Abstract
To successfully navigate through the environment animals rely on information concerning their directional heading and location. Many cells within the postsubiculum and anterior thalamus discharge as a function of the animal’s head direction (HD), while many cells in the hippocampus discharge in relation to the animal’s location. We placed lesions in the hippocampus and recorded from HD cells in the postsubiculum and anterior thalamus. Lesions of the hippocampus did not disrupt the HD cell signal in either brain area, indicating that the HD cell signal must be generated by structures external to the hippocampus. In addition, each cell’s preferred firing direction remained stable across days when the lesioned animal was placed into a novel environment. This stability appeared to weaken after several weeks of nonexposure to the new enclosure for two out of five animals, and subsequently recorded cells from these two animals established a new angular relationship between the familiar and novel environments. Our results suggest that extra-hippocampal structures are capable of creating and maintaining a novel representation of the animal’s environmental context. This representation shares features in common with mnemonic processes involving episodic memory that until now were assumed to require an intact hippocampus.
Keywords: anterior thalamic nuclei, navigation
Contemporary theories of hippocampal function postulate that the hippocampus is important in forming complex associations among environmental stimuli (1, 2). Animals with lesions of the hippocampus exhibit abnormalities in context-dependent behavior and are severely impaired in tasks requiring the effective use of spatial information (3–5). Without a hippocampus animals are expected to have difficulty using episodic information about their environment.
Information concerning the animal’s spatial orientation is thought to be conveyed by two types of allocentric spatial cells: place cells and head direction (HD) cells. While place cells are found primarily in the hippocampus and convey information about the animal’s location (6, 7), HD cells discharge as a function of the animal’s HD in the horizontal plane, independent of location within the environment (8). HD cells were originally identified in the postsubiculum but have now been observed in several other brain structures, including the anterior dorsal portion of the anterior thalamic nuclei (ATN) (9). The postsubiculum, in addition to being reciprocally connected with the ATN, also receives a major projection from the subiculum, which contains place cells and is the primary output target of the hippocampus (10, 11). Thus, hippocampal place cells could influence or contribute to the HD cell signal in the postsubiculum.
When an animal is placed into a novel context or environment, HD cells maintain their directional firing properties, but the direction of maximum discharge (preferred direction) frequently shifts to a new direction (12). If the animal is reintroduced into that environment at a later time, the HD cell’s preferred direction will return to its former direction for that particular context. Internally generated information about the animal’s movements (e.g., vestibular, proprioceptive, and motor efference copy cues) is especially influential during the animal’s initial experience in a novel environment, but thereafter environmental landmarks are the main determinants of the HD cell’s preferred direction (13). Thus, on future exposures to this new environment, the cell’s preferred direction will be controlled by the salient landmark(s) of that environment and will consistently maintain the same angular relationship with the landmark(s) over time (13). This stability could be attributed to complex perceptual processes whereby certain features of the environment always drive the cells’ preferred directions to a particular set of orientations (14, 15). These processes would presumably be independent of hippocampal function. Alternatively, the stability could be due to a mnemonic process whereby salient features of the novel environment are stored and later retrieved when the animal finds itself in that environment again. Some theories have postulated that this latter process may be hippocampal-dependent (3, 16). It is also likely that the perceptual and mnemonic processes are fundamentally interrelated since normal perception depends upon previously stored perceptions.
To investigate the role of the hippocampus in processing landmark information, as well as its role in HD cell activity, we recorded HD cells from rats with bilateral neurotoxic lesions of the hippocampus. We tested (i) whether HD cell activity is present without a hippocampus and (ii) if HD cells were identified, whether their preferred directions remain stable across multiple testing sessions when the animal was exposed to a novel environment. A stable preferred direction under such conditions would indicate that selective episodic spatial information can be stored and maintained over time without a hippocampus, either through perceptual or mnemonic processes. We report here that animals with hippocampal lesions contain HD cells, that their preferred firing directions remain stable across days in a novel environment, and that their firing properties appear to reflect the encoding and retrieval of new information about novel environments.
