Abstract
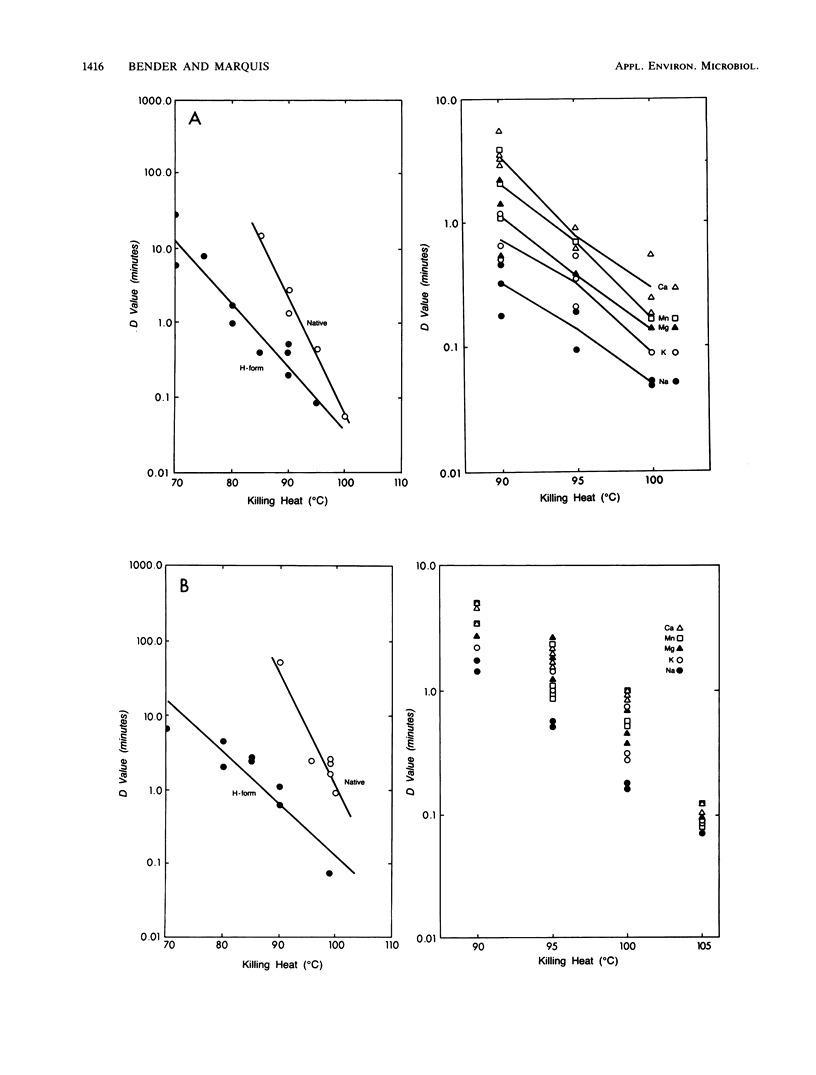
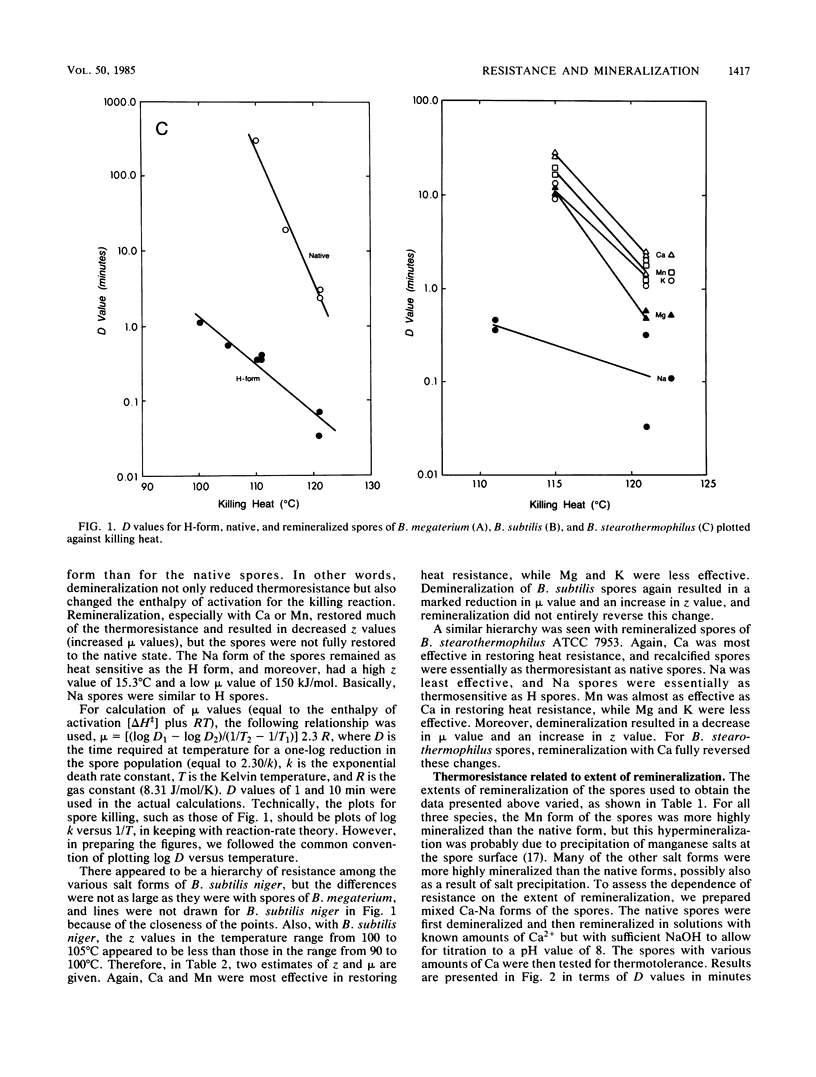
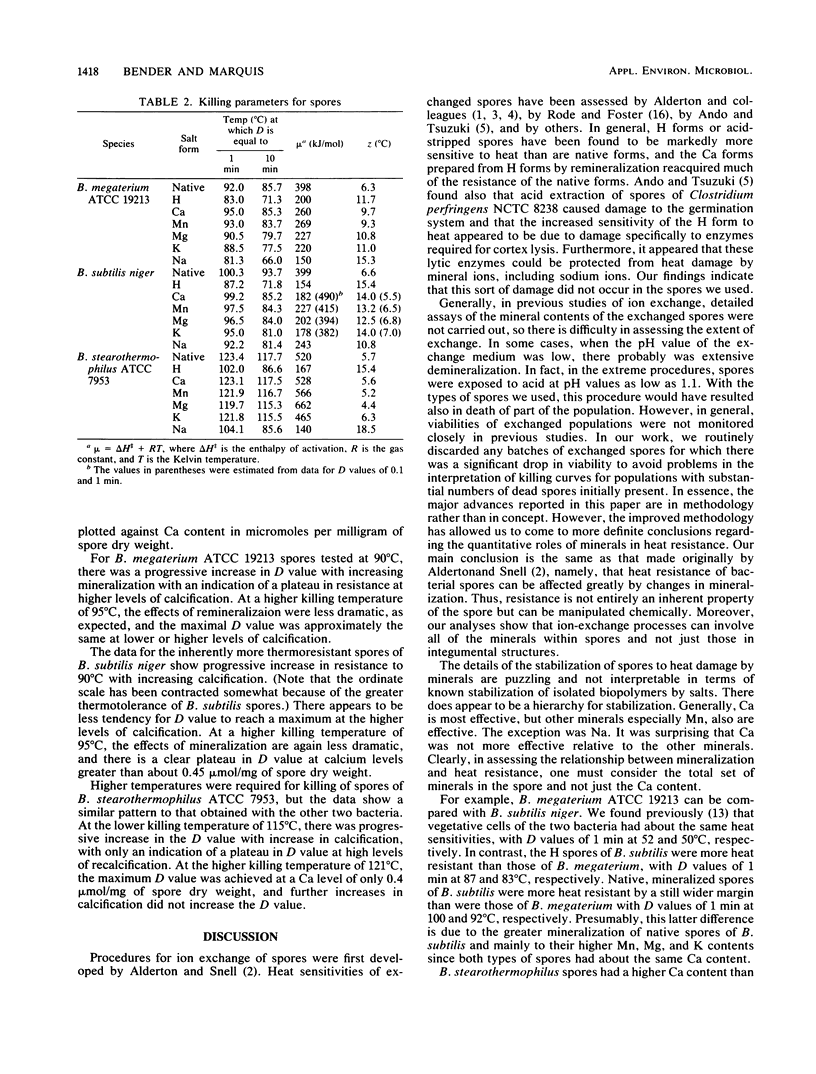
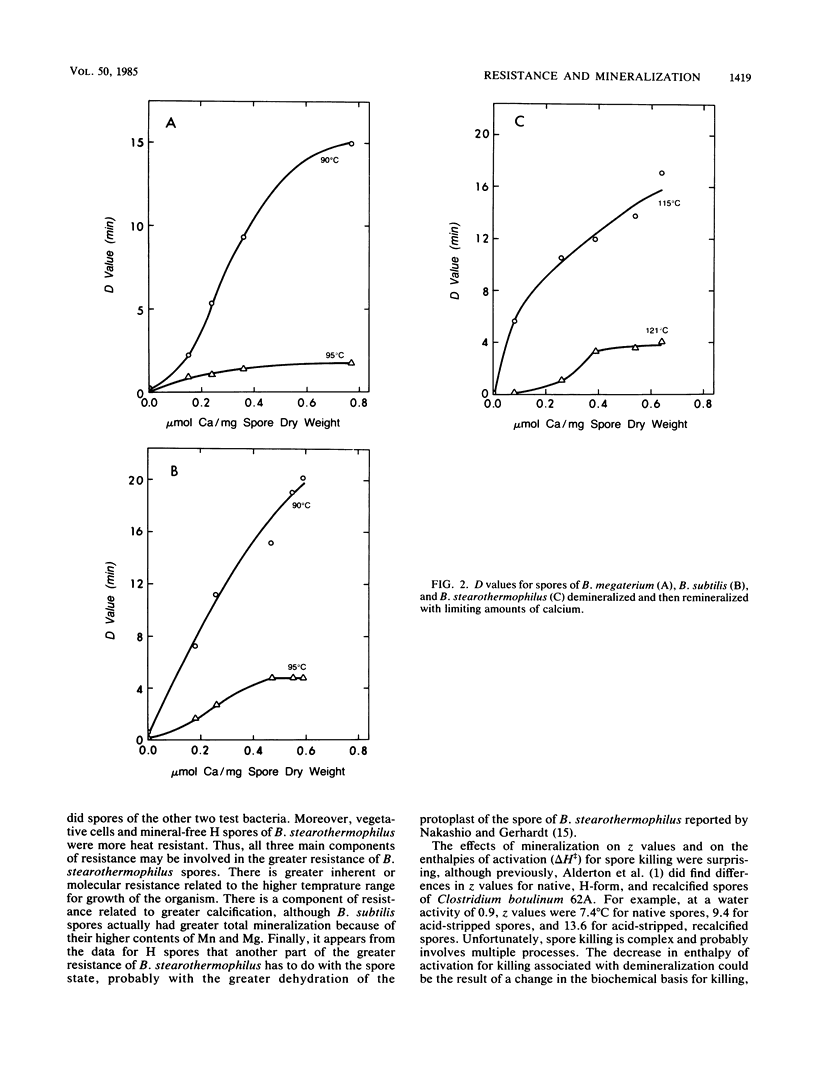
Spores of Bacillus megaterium ATCC 19213, Bacillus subtilis niger and Bacillus stearothermophilus ATCC 7953 were converted to fully demineralized, but viable, H forms by controlled acid titration. H forms were more heat sensitive than were native forms, but z values were greater for killing of H spores than those for native spores. Therefore, the differences in heat sensitivity between native and H forms decreased with increasing killing temperature. The increase in heat sensitivity associated with demineralization did not appear to be due to damage to cortex lytic enzymes of the germination system because it could not be moderated by decoating heated H spores and plating them on medium with added lysozyme. H spores could be remineralized by means of back titration with appropriate base solutions. The remineralized spores, except for the Na form, were then more heat resistant than were H spores. Ca and Mn were more