Abstract
The n-type K+ channel (n-K+, Kv1.3) in lymphocytes has been recently implicated in the regulation of Fas-induced programmed cell death. Here, we demonstrate that ceramide, a lipid metabolite synthesized upon Fas receptor ligation, inhibits n-K+ channel activity and induces a tyrosine phosphorylation of the Kv1.3 protein in Jurkat T lymphocytes. Tyrosine phosphorylation of the n-K+ channel correlated with an activation of the Src-like tyrosine kinase p56lck upon cellular treatment with the ceramide analog C6-ceramide. Because genetic deficiency of p56lck or inhibition of Src-like tyrosine kinases by herbimycin A prevented ceramide-mediated n-K+ channel inhibition and tyrosine phosphorylation, we propose a ceramide-initiated activation of p56lck resulting in tyrosine phosphorylation and inhibition of the n-K+ channel protein.
The cellular homeostasis of the immune system is regulated by the balance between cell proliferation and programmed cell death. Programmed cell death or apoptosis has been shown to be a fundamental, evolutionary conserved process involved in development and regulation of the immune response (1–4). Apoptosis of several cell types can be induced by a variety of physiological or pathophysiological stimuli, including TNF, Fas/ApoI/CD95, ceramide, reactive oxygen intermediates, ionizing radiation, heat shock, or treatment with some cytostatic drugs (5–16).
The Fas/Apo-I/CD95 receptor has been shown to be a highly important receptor in the regulation of apoptosis in mature lymphocytes (4). The major function of the Fas receptor seems to be the regulation of the peripheral immune response (4). Thus, mutations in the Fas receptor or its ligand result in the defects of lpr and gld mice characterized by lymphadenopathy, lymphoaccumulation, and autoimmune organ failure (17–19).
Recent studies suggest that the synthesis of ceramide has an important function for Fas-triggered programmed cell death (20, 21). Ceramides are known stimuli of apoptosis and are synthesized by activation of an acidic and/or neutral sphingomyelinase (20–22). Both enzymes have been shown to be activated by the Fas receptor (20–22). The regulatory mechanisms of sphingomyelinase activation by the Fas receptor are completely unknown; however, a recent report suggested that the synthesis of ceramides depends on the function of ICE-like proteases in cells transfected with the reaper protein (23). Ceramides have been shown to stimulate a variety of enzymes, including a ceramide-activated proline-directed protein kinase (24), a phosphatase (25), Jun N-terminal kinase (12, 26), Raf-K (27), and tyrosine phosphorylation (28).
We and others have previously suggested that protein tyrosine kinase activation is an essential event in Fas-induced apoptosis because inhibition of protein tyrosine kinases (29, 51) and expression of the tyrosine phosphatase FAP (30) prevent Fas-induced cell death. The Src-kinase p59fyn has been shown to associate with the Fas receptor; however, the function of this association is unknown (31, 32). Evidence for a crucial function of Src-like tyrosine kinases for Fas-induced apoptosis is also provided from knock-out mice of Fyn and Lyn showing a deficiency of programmed cell death in peripheral B and T lymphocytes (31, 33).
Other molecules activated by the Fas receptor include the small G protein p21Ras (21), phospholipase A2 (22), a serine/threonine kinase (34), Jun N-terminal kinases (35), and several members of the ICE-like protease family (36–39). We have previously demonstrated that Fas receptor ligation also results in a tyrosine kinase-dependent inhibition of the n-type K+ (n-K+) channel (40). The interaction of these molecules with ceramide has to be determined.
In the present study, we demonstrate an inhibition of the n-K+ channel (Kv1.3) in Jurkat T lymphocytes upon treatment of the cells with synthetic C6- or C2-ceramide. The inhibitory effect of ceramide correlated with tyrosine phosphorylation of the n-K+ channel and an activation of the Src-like tyrosine kinases p56lck and was absent in p56lck genetically deficient or in herbimycin A-pretreated Jurkat cells. The results point to a signaling cascade from ceramides via tyrosine kinases to the n-K+ channel.