METHODS
Behavioral training, room environment, recording techniques, and data analysis were similar to those described previously (8, 9). Briefly, food-deprived Long–Evans female rats were trained to retrieve food pellets thrown randomly into a 76-cm diameter cylinder containing a single prominent cue occupying ≈100° of arc taped to the inside wall. The cylinder was uniformly surrounded by a 2-m diameter floor-to-ceiling circular black curtain. After training, the animals were implanted with an array of recording electrodes (17) in either the postsubiculum (n = 4) or the ATN (n = 3). In addition, 0.05–0.1 μl of ibotenic acid (dissolved in 10 mg/ml K+ PBS) was injected bilaterally at nine sites per hemisphere to selectively lesion the hippocampus (18).
To encourage the use of the cue card for an orienting reference, the floor paper was changed in between each recording session to reduce the use of olfactory cues for orientation. Prior to all recording sessions, but not during presurgical training and cell screening, the animals underwent a disorientation procedure in an attempt to prevent the use of path integration mechanisms. This procedure involved gently spinning the animal back-and-forth in an opaque cardboard box for about 1 min while the experimenter walked around the periphery of the curtain and cylinder.
In addition to the cylindrical enclosure, cells were also recorded from square-, triangular-, and pentagon-shaped arenas. The square and equilateral triangle enclosures were placed in the same location within the room that the cylinder had previously occupied. A single cue card was attached to the inside wall of each enclosure and was situated at approximately the same location in the room for all three arenas. The walls measured 70 and 88 cm in length for the square and triangle, respectively, and both chambers were 51 cm high. The square was painted gray and contained a white cue card, while the triangle was painted white and contained a dark green cue card. The shape of the pentagon was irregular, having sides of 44, 40, 74, 79, and 74 cm, with a height of 51 cm. The surface area within all four environments was similar. Except for the recording of one cell, the pentagon was placed in a second recording room without a surrounding curtain. Thus, under these conditions the animal had access to visual cues within the room at the time of recording. The walls of the pentagon were light-colored plywood with a white cue card attached to one wall. The animal was placed into the enclosures from a constant location on any given day, but the entry point was varied across days. The daily chronological order of recordings from the different environments was counterbalanced across all but the first day. Statistical analyses were made using the Student’s t test (P = 0.05).
RESULTS
Firing Properties.
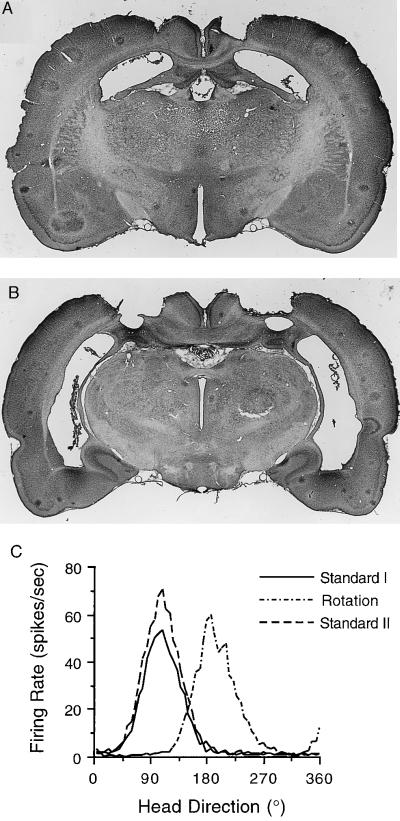
All animals (n = 7) had complete lesions of the dorsal hippocampus, while a small portion (≈10%) of the ventral hippocampus remained intact bilaterally in some animals (n = 5) (Fig. 1 A and B). This ventral portion only contained parts of the dentate and CA3 regions; thus, all animals had complete lesions of the CA1 area. In addition, the dorsal hippocampus has been shown to be the critical locus for the normal acquisition of a spatial task (19). Most animals also had extensive damage to the subiculum. Despite the complete destruction of the CA1 area, the major source of hippocampal output, HD cells were present in all animals. The response properties of cells in animals with hippocampal lesions did not differ from control animals. Quantitative measurements were obtained from 21 HD cells (11 postsubiculum, 10 ATN). The mean peak firing rates, directional firing ranges, and background firing rates across all HD cells were 38.7 spikes/sec, 101.9°, and 2.7 spikes/sec, respectively. These values were not significantly different from values reported for intact animals (8, 9). HD cells comprised 24.2% and 32.2% of all cells identified within the postsubiculum and ATN, respectively. These percentages are similar to values found in nonlesioned animals (8, 9). Fig. 1C illustrates the firing profile of a postsubicular HD cell recorded from the lesioned animal depicted in Fig. 1 A and B.
