD, Vorico(iv + topical, oral) | Abrasion | - | resolved |
| Anandi et al 2005 | 1 | solani | cutaneous | Ketoconazole (oral) | Diabetes | - | resolved |
| Cudillo et al 2005 | 1 | not spec. | disseminated | AmB-L, Vorico (oral), AmB-L | ALL | CTH | died |
| Durand et al 2005 | 1 | moniliforme | endophthalmitis | AMBD(topical), Vorico(oral) | Catarct | - | resolved |
| Lodato et al 2005 | 1 | solani | liver abscesses | ABLC | CD | SOT | resolved |
| Lin et al 2005 | 3 | solani | keratitis | Netamycin(ed) | - | - | resolved |
| solani | keratitis | Netamycin(ed) + Keto(oral) | - | - | resolved | ||
| solani | keratitis | Netamycin(ed) + Fluco(oral) | resolved | ||||
| Giaconi et al 2006 | 1 | oxysporum | keratitis | Vorico(topical) | - | - | not resolve |
| Gorman et al 20068 | 1 | oxysporum | pneumonia | Vorico(oral) | - | - | resolved |
| Hsu et al 2006 | 1 | not spec. | cutaneous | Vorico(oral) | NHL | - | resolved |
| Madariaga and Kohl 2006 | 1 | not spec. | disseminated | Vorico(iv) | Emph | PDN | - |
| Sagnelli et al 2006 | 1 | verticillioides | disseminated | Vorico(iv, oral) | ST | CTH | resolved |
| Selleslag 2006 | 1 | solani | disseminated | AmB-L | ALL | CTH | resolved |
| Stanzani et al 2006 | 1 | solani | disseminated | AmB-L + Vorico(iv, oral) | AML | BMT | resolved |
| Bunya et al 2007 | 3 | not spec. | keratitis | Vorico(topical) | - | - | resolved |
| not spec. | keratitis | Vorico(orlal + topical) | - | - | resolved | ||
| not spec. | keratitis | Vorico(oral + topical) | - | - | not resolved | ||
| Tu et al 2007 | 3 | not spec. | endolphalmitis | AMBD(topical), Vorico(iv), Posa | - | CL | resolved |
| not spec. | keratitis | AMBD(topical), Vorico(topical + oral), Posa | - | - | resolved | ||
| not spec. | endolphalmitis | Vorico(topical + iv, oral), Posa | - | CL | resolved |
Abbreviations: ABLC, amphotericin B lipid complex; AmB-L, liposomal amphotericin B; AMBD, amphotericin B deoxycholate; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; Amyl, amyloidosis; AS, Alport syndrome; BMT, allogeneic bone marrow transplantation; Caspo, caspofungin; CD, Caroli’s disease; CHT, chemotherapy; CL, contact lens; ed, eye drops; Emph, emphysema; FES, Fisher-Evans syndrome; Fluco, fluconazole; HD, Hodgkin disease; HM, hematological malignancies; Itra, itraconazole; Keto, ketoconazole; PDN, steroids; Posa, posaconazole; SOT, solid organ transplant; ST, solid tumor; Vorico, voriconazole.
The incidence of fusariosis and its mortality rate are significantly higher in patients with hematological malignancies and in those with allogeneic HSCT due to more intense immunosuppression and profound and prolonged neutropenia. The genus Fusarium comprises a large number of species (more than 20) and the most common human pathogen is Fusarium solani isolated in approximately half of the reported infections. The remaining cases of human fusariosis are caused by Fusarium oxysporum, Fusarium moniliforme, and Fusarium verticillioides, each of which account for 10%–14% of all infections. Considering the increasing incidence of Fusarium spp. infections, an increased virulence of these species cannot be excluded (Nelson et al 1994; Nucci et al 2003).
Fusarium spp. manifest an inherent resistance to a multitude of antifungal agents, making the treatment of fusariosis a challenging task especially in severely immunosupressed individuals with hematological malignancies or transplant recipients. In this patient population fusariosis is frequently fatal (Al-Abdely 2003). Despite its equivocal in vitro susceptibility and treatment failures (Arikan et al 1999), amphotericin B has remained the drug of choice for the management of disseminated fusariosis (Guarro et al 1995). Voriconazole (VRC) is a triazole antifungal agent approved by the FDA in May 2002 for the treatment of fungal infections including aspergillosis, cryptococcosis, scedosporiosis, and fusariosis since in vitro data and clinical evidence indicated activity against Fusarium spp. (Arikan et al 1999; Espinel-Ingroff et al 2001; Paphitou et al 2002; Consigny et al 2003; Herbrecht 2004).