MATERIALS AND METHODS
Cell Culture and Stimulation.
Jurkat and p56lck-deficient JCaM1.6 cells were obtained from American Type Culture Collection (Bethesda). All cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mM Hepes (pH 7.4), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin (all purchased from GIBCO/BRL), and 50 μM 2-mercaptoethanol. p56lck-reconstituted JCaM1.6 cells were maintained in 250 μg/ml hygromycin.
For activation, cells (2 × 106 or 20 × 106 per sample for cell lysates or immunoprecipitations, respectively) were washed twice in sterile Hepes/saline (132 mM NaCl/20 mM Hepes/5 mM KCl/1 mM CaCl2/0.7 mM MgCl2/0.8 mM MgSO4) and stimulated at 37°C with 10 μM synthetic C6- or C2-ceramide, inactive stereoisomer dihydro-C2-ceramide, sphingosine (Biomol, Hamburg, Germany), or the solvent dimethyl sulfoxide for the indicated times. Src-like tyrosine kinases were inhibited by 8-h incubation with 10 μM herbimycin A.
Immunoprecipitation and Immunoblotting.
Cell stimulation was terminated by lysis in 25 mM Hepes, pH 7.4/0.1% SDS/0.5% sodium deoxycholate/1% Triton X-100/125 mM NaCl/10 mM each NaF, Na3VO4, and sodium pyrophosphate/10 μg/ml each aprotinin and leupeptin (lysis buffer) for total cell lysates and for immunoprecipitation of p56lck or the n-K+ channel protein. Agarose-coupled anti-p56lck-antibodies were purchased from Upstate Biotechnology (Lake Placid, NY), and the antiserum to the n-K+ channel protein has been described (40). For control immunoprecipitates, normal rabbit immunoglobulins were used as indicated. After lysis, DNA and cell debris were pelleted by centrifugation at 20,000 × g for 15 min, and the supernatants were added to 5× SDS sample buffer and 5% 2-mercaptoethanol (total cell lysates) or subjected to immunoprecipitation overnight at 4°C. After addition of protein A/G-coupled agarose, incubation was continued for at least 60 min. Immunocomplexes were washed six times in lysis buffer, applied to kinase assays, or resuspended in SDS sample buffer (60 mM Tris, pH 6.8/2.3% SDS/10% glycerol/5% 2-mercaptoethanol). Proteins were separated by SDS/PAGE, followed by electrophoretic transfer to Immobilon filters (Millipore). Phosphotyrosine blots were performed using the monoclonal 4G10 antibody (Upstate Biotechnology). Blots were incubated overnight at 4°C with 4G10 (diluted to 1 μg/ml in Tris-buffered saline, supplemented with 0.1% Tween 20). Immunoblots were developed by incubation with horseradish peroxidase-conjugated protein G (Bio-Rad) and use of a chemoluminescence kit (Amersham). The blots were stripped for 45-min incubation in 20 mM Tris, pH 6.8/2% SDS/70 mM 2-mercaptoethanol at 70°C after primary analysis and reprobed with the immunoprecipitating antibody to test for equal amounts of immunoprecipitated protein.
p56lck Assay.
The activity of Src kinases was determined by autophosphorylation. The kinase p56lck was immunoprecipitated by using agarose-coupled anti-p56lck (Upstate Biotechnology). Immunoprecipitates were incubated overnight at 4°C as described above and washed four times in lysis buffer and twice in kinase buffer (25 mM Hepes, pH 7.0/150 mM NaCl/10 mM MnCl2/1 mM Na3VO4/5 mM DTT/0.5% Nonidet P-40). Samples were resuspended in 40 μl of kinase buffer. The reaction was initiated by addition of 10 μCi of [γ-32P]ATP (DuPont/Nen; 3,000 Ci/mmol; 1 Ci = 37 GBq) and ATP (10 μM) in kinase buffer. The samples were incubated at 30°C for 20 min, the reaction was stopped with 8 μl of reducing 5× SDS sample buffer, and SDS/PAGE was performed, followed by autoradiography. An aliquot of the immunoprecipitates was analyzed by Western blotting for the amount of p56lck in the immunoprecipitates.
