Abstract
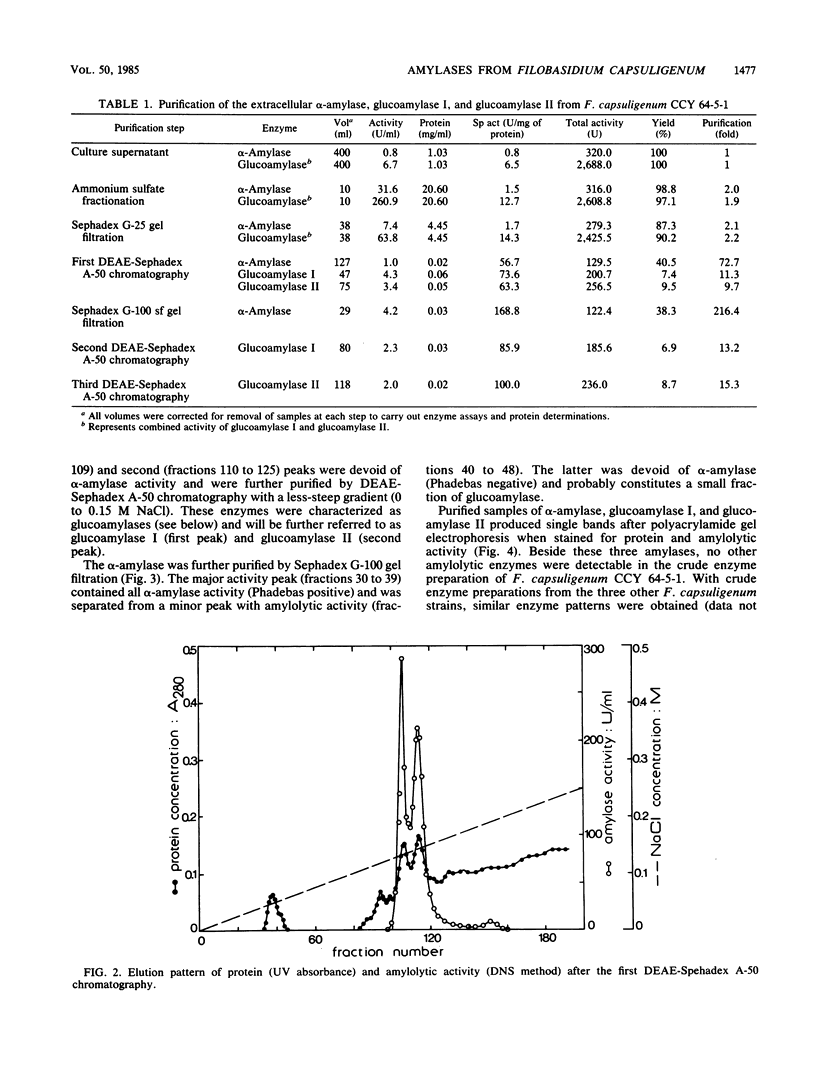
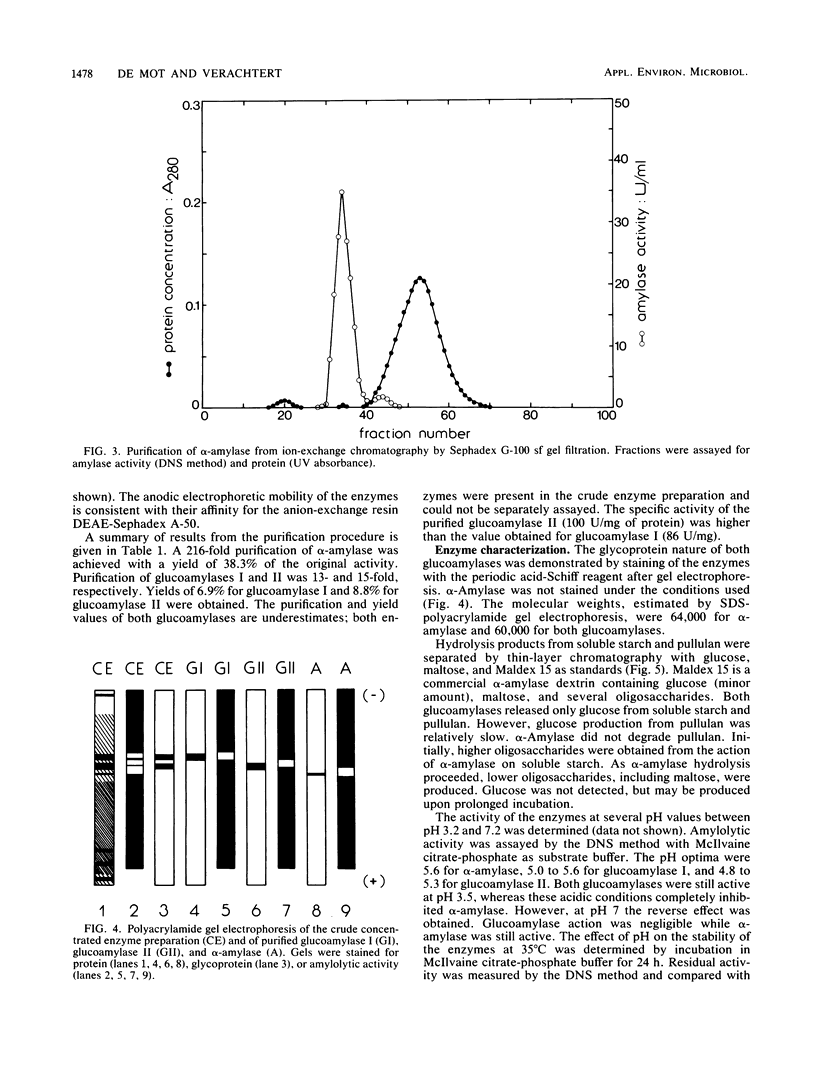
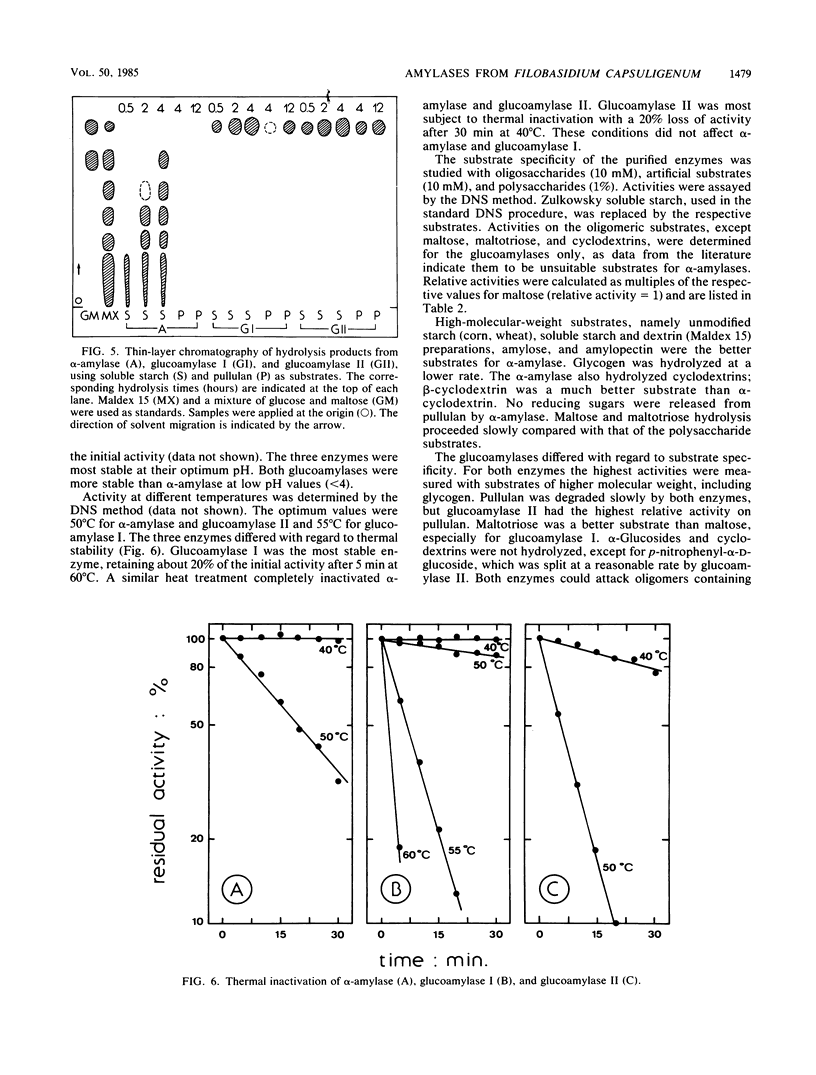
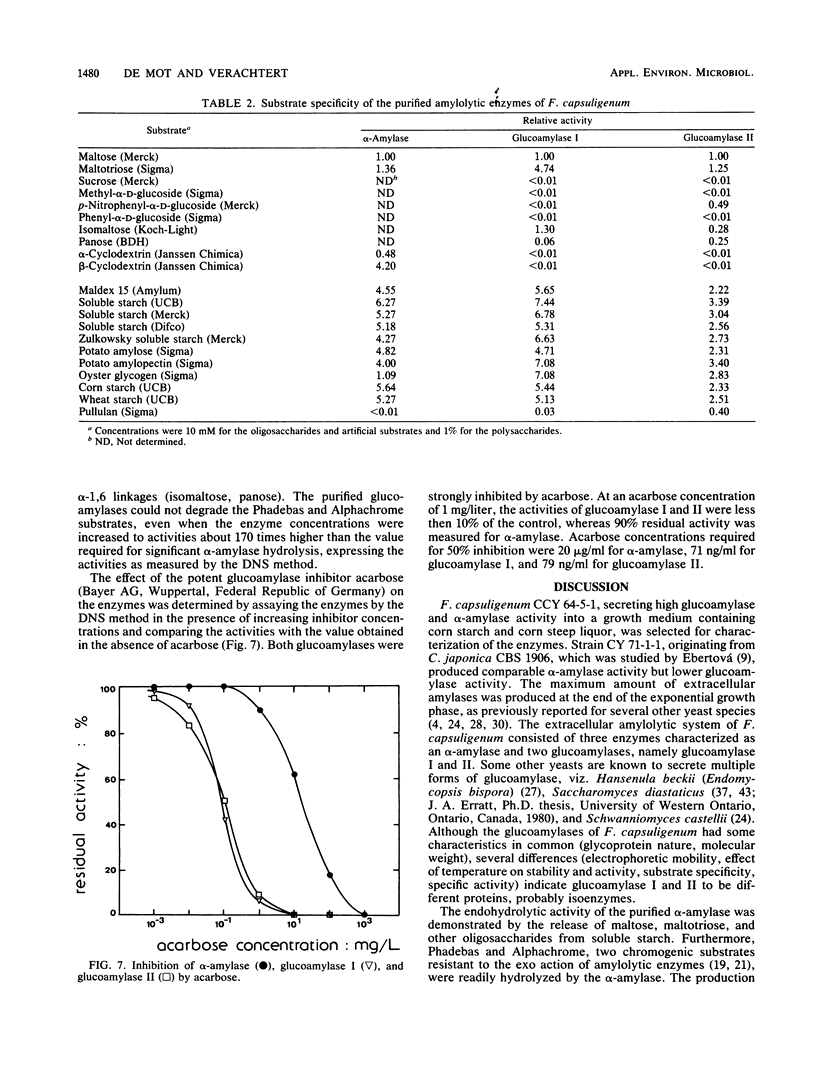
The extracellular amylolytic system of Filobasidium capsuligenum consisted of an α-amylase (1,4-α-d-glucan glucanhydrolase, EC 3.2.1.1) and two forms of glucoamylase (1,4-α-d-glucan glucohydrolase, EC 3.2.1.3). The enzymes were purified by ammonium sulfate fractionation, repeated ion-exchange chromatography (DEAE-Sephadex A-50), and gel filtration (Sephadex G-25, Sephadex G-100 sf). α-Amylase had an optimum pH of 5.6 and an optimum temperature of 50°C but was rapidly inactivated at higher temperature. The molecular weight was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be 64,000. An acarbose concentration of 20 μg/ml was required for 50% inhibition of the α-amylase. Both glucoamylases are glycoproteins of identical molecular weight (60,000) and produce only glucose by exohydrolysis. The debranching activity of the glucoamylases was evidenced with substrates containing α-1,6 linkages. The pH optima were 5.0 to 5.6 for glucoamylase I and 4.8 to 5.3 for glucoamylase II. Glucoamylase I had a higher optimum temperature (55°C) than glucoamylase II (50°C) and was also more resistant to thermal inactivation. Only low acarbose concentrations (<0.1 μg/ml) were required