Treatment of fusariosis
Fusarium spp. can cause local and, most importantly, disseminated infections in immunocompromised patients with involvement of multiple organs including the skin (Figure 1).
Figure 1.
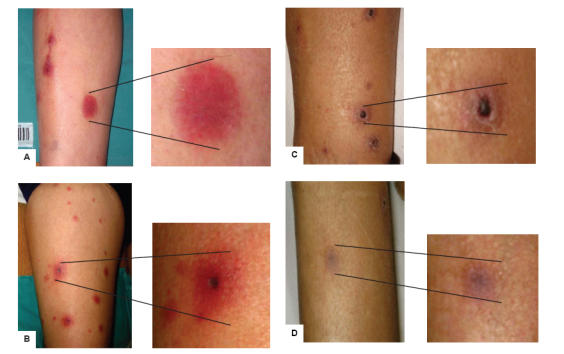
Patient with disseminated fusariosis and skin involvement. The lesions are seen most commonly on extremities and appear as widespread, violaceous (A) or erythematous indurated elements (B). With the resolution of the infection the skin lesions become darker (C) and the lesions disappear (D).
For the clinician taking care of patients with hematological malignancies disseminated fungal infections constitute one of the most difficult challenges. In this patient population Fusarium spp. is an emerging cause of non-Aspergillus mould infection and is associated with high mortality. In 1997 Boutati and Anaissie (Boutati and Anaissie 1997) described 43 patients with hematological malignancies who developed invasive Fusarium spp. infection. Thirteen of these (30%) responded to therapy with amphotericin B deoxycholate (AMBD) or its lipid formulations [liposomal amphotericin B (AmB-L); amphotericin B lipid complex (ABLC)]. The majority (70%) of patients died from the infection while a resolution was only seen in patients who ultimately recovered from cytopenia (Boutati and Anaissie 1997; Kontoyiannis et al 2004). More recently, 84 cases of Fusarium spp. infection in patients with hematological malignancies were reported (Nucci 2003). Of them, only 21% were alive 90 days after the diagnosis.
Polyenes
Current therapy for refractory invasive fungal infections caused by less-common moulds remains inadequate. Since fusariosis may mimic aspergillosis in its clinical manifestations, affected patients were usually treated with amphotericin B, an agent with poor activity in vitro against Fusarium spp. (Pfaller et al 2002), whereas evidence of a good in vivo activity is reported with ABLC (Lodato et al 2006) and AmB-L (Jensen et al 2004; Selleslag 2006).
Azoles
Itraconazole
Itraconazole was demonstrated to exert negligible activity against Fusarium spp. (Pfaller et al 2000). It has rarely been administered against Fusarium spp. infections with unequivocal results. Additionally it has been demonstrated that amphotericin B and VRC are consistantly more effective than itraconazole against Fusarium isolates (Lewis et al 2005). Itraconazole has seldom been administered for Fusarium infections with nonunivocal results (Reis et al 2000; Pereiro et al 2001; Cocuroccia et al 2003; Vincent et al 2003).
Voriconazole
VRC is an extended-spectrum, synthetic triazole derivative of fluconazole, whose mechanism of action is inhibition of the cytochrome P450 (CYP)-dependent enzyme 14-α-sterol demethylase, preventing the fungal cell membrane synthesis and causing its disruption (Denning et al 2002; Ghannoum and Kuhn 2002; Johnson and Kauffman 2003; Herbrect 2004; Scott and Simpson 2007).
Intravenous and/or oral VRC is generally well tolerated. Nevertheless, approximately half of all subjects receiving VRC experienced at least one treatment-related adverse event (Ghannoum and Kuhn 2002; Alkan et al 2004; Herbrect 2004; EMEA summary of product characteristics 2007; Scott and Simpson 2007).
In Candida spp. VRC is fungistatic, whereas in filamentous organisms it is fungicidal. VRC shows in vitro activity against a variety of yeasts, filamentous fungi and dimorphic moulds. Candida spp., Aspergillus spp., Fusarium spp., and Scedosporium spp. are the pathogens against which VRC has been approved for treatment, whereas it has little or no activity against Zygomycetes.
Susceptibility of filamentous fungi to VRC was tested by 3 studies (Johnson et al 1998; Espinel-Ingroff et al 2001; Linres et al 2005). While Johnson and Espinel-Ingroff obtained minimum inhibitory concentrations (MICs) of between 2 and 8 μg/mL, more recently Linares et al showed that Fusarium spp. display greater susceptibility to VRC (MICs 0.25–4 μg/mL) than that reported before (Linares et al 2005). Although animal data suggest a possible correlation between the efficacy of VRC and MIC values, there was no correlation between clinical outcome and MIC values in clinical trials (EMEA 2007).