Electrophysiology.
Whole-cell patch-clamp experiments (41) were performed at 30°C in a 0.4-ml perfusion chamber. The bath solution contained 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 10 mM Hepes (pH 7.4 with NaOH), and patch electrodes of 3- to 7-MΩ resistance were filled with 134 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM EGTA (10 nM free Ca2+ concentration), 10 mM Hepes, and 1 mM ATP (pH 7.4 with KOH).
An EPC-9 (HEKA Electronics, Lambrecht/Pfalz, Germany) amplifier was used for voltage clamping and current amplification. Records were low-pass filtered at a corner frequency of 3 kHz and transferred to an Atari computer by using an E9Screen data acquisition system at a sampling rate of 10 kHz and an ITC-16 interface (Instrutech, Mineola, NY). Whole-cell data were analyzed with review software (Instrutech). Cations leaving the cell correspond to outward current and are reported as upward deflections. Membrane potentials are reported as intracellular with respect to ground.
RESULTS
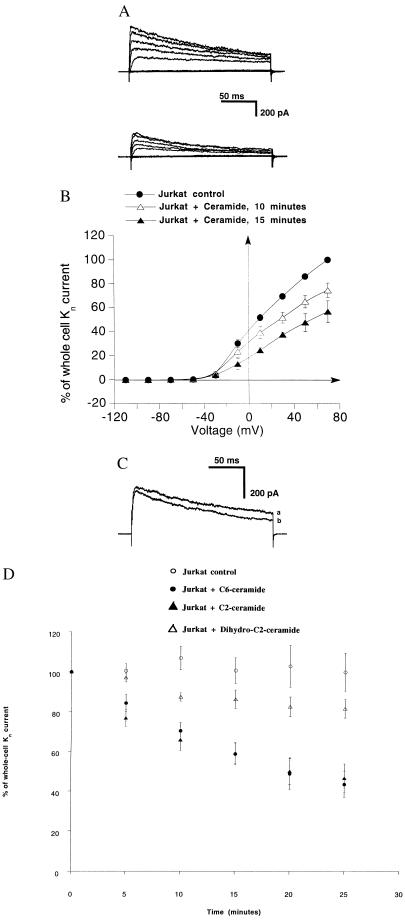
Previous studies from our group (40) showed a tyrosine kinase-dependent inhibition of the n-K+ channel upon Fas receptor ligation. Ceramide has been shown to be important in the mediation of Fas-induced programmed cell death (20, 21). To analyze the function of ceramides for n-K+ channel inactivation by the Fas receptor, we stimulated Jurkat human T lymphocytes with a synthetic ceramide (C6-ceramide) and performed whole-cell patch-clamp experiments. Fig. 1A Upper shows that depolarizing voltages elicited whole-cell outward currents exhibiting kinetics typical for n-K+ channels in lymphocytes. The current was selective for K+ and sensitive to nanomolar concentrations of the well known inhibitors charybdotoxin and margatoxin (data not shown; refs. 42 and 43). Fig. 1A Lower displays outward currents from the same experiment after incubation of Jurkat cells with 10 μM C6-ceramide. Stimulation of Jurkat cells with C6-ceramide resulted in a 41 ± 5% (n = 9) decrease of the current in 15 min and in a 67 ± 6% (n = 4) decrease 25 min after incubation of C6-ceramide. Ceramide was added to the patches between 6 and 15 min after formation of the seal. Washing the cells with control solution after stimulation with C6-ceramide did not result in recovery of channel activity (n = 3), indicating an irreversible inhibition within the experimental period. Fig. 1B shows that the whole-cell current significantly decreased for all voltages of ≥10 mV after 15 min of C6-ceramide incubation (n = 6). No spontaneous rundown of the current could be observed in 34 control experiments. Application of the lipid solvent dimethyl sulfoxide (0.1%) did not affect the channel activity.