to reduce the activity of the glucoamylases by 50%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustín J., Zemek J., Kocková-Kratochvílová A., Kuniak L. Production of alpha-amylase by yeasts and yeast-like organisms. Folia Microbiol (Praha) 1978;23(5):353–361. doi: 10.1007/BF02876434. [DOI] [PubMed] [Google Scholar]
- Clementi F., Rossi J., Costamagna L., Rosi J. Production of amylase(s) by Schwanniomyces castellii and Endomycopsis fibuligera. Antonie Van Leeuwenhoek. 1980;46(4):399–405. doi: 10.1007/BF00421986. [DOI] [PubMed] [Google Scholar]
- De Mot R., Van Oudendijck E., Verachtert H. Purification and characterization of an extracellular glucoamylase from the yeast Candida tsukubaensis CBS 6389. Antonie Van Leeuwenhoek. 1985;51(3):275–287. doi: 10.1007/BF02439937. [DOI] [PubMed] [Google Scholar]
- EBERTOVA H. STUDY OF THE FORMATION AND PROPERTIES OF THE AMYLOLYTIC SYSTEM OF CANDIDA JAPONICA. Folia Microbiol (Praha) 1963;40:333–341. doi: 10.1007/BF02906030. [DOI] [PubMed] [Google Scholar]
- Fell J. W., Statzell A. C., Hunter I. L., Phaff H. J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie Van Leeuwenhoek. 1969;35(4):433–462. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laluce C., Mattoon J. R. Development of Rapidly Fermenting Strains of Saccharomyces diastaticus for Direct Conversion of Starch and Dextrins to Ethanol. Appl Environ Microbiol. 