The efficacy and safety of VRC for the primary treatment of invasive aspergillosis in immunocompromised patients has been described in randomized, non-blind, multinational trials and in observational studies (Denning et al 2002; Ghannoum and Kuhn 2002; Herbrecht et al 2002; Herbrecht 2004; Alvarez-Lerma et al 2005; Mouas et al 2005; Scott and Simpson 2007), but also many case reports have shown the efficacy of VRC in the treatment of fusariosis (Table 1) (Reis et al 2000; Consigny et al 2003; Perfect et al 2003; Rodriguez et al 2003; Vincent et al 2003; Bigley et al 2004; Garbino et al 2004; Guimerá-MartÍn-Neda et al 2004; Guzman-Cotrilli et al 2004; Polizzi et al 2004; Durand et al 2005; Gorman et al 2006; Hsu et al 2006; Sagnelli et al 2006; Stanzani et al 2006; Bunya et al 2007). Thirty-four English language case reports were found by a computerized search of MEDLINE from January 2000 to April 2007. We found 20 disseminated fusariosis and 14 localized infections: 10 ocular involvements, 2 cutaneous lesions, 1 pneumonia, 1 peritonitis. Hematological disease was the underlying setting in 18 patients, while solid tumor and chronic emphysema affected one patient each. All the patients with disseminated infection were immunodepressed because of chemotherapy, or HSCT, or steroids administration, as shown in Table 1. The overall response to VRC for disseminated fusariosis was 63% (12/19 evaluable). Treatment with VRC was initiated in 19 patients with the iv loading dose of 6 mg/kg bid, followed by the maintenance dose of 4 mg/kg bid. The switch to the oral treatment (200 mg bid) was made in 18 patients. Only one patient received the oral formulation of VRC from the start. For localized infections, VRC was administered iv to 5 patients, eventually followed by oral administration for long-term maintenance. Topical VRC preparation could be added. In 6 patients oral voriconazole was administered from the beginning, while 2 patients received only topical formulations. In 3 patients with disseminated fusariosis, combined therapy consisting in voriconazole and AmB-L was administered, while VRC was given as salvage treatment in 4 patients with disseminated fusariosis (data shown in Table 1).
Recently, Perfect et al reported a multicenter, open-label, clinical study to assess the efficacy and safety of VRC for the treatment of less-common, emerging or refractory invasive fungal infections (Perfect et al 2003). Three-hundred and one immunocompromised patients were studied. The drug was administered iv at recommended dosages for at least 3 days, and the median duration of iv treatment was 18 days (range 1–138 days). Thereafter patients could be switched to oral treatment. The median duration of oral administration was 69 days (range 1–326 days). In the study they treated 11 fusariosis, showing 45% of satisfactory global response with respect to the high mortality rate (70%) for disseminated fusariosis treated with other therapies (Krcmery et al 1997; Boutati and Anaissie 1997; Perfect et al 2003). These results were consistent with data from previous reports focused on the use of VRC in critically ill patients. They confirm its good profile concerning safety and tolerability, with an incidence of treatment-related toxicities, such as visual disturbances and rashes, requiring suspension for 3.5% of patients, but none of the toxicities were severe. Also liver function abnormalities were noted in >10% of patients, but only 2.4% had their treatment discontinued, confirming previously reported data (Potoski and Brown 2002).
Posaconazole
Posaconazole (PSC) is a potent extended spectrum tiazole that has been shown to be highly active against yeasts and moulds, including Fusarium spp. (Espinel-Ingrof et al 2004; Torres et al 2005). Recently, Raad et al described a clinical experience with PSC utilized for refractory fusariosis or for patients intolerant to conventional antifungal therapy in 21 cases, with an overall response of 48%. This result is comparable with those seen with VRC, and prolonged neutropenia was an unfavorable risk factor of non-response (20% in patients who recovered from myelosuppression versus 67% in patients who did not recover) (Raad et al 2006). Moreover, 4 cases of ocular infection and 1 case of pneumonia by Fusarium spp. were described, treated and resolved with PSC after failure of treatment with VRC (iv, oral, and topical formulation) and other antifungal drugs (Sponsel et al 2002; Herbrecht et al 2004; Tue et al 2007).
Figure 2.