Figure 1.
C6-ceramide (10 μM) specifically inhibits voltage-gated n-K+ channels of Jurkat lymphocytes. (A) Shown are outward whole-cell currents elicited by 200-ms voltage pulses (from −110 to 70 mV, in 20 mV steps) delivered at 30-s intervals from a Vhold of −70 mV. (Upper) Currents in control condition. (Lower) Currents 17 min after incubation with 10 μM C6-ceramide. (B) Current–voltage relationship of whole-cell currents in control conditions (n = 6), 10 min (n = 6) and 15 min (n = 3) after incubation with 10 μM C6-ceramide, respectively. Similar to the results in A, the current was significantly decreased upon cellular incubation with C6-ceramide. Currents are expressed as the percent of the peak currents at +70 mV under control conditions before addition of C6-ceramide. All data are expressed as arithmetic means ± SE. The differences in currents between control and incubated cells are significant at −10 mV after 15 min of incubation (two-tailed t test for independent samples was higher than 2.9; P <0.02; degree of freedom = 7). (C) In contrast to C6-ceramide, the treatment of Jurkat cells with dihydro-C2-ceramide did not result in a significant change of the current. Shown are outward currents elicited by 200-ms depolarizing pulses to +40 mV from Vhold at −70 mV in 100-s intervals in the control condition (current a) and 20 min after incubation of Jurkat cells with 10 μM dihydro-C2-ceramide (current b). (D) C6- and C2-ceramides, but not dihydro-C2-ceramide, induce a rapid and sustained inhibition of n-K+ channel currents. Peak currents at +40 mV are expressed as the percent of the respective currents at zero time (corresponds to the time of the addition of the substance). The pulse protocol described for C was applied in all experiments. All data are expressed as arithmetic means ± SE. ○, Currents in control conditions (n = 34, 30, 25, 22, and 18); • and ▴, currents after incubation with 10 μM C6-ceramide (n = 11, 10, 9, 5, and 4) or C2-ceramide (n = 5, 5, 5, 4, and 4), respectively, for the indicated times. Differences between these groups are already significant 5 min after C2- or C6-ceramide addition (two-tailed t tests were higher than 3.7; P < 0.0034). ▴, Currents in Jurkat cells after incubation with 10 μM dihydro-C2-ceramide (n = 12, 11, 12, 9, and 7). Differences between currents after incubation with C2 or C6-ceramide and dihydro-C2-ceramide, respectively, are already significant 5 min after starting the stimulation (t test were higher than 2.7; P < 0.013), whereas differences between control condition and dihydro-C2-ceramide-treated cells are not significant during the whole experimental period (P < 0.085).
Similarly to the effect of Fas stimulation (40), C6-ceramide also caused a decrease of the open probability of the n-K+ channel without affecting the unitary current in the outside-out excised patch configuration (n = 2).
To assess the specificity of the effect of C6-ceramide, experiments were performed by incubating the cells with 10 μM C2-ceramide, a second, active ceramide analog, and 10 μM dihydro-C2-ceramide, an inactive stereoisomer of C2- and C6-ceramides. dihydro-C2-ceramide induced a slight (not significant in respect to control cells) 14 ± 5% or 19 ± 5% reduction of whole-cell currents 15 or 25 min, respectively, after addition of the drug. Fig. 1C shows representative current traces before and after 20 min of incubation with dihydro-C2-ceramide from the same experiment. The whole-cell currents as a function of the time after C2- or C6-ceramide and dihydro-C2-ceramide incubation as well as under control conditions are reported in Fig. 1D. In summary, these results show a specific inhibition of the n-K+ channel by C6- or C2-ceramide. Furthermore, 10 μM sphingosine, which has similarity to ceramide and structurally inhibits K+ channels in smooth muscle cells (44), did not have a significant inhibitory effect on n-K+ channel currents (21 ± 12%; n = 2; data not shown).
