Abstract
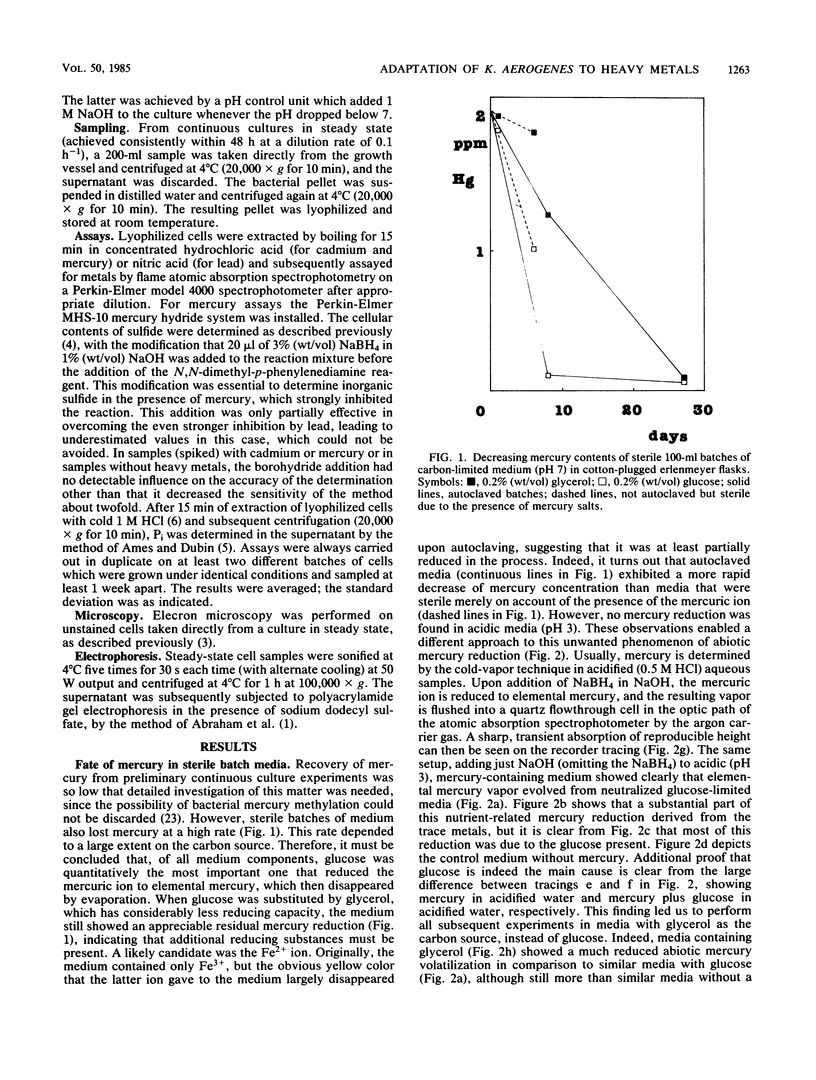
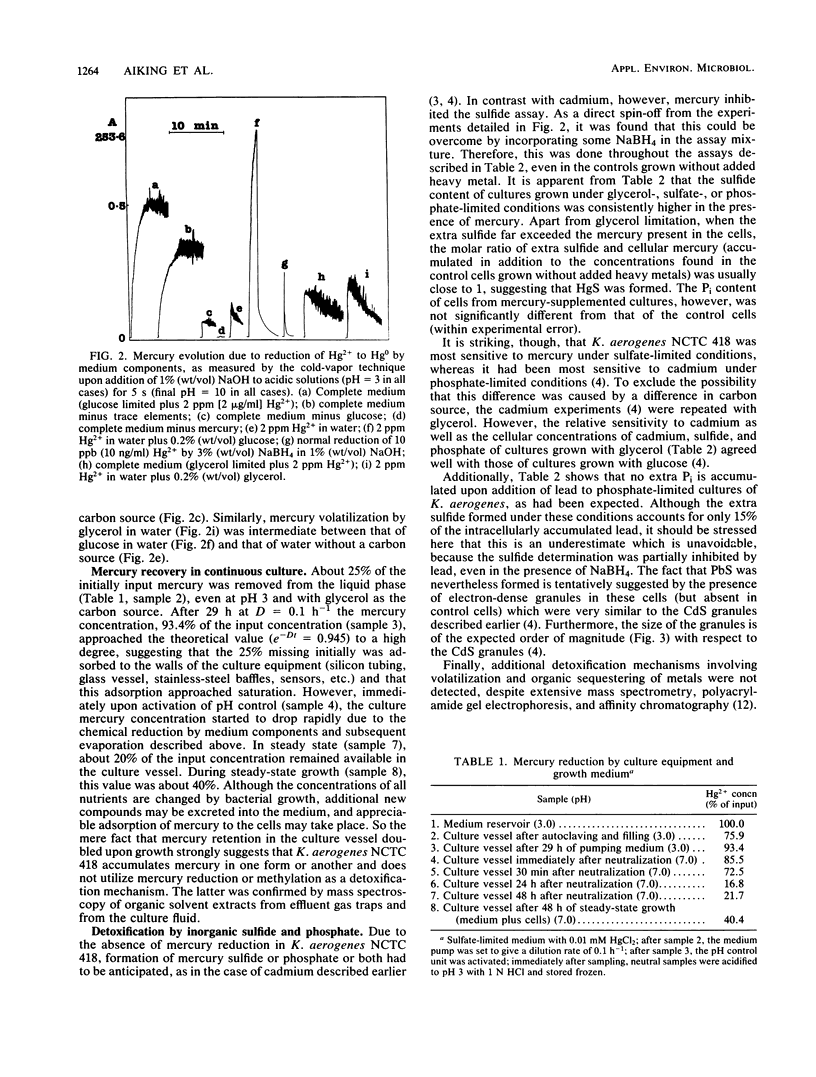
Klebsiella aerogenes NCTC 418 growing in the presence of cadmium under glucose-, sulfate-, or phosphate-limited conditions in continuous culture exhibited sulfide formation and Pi accumulation as the only demonstrable detoxification mechanisms. In the presence of mercury under similar conditions only HgS formation could be confirmed, by an increased sensitivity to mercury under sulfate-limited conditions, among others. The fact that the cells were most sensitive to cadmium under conditions of phosphate limitation and most sensitive to mercury under conditions of sulfate limitation led to the hypothesis that these inorganic detoxification mechanisms generally depended on a kind of "facilitated precipitation". The process was coined thus because heavy metals were probably accumulated and precipitated near the cell perimeter due to the relatively high local concentrations of sulfide and phosphate there. Depending on the growth-limiting nutrient, mercury proved to be 25-fold (phosphate limitation), 75-fold (glycerol limitation), or 150-fold (sulfate limitation) more toxic than cadmium to this organism. In the presence of lead, PbS formation was suggested. Since no other detoxification mechanisms were detected, for example, rendering heavy metal ions innocuous as metallo-organic compounds, it was concluded that formation of heavy metal precipitates is crucially important to this organism. In addition, it was observed that several components of a defined mineral medium were able to reduce mercuric ions to elemental mercury. This abiotic mercury volatilization was studied in detail, and its general and environmental implications are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Aiking H., Kok K., van Heerikhuizen H., van 't Riet J. Adaptation to Cadmium by Klebsiella aerogenes Growing in Continuous Culture Proceeds Mainly via Formation of Cadmium Sulfide. Appl Environ Microbiol. 1982 Oct;44(4):938–944. doi: 10.1128/aem.44.4.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiking H., Stijnman A., van Garderen C., van Heerikhuizen H., van 't Riet J. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl Environ Microbiol. 1984 Feb;47(2):374–377. doi: 10.1128/aem.47.2.374-377.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy M. K., Noyes O. R. Formation of methyl mercury by bacteria. Appl Microbiol. 1975 Sep;30(3):424–432. doi: 10.1128/am.30.3.424-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli M. B., Mitra R. S. Cadmium-binding component in Escherichia coli during accommodation to low levels of this ion. Appl Environ Microbiol. 