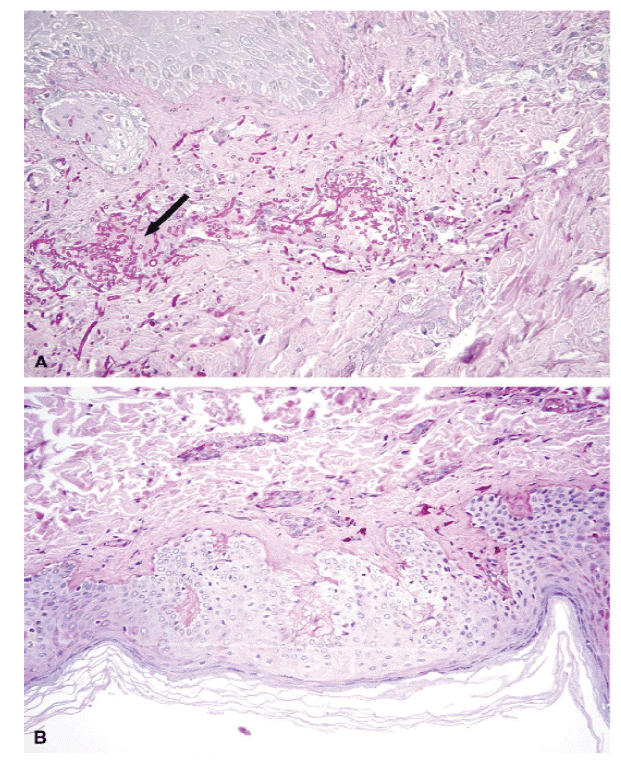
Biopsy of nodular skin lesion at the diagnosis (A) and after treatment (B).
PSC is administered as an oral suspension with food and this could limit its use in critically ill patients.
These results suggest PSC can be considered an appropriate alternative antifungal therapy to amphotericin B formulation, leading to results similar to those once found with VRC.
Echinocandins
Fusarium spp. are usually resistant to echinocandins by standard susceptibility testing (Spellberg et al 2006). Instead, a case of fungemia sustained by Fusarium spp. resistant to amphotericin B was resolved with caspofungin at standard doses in a patient with acute myeloid leukemia (Apostolidis et al 2003). A recent murine model disclosed that caspofungin at 1 mg/kg/day improved survival during active fusariosis, despite lack of reduction in fungal burden (Spellberg et al 2006), suggesting a potential role in the treatment of human fusariosis.
Future perspectives
The in vitro interaction of itraconazole, amphotericin B and VRC with anidulafungin against Aspergillus spp. and Fusarium spp. was recently evaluated (Philip et al 2005). Anidulafungin belongs to the echinocandins antifungal drugs class. The data showed that all drug combinations suggested indifference against Fusarium spp, but not antagonism or synergism. A previous study reported a potential synergistic to additive effect of caspofungin in combination with amphotericin B against Fusarium spp. (Dismukes et al 2000). Also the combination of VRC and micafungin was tested showing a synergistic effect against Fusarium spp. (Heyn et al 2005), but clinical studies are needed to confirm these data.
Nystatin is classified among the most efficient antifungal agents, widely used since 1950s, but insoluble in water. The in vitro activity of polymeric complexes of nystatin was investigated against growth inhibition and spore germination of Fusarium oxysporum. These complexes of nystatin were 3–25 times more active than nystatin against spore germination and were effective inhibitors of mycelial growth (Charvalos et al 2002). Their use in the clinical setting has not yet been investigated.
Conclusions
Invasive fusariosis is an emerging cause of mould infections in immunocompromised patients, with usually poor prognosis being Fusarium spp. resistant to most available antifungal agents (Nucci 2003). In vitro susceptibility testing may be the only clue in the choice of the appropriate antifungal agent (Al-Abdely 2004). The only antifungal drugs effective against Fusarium spp, as evidenced by their relatively low MICs, are amphotericin B, nystatin, ketoconazole, VRC, and PSC (Espinel-Ingroff 1998; Lewis et al 2005; Teixeira et al 2005; Cuenca-Estrella et al 2006), whereas fluconazole, itraconazole, and the echinocandins are not active alone against Fusarium spp. (Espinel-Ingroff 1998; Marco et al 1998; Pfaller et al 1998; Lewis et al 2005). VRC has a slightly broader spectrum of activity against most moulds, showing very good in vitro activity against Aspergillus spp., Fusarium spp, Scedosporium spp. (Denning et al 2003; Lewis et al 2005; Linares et al 2005). Many case reports have demonstrated the efficacy of VRC in the treatment of fusariosis (Reis et al 2000; Consigny et al 2003; Perfect et al 2003; Rodriguez et al 2003; Vincent et al 2003; Bigley et al 2004; Garbino et al 2004; Guimerá-MartÍn-Neda et al 2004; Guzman-Cotrilli et al 2004; Polizzi et al 2004; Durand et al 2005; Gorman et al 2006; Hsu et al 2006; Sagnelli et al 2006; Stanzani et al 2006; Bunya et al 2007), with a 63% overall response for disseminated fusariosis, while Perfect et al reported a 45% satisfactory global response, with respect to the high mortality rate (70%) for patients treated with other antifungal drugs (Perfect et al 2003).