Figure 1.
(A and B) Coronal brain sections stained with cresyl violet are shown from a representative hippocampal lesioned animal. Sections in A and B are approximately −2.4 and −5.2 mm posterior to bregma, respectively. Note that the most ventral portion of CA3 hippocampus was spared in this animal. (C) Firing rate vs. HD plots from a postsubicular HD cell recorded over three sessions within the novel pentagon-shaped environment. Following a 90° counterclockwise rotation of the cue card with the animal out of view, the cell’s preferred direction shifted a corresponding amount.
Cue Card Rotation in the Cylinder.
After identifying a HD cell, we tested whether the cue card still exerted control over the cell’s preferred direction by rotating the card 90° in the absence of the rat. In response to rotation of the cue card, the cell’s preferred directions also shifted a similar amount (Fig. 1C); for 90° card rotations the mean shift of the preferred directions was 77.1 ± 2.7°, (range 66°-96°, n = 13). The small undershift has also been observed in previous studies on nonlesioned animals (12). These results indicate that, as with nonlesioned animals, the cue card is still able to exert stimulus control over the cells’ preferred directions.
Novel Enclosures.
If a cell’s preferred direction was determined solely with respect to the cue card in lesioned animals, we would expect the preferred direction to be invariant across different shaped environments, as long as the cue card remained at the same location. We tested this hypothesis by recording HD cells in novel shaped environments (square, triangle, or irregular pentagon) with the cue card at a constant location with respect to the recording room. In general, the cell’s preferred direction did not differ very much between the square-shaped apparatus and the cylinder (only 2 of 11 cells tested in both the cylinder and square had a difference >18°). These results are similar to findings reported from intact animals (12) and show that HD cells from both lesioned and nonlesioned animals have similar responses to the square and cylindrical enclosures. In contrast, when 17 cells from six animals were monitored in the triangle or pentagon enclosures the mean absolute change in preferred direction between the cylinder and novel environment was 120.4 ± 8.4° (range 36–174°). Similar findings were also reported for HD cells in nonlesioned animals when they were monitored in a rectangular-shaped environment (12). Note that both the square and the rectangle contain similar visual features: the four 90° angles of the corners, the colored walls, and the white cue cards. Despite these similarities, when compared with the cell’s preferred direction in the cylinder, the preferred direction usually only shifted its orientation in the rectangle. This finding suggests that HD cells are sensitive to the global geometric properties of the environment in addition to simple sensory features such as the cue card.
Rotations of the cue card in each of the novel-shaped environments led to a near-equal shift in the cell’s preferred direction (n = 6). The mean deviation between the cell’s preferred direction and the expected preferred direction following card rotation was 18.0 ± 6.0° and was not significantly different from values observed in control animals (9, 12). This result indicates that the newly established preferred direction can still be controlled by the salient features of the environment.
We also studied how consistent the cell’s preferred direction would be in each environment over repeated recording sessions because it is possible that a preferred direction was arbitrarily selected upon each introduction to the environment and then maintained using path integration mechanisms during the 8-min recording session. For the cylinder environment, the mean absolute difference in preferred direction between two sessions conducted on different days for 12 cells was 14.0 ± 1.7°. Fig. 2A illustrates the preferred directions of HD cells recorded in the cylinder on 2 consecutive days. With the exception of one cell, the preferred directions of all HD cells shifted by ≤18° across days, which is comparable to results reported for nonlesioned animals (8, 9).