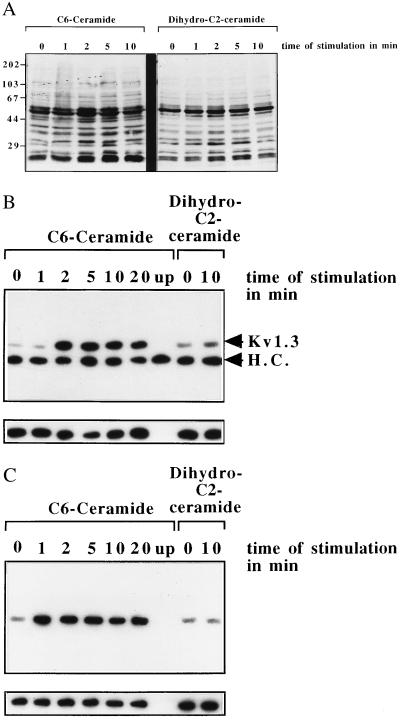
Because tyrosine phosphorylation of the n-K+ channel seems to inhibit the current of the channel (40), we tested whether C6- or C2-ceramide induces cellular tyrosine phosphorylation and phosphorylation of the n-K+ channel protein. To this end, phosphotyrosine blots of whole-cell lysates were performed (Fig. 2A). The data show a tyrosine phosphorylation of at least 12 proteins upon cellular treatment with C6-ceramide, whereas the inactive stereoisomer dihydro-C2-ceramide did not induce cellular tyrosine phosphorylation (Fig. 2A). Tyrosine phosphorylation of the n-K+ channel by C6-ceramide was directly tested by immunoprecipitation of the n-K+ channel protein. The results (Fig. 2B) show an approximately 10-fold increase of tyrosine phosphorylation of the n-K+ channel protein upon treatment of the cells with C6-ceramide, whereas no increase of tyrosine phosphorylation of the n-K+ channel protein could be detected after addition of the inactive stereoisomer dihydro-C2-ceramide (Fig. 2B). A similar tyrosine phosphorylation of the n-K+ channel was observed upon treatment of Jurkat cells with 10 μM C2-ceramide (data not shown). The increased tyrosine phosphorylation in whole-cell lysates and of the n-K+ channel protein correlated with a stimulation of the Src-like tyrosine kinase p56lck upon stimulation of the cells with C6-ceramide (Fig. 2C), whereas incubation with the inactive stereoisomer dihydro-C2-ceramide did not result in a significant stimulation of p56lck (Fig. 2C).
Figure 2.
(A) Stimulation of Jurkat with C6-ceramide results in an increase of cellular tyrosine phosphorylation. Cellular treatment with dihydro-C2-ceramide did not trigger cellular tyrosine phosphorylation. Jurkat cells were stimulated with 10 μg/ml C6-ceramide for the indicated time, and the lysates were centrifuged and resuspended in 5× SDS sample buffer. Samples were separated by 10% SDS/PAGE and blotted, and the blots were analyzed for tyrosine phosphorylation by using monoclonal anti-phosphotyrosine-antibodies 4G10. (B) C6-ceramide induces tyrosine phosphorylation of the n-K+ channel protein. The Kv1.3 protein was immunoprecipitated from C6-ceramide stimulated or unstimulated cells and the immunoprecipitates were analyzed for tyrosine phosphorylation as described above. The band at approximately 50 kDa is due to a reaction of the heavy chain (H.C.) of the immunoprecipitating antibody with the protein G–horseradish peroxidase complex used for enhanced chemiluminescence development. Stimulation of the cells with dihydro-C2-ceramide does not result in a significant increase of n-K+ channel tyrosine phosphorylation showing the specificity of the stimulation. The blots were stripped after primary analysis and reprobed with the immunoprecipitating antibody, i.e., anti-Kv1.3, to show similar amounts of protein in all lanes (small blots). (C) Stimulation of Jurkat cells with 10 μM C6-ceramide induces an activation of the Src-like tyrosine kinase p56lck, which is not activated by the inactive stereoisomer dihydro-C2-ceramide. Lck was immunoprecipitated from stimulated or unstimulated cells by using anti-p56lck coupled to agarose, the immunoprecipitates were incubated with 10 μCi per sample of [γ-32P]ATP, and the autophosphorylation was determined by blotting and autoradiography. Reprobing the blots with anti-p56lck shows similar amounts of immunoprecipitated kinase in all lanes.