PSC is a new potent extended spectrum tiazole that has been shown to be active against Fusarium spp. (Espinel-Ingrof et al 2004; Torres et al 2005; Raad et al 2006). However, no clinical trials have hitherto compared PSC with VRC or addressed the relative use of these two broad spectrum tiazole agents in the management of fusariosis. Polymeric complexes of nystatin have been investigated against Fusarium oxysporum, but there have been no published clinical trials.
In conclusion, treatment of emerging invasive fungal infections is a major challenge, with no standardized therapy and high mortality rates. VRC seems to be the most promising antifungal agent for the treatment of disseminated fusariosis in immunocompromised subjects, but more clinical evidence is required.
References
- Al-Abdely HM. Management of rare fungal infections. Curr Opin Infect Dis. 2004;17:527–32. doi: 10.1097/00001432-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Alkan Y, Haefeli WE, Burhenne J, et al. Voriconazole-induced QT interval prolongation and ventricular tachycardia: a non-concentration-dependent adverse effect. Clin Infect Dis. 2004;39:e49–52. doi: 10.1086/423275. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lerma F, Nicolas-Arfelis JM, Rodriguez-Borregan JC, et al. Clinical use and tolerability of voriconazole in the treatment of fungal infections in critically ill patients. J Chemother. 2005;17:417–27. doi: 10.1179/joc.2005.17.4.417. [DOI] [PubMed] [Google Scholar]
- Anaissie E, Kantarjian H, Jones P, et al. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer. 1986;57:2141–5. doi: 10.1002/1097-0142(19860601)57:11<2141::aid-cncr2820571110>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Anandi V, Vishwanathan P, Sasikala S, et al. Fusarium solani breast abscess. Indian J Med Microbiology. 2005;23:198–9. doi: 10.4103/0255-0857.16596. [DOI] [PubMed] [Google Scholar]
- Apostolidis J, Bouzani M, Platsouka E, et al. Resolution of fungemia due to Fusarium spp. in a patient with acute leukemia treated with caspofungin. Clin Infect Dis. 2003;36:1349–50. doi: 10.1086/374895. [DOI] [PubMed] [Google Scholar]
- Arikan S, Lozano-Chiu M, Paetznick V, et al. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J Clin Microbiol. 1999;37:3946–51. doi: 10.1128/jcm.37.12.3946-3951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B, McCarthy H, Wilkins B, et al. Fatal disseminated Fusarium infection in acute lymphoblastic leukemia in complete remission. J Clin Pathol. 2001;54:488–90. doi: 10.1136/jcp.54.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley VH, Duarte RF, Gosling RD, et al. Fusarium dimerum infection in a stem cell transplant recipient treated successfully with voriconazole. Bone Marrow Transplant. 2004;34:815–17. doi: 10.1038/sj.bmt.1704660. [DOI] [PubMed] [Google Scholar]
- Bodey GP, Boktour M, Mays S, et al. Skin lesions associated with Fusarium infection. J Am Acad Dermatol. 2002;47:659–66. doi: 10.1067/mjd.2002.123489. [DOI] [PubMed] [Google Scholar]
- Boutati E, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- Bunya VY, Hammersmith KM, Rapuano CJ, et al. Topical and oral voriconazole in the treatment of fungal keratitis. Am J Ophthalmol. 2007;143:151–3. doi: 10.1016/j.ajo.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Charvalos E, Tsatsakis A, Tzatzarakis M, et al. New nystatin polymeric complexes and their in vitro antifungal evaluation in a model study with Fusarium oxysporum. Mycopathologia. 2002;153:15–19. doi: 10.1023/a:1015252121285. [DOI] [PubMed] [Google Scholar]
- Cocuroccia B, Gaido J, Gubinelli E, et al. Localized cutaneous hyalohyphomycosis caused by Fusarium spp. infection in a renal transplant patient. J Clin Microbiology. 2003;41:905–7. doi: 10.1128/JCM.41.2.905-907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigny S, Dhedin N, Datry A, et al. Successful voriconazole treatment of disseminated Fusarium infection in an immunocompromised patient. Clin Infect Dis. 2003;37:311–13. doi: 10.1086/375842. [DOI] [PubMed] [Google Scholar]
- Cudillo L, Girmenia C, Santilli S, et al. Brekthrough fusariosis in a patient with acute lymphoblastic leukemia receiving voriconazole prophylaxis. Clin Infect Dis. 2005;40:1212–13. doi: 10.1086/428849. [DOI] [PubMed] [Google Scholar]
- Cuenca-Estrella M, Gomez-Lopez A, Mellado E, et al. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother. 2006;50:917–21. doi: 10.1128/AAC.50.3.917-921.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- Denning DW, Kibbler CC, Barnes RA, et al. British Society for Medical Mycology proposed standards of care for patients with invasive fungal infections. Lancet Infect Dis. 2003;3:230–40. doi: 10.1016/s1473-3099(03)00580-2. [DOI] [PubMed] [Google Scholar]
- Dismukes WE. Introduction to antifungal drugs. Clin Infect Dis. 2000;30:653–7. doi: 10.1086/313748. [DOI] [PubMed] [Google Scholar]
- Durand ML, Kim IK, D’Amico DJ, et al. Successful treatment of Fusarium endophtalmitis with voriconazole and Aspergillus endophtalmitis with voriconazole plus caspofungin. Am J Ophtalmology. 2005;140:552–4. doi: 10.1016/j.ajo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A. Comparison of In vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–6. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A, Fothergill A, Ghannoum M, et al. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M38-A Document) for amphotericin B, itraconazole, posaconazole and voriconazole. J Clini Microbiol. 2005;43:5243–6. doi: 10.1128/JCM.43.10.5243-5246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Agency for the Evaluation of Medical Products. VFEND: summary of product characteristics [online] 2007 URL: http://www.emea.eu.int.