1981 Jan;41(1):46–50. doi: 10.1128/aem.41.1.46-50.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura I., Funaba T., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. II. NADPH-dependent reduction of mercuric chloride and vaporization of mercury from mercuric chloride by a multiple drug resistant strain of Escherichia coli. J Biochem. 1971 Dec;70(6):895–901. doi: 10.1093/oxfordjournals.jbchem.a129719. [DOI] [PubMed] [Google Scholar]
- Komura I., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. I. Vaporization of a mercury compound from mercuric chloride by multiple drug resistant strains of Escherichia coli. J Biochem. 1971 Dec;70(6):885–893. doi: 10.1093/oxfordjournals.jbchem.a129718. [DOI] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Falkinham J. O., 3rd Plasmid-encoded mercuric reductase in Mycobacterium scrofulaceum. J Bacteriol. 1984 Feb;157(2):669–672. doi: 10.1128/jb.157.2.669-672.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson B. H., Barkay T., Colwell R. R. Role of plasmids in mercury transformation by bacteria isolated from the aquatic environment. Appl Environ Microbiol. 1979 Sep;38(3):478–485. doi: 10.1128/aem.38.3.478-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. J., Porter F. D., Rubinstein J., Silver S. Mercuric reductase enzyme from a mercury-volatilizing strain of Thiobacillus ferrooxidans. J Bacteriol. 1982 Sep;151(3):1230–1236. doi: 10.1128/jb.151.3.1230-1236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Hou H. S., Imura N. Involvement of mercury methylation in microbial mercury detoxication. Arch Microbiol. 1982 Mar;131(2):176–177. doi: 10.1007/BF01054003. [DOI] [PubMed] [Google Scholar]
- Pan-Hou H. S., Imura N. Role of hydrogen sulfide in mercury resistance determined by plasmid of Clostridium cochlearium T-2. Arch Microbiol. 1981 Mar;129(1):49–52. doi: 10.1007/BF00417179. [DOI] [PubMed] [Google Scholar]
- Pan-Hou H. S., Nishimoto M., Imura N. Possible role of membrane proteins in mercury resistance of Enterobacter aerogenes. Arch Microbiol. 1981 Oct;130(2):93–95. doi: 10.1007/BF00411057. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Tuovinen O. H. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol Rev. 1984 Jun;48(2):95–124. doi: 10.1128/mr.48.2.95-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Vonk J. W., Sijpesteijn A. K. Studies on the methylation of mercuric chloride by pure cultures of bacteria and fungi. Antonie Van Leeuwenhoek. 1973;39(3):505–513. doi: 10.1007/BF02578894. [DOI] [PubMed] [Google Scholar]