Figure 2.
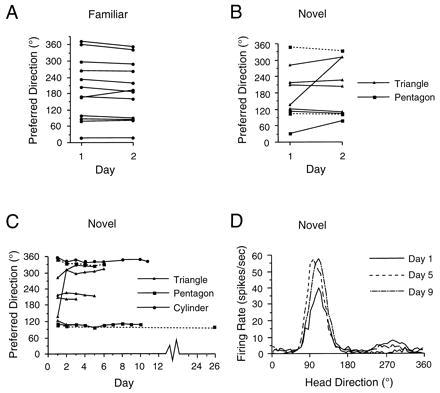
HD cell recordings across days in familiar and novel enclosures. (A) HD cells’ preferred directions from lesioned animals are shown for two consecutive days recorded within the cylinder. Most cells had stable preferred directions across days in the familiar enclosure. (B and C) Graphs plotting the cells’ preferred directions recorded across two days (B) or multiple days (C) in a novel enclosure. Except for one cell, the preferred directions in these novel enclosures were also generally stable across days. For comparison, daily sessions recorded in the familiar cylinder are shown for one cell in C. The dashed lines indicate two cells that were monitored in a different recording room than the sessions conducted in the cylinder. The preferred direction of the cell that was initially unstable in the triangle had a stable preferred direction in the pentagon when the animal was monitored in the second testing room. (D) Firing rate vs. HD plots at three time points for one ATN HD cell monitored across nine days in a novel enclosure. The increased peak firing rate observed for days 5 and 9 was attributed to better electrical isolation of the cell, and was not observed in other HD cells.
By recording HD cells in a novel environment across days, we presented a stronger challenge to the notion that the hippocampus is not necessary for stable HD cell activity because the animal has never experienced this context with an intact hippocampus. Importantly, as with recordings in the cylinder, there was little change in each cell’s preferred direction across days in the triangle or pentagon. The preferred directions from nine cells recorded in novel enclosures over 2 days are presented in Fig. 2B. The mean difference in the preferred direction over two days was 34.0 ± 17.4°, although this value is skewed by the results from two cells (the other seven cells had directional shifts ≤30°). A Student’s t test showed there was no significant difference between the directional shifts in the cylinder and the novel enclosures.
Fig. 2C shows the preferred directions of all cells in the novel environments recorded over as many as 11 days. Note that the one cell that showed a large directional shift between days 1 and 2 remained stable for the subsequent 4 days. The stability observed in the preferred direction across days cannot be attributed to prelesion familiarity with other unintentional cues in the recording room because two cells (indicated by dotted lines in Fig. 2C) were monitored in a second room containing different cues and no surrounding curtain. The firing rate vs. HD functions from one cell are plotted for three recording sessions on different days in Fig. 2D. The variability in the preferred direction across more than 2 days was analyzed by compiling a 10-cell matrix containing the differences in preferred directions between all binary comparisons for the first 3–5 days and then calculating a mean value for each cell. For the eight cells recorded for at least 3 days in the novel enclosure, the mean difference was 16.6 ± 8.4°. This value was not significantly different compared with the mean value (12.5 ± 3.6°) from seven cells recorded in the cylinder over 3–5 days. In sum, even though the animal’s entire experience within the novel environment occurred without a hippocampus, HD cells were still able to maintain a consistent preferred direction across days. This result suggests that extra-hippocampal structures can encode novel spatial relationships and accurately maintain this representation across several days.
Multiple Recordings from the Same Animal.
We were also interested in the response of HD cells recorded at different times from individual animals within the various environments. Previous studies in nonlesioned animals have shown that whenever two HD cells are recorded simultaneously the angular relationship between their preferred directions remains constant following various environmental manipulations (12, 13). Thus, by examining the response pattern for one cell we can make inferences regarding how the HD cell network in a particular brain area responds to a given enclosure, as well as the relations between different enclosures. If the visual features of an environment drive the directional network to a particular set of preferred directions, then the absolute difference between a cell’s preferred direction in the two environments should remain constant across all HD cells from an individual animal. This property was usually evident for postsubicular HD cells in nonlesioned animals (see table 2 of ref. 12), although inconsistencies have been observed occasionally in previous studies (unpublished observations). If this property of maintaining a consistent absolute difference between environments is not found in lesioned animals, then (i) the preferred directions are uncoupled in lesioned animals, or (ii) the same environmental features can drive the HD cell network to a different set of values on different occasions.