1984 Jul;48(1):17–25. doi: 10.1128/aem.48.1.17-25.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath P., Shenoy B. C., Raghavendra Rao M. R. Fungal glucoamylases. J Appl Biochem. 1983 Aug-Oct;5(4-5):235–260. [PubMed] [Google Scholar]
- McCleary B. V. New chromogenic substrates for the assay of alpha-amylase and (1 leads to 4)-beta-D-glucanase. Carbohydr Res. 1980 Nov 1;86(1):97–104. doi: 10.1016/s0008-6215(00)84584-x. [DOI] [PubMed] [Google Scholar]
- Moseley M. H., Keay L. Purification and characterization of the amylase of B. subtilis NRRL B3411. Biotechnol Bioeng. 1970 Mar;12(2):251–271. doi: 10.1002/bit.260120207. [DOI] [PubMed] [Google Scholar]
- Onishi M. Studies of the interaction of substrate analogues with bacterial liquefying alpha-amylase by means of spectrophotometry and steady state kinetics. J Biochem. 1971 Jan;69(1):181–189. doi: 10.1093/oxfordjournals.jbchem.a129446. [DOI] [PubMed] [Google Scholar]
- Oteng-Gyang K., Moulin G., Galzy P. A study of amylolytic system of Schwanniomyces castelii. Z Allg Mikrobiol. 1981;21(7):537–544. doi: 10.1002/jobm.3630210707. [DOI] [PubMed] [Google Scholar]