- Garbino J, Uckay I, Rohner P, et al. Fusarium peritonitis concomitant to kidney transplantation successfully managed with voriconazole: case report and review of the literature. Transpl Int. 2005;18:613–18. doi: 10.1111/j.1432-2277.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA, Kuhn DM. Voriconazole – better chances for patients with invasive mycoses. Eur J Med Res. 2002;7:242–56. [PubMed] [Google Scholar]
- Giaconi JA, Marangon FB, Miller D, et al. Voriconazole and fungal keratitis: a report of two treatment failures. J Ocul Pharmacol Ther. 2006;22:437–9. doi: 10.1089/jop.2006.22.437. [DOI] [PubMed] [Google Scholar]
- Gorman SR, Magiorakos AP, Zimmerman SK, et al. Fusarium oxysporum pneumonia in an immunocompetent host. South Med J. 2006;99:613–16. doi: 10.1097/01.smj.0000217160.63313.63. [DOI] [PubMed] [Google Scholar]
- Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–54. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- Guimerá-Martí-Neda F, García-Bustínduy M, Noda-Cabrera A, et al. Cutaneous infection by Fusarium: successful treatment with oral voriconazole. British J Dermatol. 2004;150:770–95. doi: 10.1111/j.0007-0963.2004.05878.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Cottrill JA, Xiaotian Zheng, Chadwick EG. Fusarium solani endocarditis successfully treated with liposomial amphotericin B and voriconazole. Pediatric Infect Dis J. 2004;23:1059–60. doi: 10.1097/01.inf.0000143649.90952.41. [DOI] [PubMed] [Google Scholar]
- Hamaki T, Kami M, Kishi A, et al. Vesicles as initial skin manifestation of disseminated fusariosis after non-myeloablative stem cell transplantation. Leuk Lymphoma. 2004;45:631–3. doi: 10.1080/1042819031000151941. [DOI] [PubMed] [Google Scholar]
- Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- Herbrecht R. Voriconazole: therapeutic review of a new azole antifungal. Expert Rev Anti Infect Ther. 2004;2:485–97. doi: 10.1586/14787210.2.4.485. [DOI] [PubMed] [Google Scholar]
- Herbrecht R, Kessler R, Kravanja C, et al. Successful treatment of Fusarium proliferatum pneumonia with posaconazole in a lung transplant recipient. J Heart Lung Transplant. 2004;23:1451–4. doi: 10.1016/j.healun.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Heyn K, Tredup A, Salvenmoser S, et al. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp and Fusarium solani. J Antimicrob Chemother. 2005;49:5157–9. doi: 10.1128/AAC.49.12.5157-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CK, Hsu MM, Lee JY. Fusariosis occurring in an ulcerated cutaneous CD8+ T cell lymphoma tumor. Eur J Dermatol. 2006;16:297–301. [PubMed] [Google Scholar]
- Jensen TG, Gahrn-Hansen B, Arendrup M, et al. Fusarium fungemia in immunocompromised patients. Clin Microbiol Infect. 2004;10:499–501. doi: 10.1111/j.1469-0691.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Szekely A, Warnock DW. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998;42:741–5. doi: 10.1093/jac/42.6.741. [DOI] [PubMed] [Google Scholar]
- Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36:630–7. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- Khoury H, Ball NJ. Disseminated fusariosis in a patient with acute leukemia. Br J Haematol. 2003;120:1. doi: 10.1046/j.1365-2141.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- Kivivouri SM, Hovi L, Vetternanta K, et al. Invasive fusariosis in two transplanted children. Eur J Pediatr. 2004;163:692–3. doi: 10.1007/s00431-004-1530-x. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Bodey GP, Hanna H, et al. Outcome determinants of fusariosis in a tertiary care cancer center: the impact of neutrophil recovery. Leuk Lymphoma. 2004;45:139–41. doi: 10.1080/1042819031000149386. [DOI] [PubMed] [Google Scholar]
- Krcmery V, Jr, Jesenska Z, Spanik S, et al. Fungaemia due to Fusarium spp. in cancer patients. J Hosp Infect. 1997;36:223–8. doi: 10.1016/s0195-6701(97)90197-3. [DOI] [PubMed] [Google Scholar]