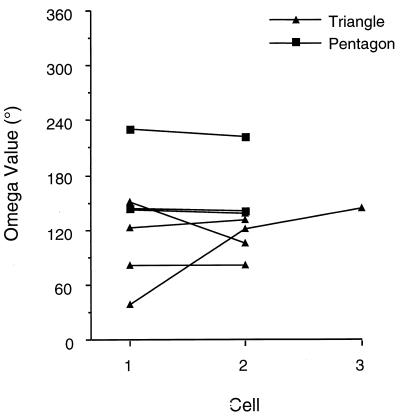
To address these issues, we analyzed the difference between the preferred firing directions of HD cells recorded from an animal in the cylinder and novel enclosures. Each cell’s preferred direction in the cylinder was subtracted from its preferred direction in the novel enclosure. The absolute value of this difference (Ω) was then compared across cells within the same animal. Because Ω was plotted in each novel enclosure, an animal could have two series of Ω values, one for the triangle and one for the pentagon. Fig. 3 shows that the value of Ω remained relatively constant across cells recorded from the same animal. However, two animals showed marked changes in Ω and a third animal with a partial hippocampal lesion that was not included in the results above also exhibited an unstable Ω value in the pentagon (96°, data not shown).
Figure 3.
Plot of Ω values across different cells within individual animals. Ω was determined by calculating the angular difference between a cell’s preferred firing direction in the novel enclosure and its firing direction in the cylinder. The sessions being compared were recorded on the same day, usually within 1 hr of each other. The Ω values remained relatively constant across most cells, although there were cases where Ω varied considerably.
The mean number of days separating the two recording sessions was 19.3 ± 5.0 days (range: 2–56 days). Unfortunately, we could not determine whether there was a good correlation between the number of days separating the recording sessions and the amount of change in Ω across two cells because most of the recording sessions were separated by a similar number of days (≈19). Nonetheless, the magnitude and frequency of observed changes in Ω for some animals suggests that the physical layouts of the environments do not always determine the specific preferred directions for HD cells or the magnitude of change in the preferred direction between two environments.
On seven occasions in three animals we simultaneously monitored two HD cells during both the cylinder and novel enclosure sessions. On all occasions the shift in one cell’s preferred direction between enclosures was paralleled in the second cell, such that the angular difference between the two cells’ preferred directions remained similar across recording sessions (mean angular difference = 6.7 ± 1.9°, range: 1–17°; with one exception, all angular differences were <9°). This result indicates that directional cells in lesioned animals were able to shift their preferred directions in register.
Taken together, these results imply that when the animal experienced the novel enclosure on a daily basis, the cells’ preferred directions were stable over several days. However, occasionally after an intervening interval when the animal was not exposed to the novel enclosure, the preferred directions of subsequently recorded cells had different angular relationships between the cylinder and the novel enclosure. The newly established angular relationship between environments typically remained stable again over the next few days. Because the cells’ preferred directions remained in register in lesioned animals, this result implies that if the previously recorded HD cell were monitored after the intervening period, then we would have observed a new angular relationship for this cell’s preferred directions in the two enclosures. This inference indicates that the preferred directions of HD cells in lesioned animals showed stability on a short term basis (on the order of several days) but were occasionally unstable over longer time periods (on the order of weeks) when the animals were not exposed to the enclosure during the intervening period.