effective in restoring resistance than were Mg and K. Generally, the remineralized forms (except for the Na form) had z values greater than those of the native forms but still less than those of the H forms. At lower killing temperatures, the reinstatement of resistance could be related to the extent of remineralization. However, at higher killing temperatures, only a fraction of the mineral was effective in restoring resistance, and higher levels of remineralization did not result in greater resistance. Mineralization is clearly an important factor in spore heat resistance, but the relationship between resistance and mineralization is complex and dependent on killing temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDERTON G., THOMPSON P. A., SNELL N. HEAT ADAPTATION AND ION EXCHANGE IN BACILLUS MEGATERIUM SPORES. Science. 1964 Jan 10;143(3602):141–143. doi: 10.1126/science.143.3602.141. [DOI] [PubMed] [Google Scholar]
- Alderton G., Chen J. K., Ito K. A. Heat resistance of the chemical resistance forms of Clostridium botulinum 62A spores over the water activity range 0 to 0.9. Appl Environ Microbiol. 1980 Sep;40(3):511–515. doi: 10.1128/aem.40.3.511-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton G., Snell N. Bacterial spores: chemical sensitization to heat. Science. 1969 Mar 14;163(3872):1212–1213. doi: 10.1126/science.163.3872.1212. [DOI] [PubMed] [Google Scholar]
- Ando Y., Tsuzuki T. Mechanism of chemical manipulation of the heat resistance of Clostridium perfringens spores. J Appl Bacteriol. 1983 Apr;54(2):197–202. doi: 10.1111/j.1365-2672.1983.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E., Child S. Z., Bender G. R. Dielectric properties of native and decoated spores of Bacillus megaterium. J Bacteriol. 1979 Dec;140(3):917–928. doi: 10.1128/jb.140.3.917-928.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlin J. H., Slepecky R. A. Mechanism of the Heat Sensitization of Bacillus subtilis Spores by Ethidium Bromide. Appl Environ Microbiol. 1985 Jun;49(6):1396–1400. doi: 10.1128/aem.49.6.1396-1400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa T., Beaman T. C., Pankratz H. S., Nakashio S., Corner T. R., Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984 Aug;159(2):624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R. Mineralization and heat resistance of bacterial spores. J Bacteriol. 1985 Feb;161(2):789–791. doi: 10.1128/jb.161.2.789-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashio S., Gerhardt P. Protoplast dehydration correlated with heat resistance of bacterial spores. J Bacteriol. 1985 May;162(2):571–578. doi: 10.1128/jb.162.2.571-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Quantitative aspects of exchangeable calcium in spores of Bacillus megaterium. J Bacteriol. 1966 Apr;91(4):1589–1593. doi: 10.1128/jb.91.4.1589-1593.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson R. A., Nealson K. H. Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol. 1982 Aug;151(2):1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. R., Ellar D. J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978 Jul;135(1):133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J Bacteriol. 1978 Jun;134(3):699–705. doi: 10.1128/jb.134.3.699-705.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