- Lewis R, Wiederhold NP, Klepser ME. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillusm, Fusarium and Scedosporium spp. Antimicrob Agents Chemother. 2005;49:945–51. doi: 10.1128/AAC.49.3.945-951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Chu PH, Kuo YH, et al. Clinical experience in managing Fusarium solani keratitis. Int Clin Pract. 2005;59:549–54. doi: 10.1111/j.1368-5031.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Linares MJ, Charriel G, SolÍ F, et al. Susceptibility of filamentous fungi to voriconazole tested by two microdilution methods. J ClinMicrobiol. 2005;43:250–3. doi: 10.1128/JCM.43.1.250-253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden P, Williams P, Chan KM. Efficacy and safety of amphotericine B lipid complex injection (ABLC) in solid organ transplant recipients with invasive fungal infection. Clin Transpl. 2000;14:329–39. doi: 10.1034/j.1399-0012.2000.140409.x. [DOI] [PubMed] [Google Scholar]
- Lodato F, Tamé MR, Montagnani M, et al. Systemic fungemia and hepatic localization of Fusarium solani in a liver transplanted patient: an emerging fungal agent. Liver Transpl. 2006;12:1711–14. doi: 10.1002/lt.20899. [DOI] [PubMed] [Google Scholar]
- Madariaga MG, Kohl S. Disseminated fusariosis presenting with pulmonary nodules following a line infection. Braz J Infect Diseases. 2006;10:426. doi: 10.1590/s1413-86702006000600014. [DOI] [PubMed] [Google Scholar]
- Marco F, Pfaller MA, Messer SA, et al. Antifungal activity of a new triazole, voriconazole (UK-109, 496), compared with three other antifungal agents tested against clinical isolates of filamentous fungi. Med Mycol. 1998;36:433–6. [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Mouas H, Lutsar I, Dupont B, et al. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin Infect Dis. 2005;40:1141–47. doi: 10.1086/428734. [DOI] [PubMed] [Google Scholar]
- Musa MO, Al Eisa A, Halim M, et al. The spectrum of Fusarium infection in immunocompromised patients with hematological malignancies and in non-immunocompromised patients: a single institution experience over 10 years. Br J Haematol. 2000;108:544–8. doi: 10.1046/j.1365-2141.2000.01856.x. [DOI] [PubMed] [Google Scholar]
- Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M, Anaissie EJ. Cutaneous infection by Fusarium species in healthy and immunocompromised host: implication for diagnosis and management. Clin Infect Dis. 2002;35:909–20. doi: 10.1086/342328. [DOI] [PubMed] [Google Scholar]
- Nucci M. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr Opin Infect Dis. 2003;16:607–12. doi: 10.1097/00001432-200312000-00015. [DOI] [PubMed] [Google Scholar]
- Nucci M, Anaissie EJ, Queiroz-Telles F, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98:315–19. doi: 10.1002/cncr.11510. [DOI] [PubMed] [Google Scholar]
- Nucci M, Marr KA, Queiroz-Telles F, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38:1237–42. doi: 10.1086/383319. [DOI] [PubMed] [Google Scholar]
- Oliveira JS, Kerbauy FR, Colombo AL, et al. Fungal infections in marrow transplant recipients under antifungal prophylaxis with fluconazole. Braz J Med Biol Res. 2002;35:789–98. doi: 10.1590/s0100-879x2002000700005. [DOI] [PubMed] [Google Scholar]
- Paphitou NI, Ostrosky-Zeichner L, Paetznick VL, et al. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob Agents Chemother. 2002;46:3298–300. doi: 10.1128/AAC.46.10.3298-3300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereiro M, Jr, Abalde MT, Zulaica A, et al. Chronic infection due to Fusarium oxysporum mimicking lupus vulgaris: case report and review of cutaneous involvement in fusariosis. Acta Derm Venereol. 2001;81:51–3. doi: 10.1080/00015550117819. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Marr KA, Walsh TJ, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36:1122–31. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Marco F, Messer SA, et al. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743, 792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251–5. doi: 10.1016/s0732-8893(97)00246-0. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Messer SA, Mills K, et al. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J Clin Microbiol. 2000;38:3359–61. doi: 10.1128/jcm.38.9.3359-3361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Messer SA, Hollis RJ, et al. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob Agents Chemother. 