DISCUSSION
These findings show that the hippocampus is neither necessary for the occurrence of HD cell activity in the postsubiculum and ATN, nor necessary for transforming an egocentric spatial representation to an allocentric representation in the brain. Since place cells are found predominantly in the hippocampus, this finding is consistent with the notion that the HD cell system is functionally independent of the place cell system (12). Recent studies have suggested that the HD cell signal in the postsubiculum is generated in the ATN or in structures afferent to it (20). Although the hippocampus is not required to generate the HD cell signal, it could influence HD cell activity either through its role in spatial memory (5) or in processing relationships amongst different stimuli (3, 21). In addition, some studies have suggested that the hippocampus may be involved in recognition (ref. 22, but see ref. 23) or novelty detection processes (5, 24). Accordingly, it is possible that the hippocampus might be involved in encoding and maintaining a stable preferred firing direction in new environments where the rat is confronted with novel stimuli and then recognizes them in subsequent sessions. However, our results show that the establishment of a new preferred direction in a novel enclosure was possible without a hippocampus, and that this new direction was maintained across several days.
It was not surprising that a cell’s preferred direction was stable across days in the cylinder, because the animal experienced this enclosure before the surgical lesion. But we did not expect to find the cell’s preferred direction to be stable across days in enclosures that were only experienced after the lesion. Recall that animals without a hippocampus should have little, if any, memory of their experiences in the novel enclosures. Thus, it was interesting that the stability we observed spanned a period (several days) that usually results in memory impairments in animals with hippocampal damage (3, 25). This stability could be attributed to several possibilities. First, it is possible that the perceptual features for an enclosure with a particular shape could always drive the directional network to a particular set of values as some network models have hypothesized (14, 15). If this possibility were the case, then there might be an expectation of equivalent directional shifts between the cylinder and the novel enclosure across all cells recorded in a given animal. This result did not always occur (see Fig. 3). Furthermore, Knierim et al. (26) reported a high frequency of shifts in a cell’s preferred direction in the same environment when the animal experienced a disorientation procedure before being placed in the apparatus. If the perceptual features of the environment were driving the cell’s preferred direction to a particular value, then, despite the disorientation procedure, the HD cell should consistently contain a similar preferred direction. It has also been postulated that each HD cell may become bound, through experience, to a set of independent visual feature detectors (14). Were this process the case, then, given the present results, the binding would still need to occur independent of hippocampal processes. Furthermore, because some experience in the environment is involved over time, this event implies that the neurons must have modified their synaptic weights or connections to have stored this information. This process, by definition, can be considered a form of memory. We cannot exclude, however, the possibility that perceptual processes account for the stability we observed in the novel enclosures. Taken together, though, our results provide a challenge to the hypothesis that perceptual processes alone, without a mnemonic component, can explain these findings.
A second possibility that may account for the stability is that cues within the recording room, which were experienced presurgery, could have stabilized the preferred direction in the novel enclosures. However, this explanation does not account for the finding that the cells, including two cells that were monitored in a different room, established new preferred directions relative to the recording room in the triangle and pentagon enclosures. Furthermore, the results from the triangle and pentagon rotation sessions showed that the cues in these enclosures were sufficiently salient to control the two cells’ preferred directions and suggested that secondary cues exert, at best, a weak influence over cell discharge.
A third possibility is that animals could have used path integration as a strategy to maintain their directional heading when brought into the recording room (26, 27). However, the disorientation procedures performed before entering the novel enclosures presumably rendered path integration ineffective. Even if path integration mechanisms were capable of maintaining the preferred directions in lesioned animals, the establishment of different preferred directions in novel environments argues against this possibility.
Alternatively, the stability observed across days might be attributable to a mnemonic process present in the cortex of hippocampal-lesioned animals that is sensitive to the spatial relationships among features of the environment. Such a cortical process could account for the apparent normal encoding and retrieval of selective spatial features. However, this spatial information could deteriorate after the absence of further experience in the novel enclosures. It was therefore noteworthy that by inference the stability observed in the cell’s preferred direction across a few days appeared to degrade for some animals over longer time periods (weeks). It is possible that the instability in Ω over longer time periods may be attributable to loss of the hippocampus, although one intact animal was shown to have changes in Ω when HD cells were monitored in a cylinder and rectangular enclosure (see table 2 in ref. 12). The issue of whether the stability in Ω increases with more exposure to the enclosures warrants further study as it will provide information on how reference frames are used in guiding behavior.