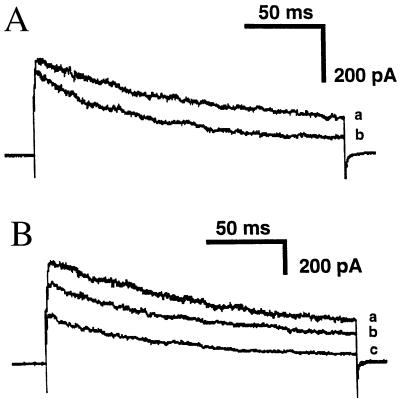
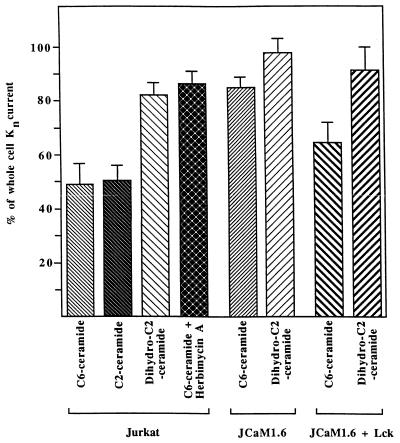
Because inhibition of n-K+ channels by Fas stimulation was prevented in p56lck-deficient or herbimycin A-treated Jurkat cells (JCaM1.6 cells; refs. 40 and 45), we tested the effect of C6-ceramide on the function and the phosphorylation status of the n-K+ channel in these cells. Fig. 3A illustrates n-K+-current traces from an experiment on JCaM1.6 cells before (current a) and after (current b) 20 min of incubation with 10 μM C6-ceramide and shows that the inhibition of the currents in these cells is significantly less than in normal Jurkat lymphocytes (see also Fig. 4). Reconstitution of the non-receptor membrane-bound tyrosine kinase p56lck into JCaM1.6 cells partially restored the ability of C6-ceramide to inhibit n-K+ channels as shown in the original traces of Fig. 3B (see also Fig. 1D). Fig. 4 illustrates the percentage of whole-cell K+ currents in Jurkat, JCaM1.6, p56lck-reconstituted JCam1.6 (JCaM1.6/Lck+), and herbimycin A-treated cells after 20-min incubation with 10 μM C6-ceramide or 10 μM dihydro-C2-ceramide. Differences are statistically significant (P values are ≤0.01 in two-tailed Student’s t test) between the effect of C6-ceramide and dihydro-C2-ceramide on Jurkat lymphocytes and between the effect of C6-ceramide on Jurkat and JCaM1.6 cells or herbimycin A-treated Jurkat cells. The extent of n-K+ channel inhibition by C6-ceramide was significantly different (P values ≤ 0.05) between JCaM1.6 and JCaM1.6/Lck+ cells, whereas no significant difference of n-K+ channel inactivation in Jurkat and JCaM1.6/Lck+ cells could be measured. The data also show that C6-ceramide and C2-ceramide induce a very similar inhibition of n-K+ channels.