2002;46:1032–7. doi: 10.1128/AAC.46.4.1032-1037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip A, Odabasi Z, Rodriguez J, et al. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp and Fusarium spp. Antimicrob Agents Chemother. 2005;49:3572–4. doi: 10.1128/AAC.49.8.3572-3574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi A, Siniscalchi C, Mastromarino A, et al. Effect of voriconazole on a corneal abscess caused by Fusarium. Acta Ophtalmol Scand. 2004;82:762–4. doi: 10.1111/j.1600-0420.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- Potoski BA, Brown J. The safety of voriconazole. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/343746. [DOI] [PubMed] [Google Scholar]
- Raad I, Hachem RY, Herbrecht R, et al. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematological malignancies and other conditions. Clin Infect Dis. 2006;42:1398–403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- Reis A, Sundmacher R, Tintelnot K, et al. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br J Ophthalmol. 2000;84:932–33. doi: 10.1136/bjo.84.8.928d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CA, Luján-Zilbermann J, Wooderd P. Successful treatment of disseminated fusariosis. Bone Marrow Transpl. 2003;31:411–12. doi: 10.1038/sj.bmt.1703857. [DOI] [PubMed] [Google Scholar]
- Sagnelli C, Fumagalli L, Prigitano A, et al. Successful voriconazole therapy of disseminated Fusarium verticillioides infection in an immunocompromised patient receiving chemotherapy. J Antimicrob Chemother. 2006;57:796–8. doi: 10.1093/jac/dkl016. [DOI] [PubMed] [Google Scholar]
- Sampathkumar P, Paya CV. Fusarium infection after solid organ transplant. Clin Infect Dis. 2001;32:1237–40. doi: 10.1086/319753. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs. 2007;67:269–98. doi: 10.2165/00003495-200767020-00009. [DOI] [PubMed] [Google Scholar]
- Selleslag D. A case of fusariosis in an immunocompromised patient successfully treated with liposomal amphotericin B. Acta Biomed. 2006;77(Suppl 2):32–5. [PubMed] [Google Scholar]
- Singh N. Impact of current transplantation practices on the changing epidemiology of infections in transplant recipients. Lancet Infect Dis. 2003;3:156–61. doi: 10.1016/s1473-3099(03)00546-2. [DOI] [PubMed] [Google Scholar]
- Spellberg B, Schwartz J, Fu Y, et al. Comparison of antifungal treatments for murine fusariosis. J Antimicrob Chemother. 2006;58:973–9. doi: 10.1093/jac/dkl378. [DOI] [PubMed] [Google Scholar]
- Sponsel WE, Graybill JR, Nevarez HL, et al. Ocular and systemic posaconazole (SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br J Ophthalmol. 2002;86:829–30. doi: 10.1136/bjo.86.7.829-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzani M, Vianelli N, Bandini G, et al. Successful treatment of disseminated Fusariosis after allogeneic hematopoietic stem cell transplantation with the combination of voriconazole and liposomial amphotericin. B J Infect. 2006;53:e243–6. doi: 10.1016/j.jinf.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Teixeira AB, Silva M, Lyra L, et al. Antifungal susceptibility and pathogenic potential of environmental isolated filamentous fungi compared with colonizing agents in immunocompromised patients. Mycopathologia. 2005;160:129–35. doi: 10.1007/s11046-005-0117-z. [DOI] [PubMed] [Google Scholar]
- Torres HA, Hachem RY, Chemaly RF, et al. Posaconazole: a broad-spectrum tiazole antifungal. Lancet Infect Dis. 2005;5:775–85. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- Tu EY, McCartney DL, Beatty RF, et al. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592) Am J Ophtalmol. 2007;143:222–7. doi: 10.1016/j.ajo.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Cabrero JE, Greene JN, et al. Successful voriconazole therapy of disseminated Fusarium solani in the brain of a neutropenic cancer patient. Cancer Control. 2003;10:414–19. doi: 10.1177/107327480301000511. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Groll A, Hiemenz J, et al. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- Young CN, Meyers AM. Opportunistic fungal infection by Fusarium oxysporum in a renal transplant patient. Sabouraudia. 1979;17:219–23. [PubMed] [Google Scholar]