In conclusion, extra-hippocampal structures can encode episodic information concerning the spatial features of an environment, and animals can use this information to determine their directional heading. Our findings suggest that other brain structures can process abstract spatial information in novel environments and maintain this relationship over several days. Given that the posterior parietal and cingulate cortices are involved in spatial information processing (28–31) and that mechanisms of neural plasticity, such as long-term potentiation, have been identified in the neocortex (32), it is possible that areas of the posterior neocortex are capable of encoding this landmark information.
Acknowledgments
We thank P. Dudchenko, J. Goodridge, R. Stackman, and M. Glickstein for helpful comments during this project. This work was supported by National Institute of Mental Health Grants MH48924 and MH01286.
ABBREVIATIONS
- HD
head direction
- ATN
anterior thalamic nucleus
References
- 1.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum H, Otto T, Cohen N J. Behav Brain Sci. 1994;17:449–518. [Google Scholar]
- 3.Kim J J, Fanselow M S. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 4.Morris R G M, Garrud P, Rawlins J N P, O’Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 6.O’Keefe J. Prog Neurobiol. 1979;13:419–439. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Mara S M. Prog Neurobiol. 1995;45:253–274. doi: 10.1016/0301-0082(94)00050-r. [DOI] [PubMed] [Google Scholar]
- 8.Taube J S, Muller R U, Ranck J B., Jr J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taube J S. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Groen T, Wyss J M. Brain Res. 1992;529:165–177. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- 11.Sharp P E, Green C. J Neurosci. 1994;14:2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taube J S, Muller R U, Ranck J B., Jr J Neurosci. 1990;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube J S, Burton H L. J Neurophysiol. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- 14.Skaggs W E, Knierim J J, Kudrimoti H S, McNaughton B L. In: Advances in Neural Information Processing Systems. Tesauro G, Touretzky D S, Leen T K, editors. Vol. 7. Cambridge, MA: MIT Press; 1995. pp. 173–180. [PubMed] [Google Scholar]
- 15.Touretzky D S, Redish A D. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Nadel L, Willner J. Physiol Psychol. 1980;8:218–228. [Google Scholar]
- 17.Kubie J L. Physiol Behav. 1984;32:115–118. doi: 10.1016/0031-9384(84)90080-5. [DOI] [PubMed] [Google Scholar]
- 18.Jarrard L E. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 19.Moser M-B, Moser E I, Forrest E, Andersen P, Morris R G M. Proc Natl Acad Sci USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taube J S, Goodridge J P, Golob E J, Dudchenko P A, Stackman R W. Brain Res Bull. 1996;40:477–486. doi: 10.1016/0361-9230(96)00145-1. [DOI] [PubMed] [Google Scholar]
- 21.Cohen N J, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 22.Gaffan D, Harrison S. Exp Brain Res. 1989;74:202–212. doi: 10.1007/BF00248293. [DOI] [PubMed] [Google Scholar]
- 23.Meunier M, Hadfield W, Bachevalier J, Murray E A. J Neurophysiol. 1996;75:1190–1205. doi: 10.1152/jn.1996.75.3.1190. [DOI] [PubMed] [Google Scholar]
- 24.Knight R T. Nature (London) 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 25.Eichenbaum H, Otto T, Cohen N J. Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 26.Knierim J J, Kudrimotti H S, McNaughton B L. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow J S. J Theor Biol. 1964;6:76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- 28.Mishkin M, Ungerleider L G, Mack K. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- 29.Chen L L, Lin L H, Green E J, Barnes C A, McNaughton B L. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 30.Olson C R, Musil S Y, Goldberg M E. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birkhauser; 1994. pp. 366–380. [Google Scholar]
- 31.Brotchie P R, Andersen R A, Snyder L H, Goodman S J. Nature (London) 1995;375:232–235. doi: 10.1038/375232a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee K S. Brain Res. 1982;239:617–623. doi: 10.1016/0006-8993(82)90538-8. [DOI] [PubMed] [Google Scholar]