- Pringle J. R., Mor J. R. Methods for monitoring the growth of yeast cultures and for dealing with the clumping problem. Methods Cell Biol. 1975;11:131–168. doi: 10.1016/s0091-679x(08)60320-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues de Miranda L. Filobasidum capsuligenum nov. comb. Antonie Van Leeuwenhoek. 1972;38(1):91–99. doi: 10.1007/BF02328080. [DOI] [PubMed] [Google Scholar]
- Ruttloff H., Friese R., Kupke G., Täufel A. Differenzierung und Charakterisierung von Glucoamylase-Isoenzymen aus Endomycopsis bispora. Z Allg Mikrobiol. 1969;9(1):39–47. doi: 10.1002/jobm.3630090106. [DOI] [PubMed] [Google Scholar]
- Ruttloff H., Täufel A., Friese R., Zickler F. Aur Ausscheidung von Glucoamylase-Isoenzymen durch einen Stamm der Gattung Endomycopsis. Z Allg Mikrobiol. 1970;10(5):335–340. [PubMed] [Google Scholar]
- Spencer-Martins I. Extracellular Isoamylase Produced by the Yeast Lipomyces kononenkoae. Appl Environ Microbiol. 1982 Dec;44(6):1253–1257. doi: 10.1128/aem.44.6.1253-1257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Koyama S., Takeo K., Kuge T. Kinetic studies on the hydrolyses of alpha-, beta-, and gamma-cyclodextrins by Taka-amylase A. J Biochem. 1974 Jul;76(1):57–63. doi: 10.1093/oxfordjournals.jbchem.a130559. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson J. J., Ingledew W. M. Isolation and characterization of Schwanniomyces alluvius amylolytic enzymes. Appl Environ Microbiol. 1982 Aug;44(2):301–307. doi: 10.1128/aem.44.2.301-307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