Figure 3.
Deficiency of p56lck in JCaM1.6 cells prevents inhibition of n-K+ current by C6-ceramide. Retransfection of p56lck reconstitutes the inhibitory effect of synthetic ceramides. (A) Outward currents elicited by 200-ms depolarizing pulses to +40 mV delivered from Vhold −70 mV at 100-s intervals in control conditions (current a) and after 20-min incubation with 10 μM C6-ceramide (current b) in JCaM1.6 cells. (B) Representative traces of n-K+ currents elicited by the pulse protocol described in A under control conditions (current a) and after 15-min (current b) or 25-min (current c) incubation with 10 μM C6-ceramide in JCaM1.6/Lck+ cells.
Figure 4.
Genetic or pharmacological inhibition of p56lck prevents inactivation of the n-K+ channel by synthetic ceramides. The figure shows alterations of peak currents in percent of control peak currents (at +40 mV) 20 min after addition of 10 μM C6- or C2-ceramide or 10 μM dihydro-C2-ceramide in Jurkat cells, in p56lck-deficient JCaM1.6 cells, in p56lck-reconstituted JCaM1.6 cells, or in herbimycin A-pretreated Jurkat cells. Differences are significant (two tailed t tests) between the groups Jurkat + C6/C2-ceramide and Jurkat + dihydro-C2-ceramide and between Jurkat + C6-ceramide and JCaM1.6 + C6-ceramide or Jurkat + herbimycin A + C6-ceramide, respectively. The differences are also significant between JCaM1.6 + C6-ceramide and JCaM1.6/Lck+ + C6-ceramide. No significant difference could be detected among the groups Jurkat + C6-ceramide, Jurkat + C2-ceramide, and JCaM1.6/Lck+ + C6-ceramide.
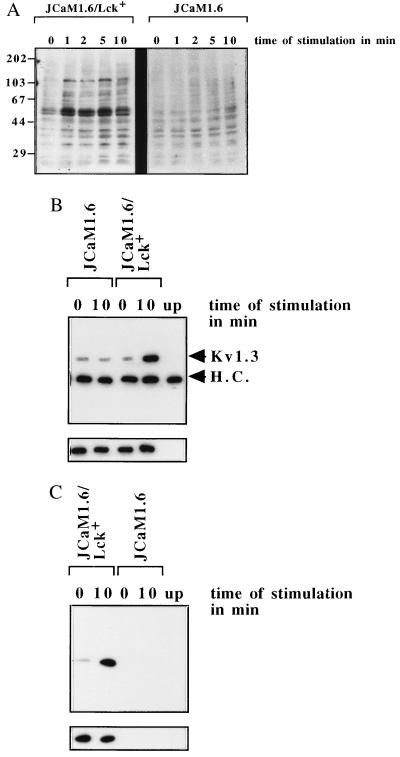
The experiments using p56lck-deficient JCaM1.6 and herbimycin A-treated Jurkat cells point to an important function of Src-like tyrosine kinases in the mediation of the inhibitory effect of C6-ceramide on the n-K+ channel. The lack of inhibition of the n-K+ channel by C6-ceramide in JCaM1.6 cells correlated with a failure to significantly increase tyrosine phosphorylation in whole-cell lysates (Fig. 5A) and the tyrosine phosphorylation of the n-K+ channel protein itself (Fig. 5B). A similar inhibition of C6-ceramide-induced n-K+ channel tyrosine phosphorylation was observed after pretreatment with herbimycin A (data not shown). In accordance with the data obtained in the patch-clamp experiments, retransfection of p56lck into JCaM1.6 cells restored the tyrosine phosphorylation of cellular proteins (Fig. 5A), including the n-K+ channel protein (Fig. 5B). Cellular tyrosine phosphorylation in JCaM1.6/Lck+ correlated with a restored activation of p56lck in the retransfected cells (Fig. 5C), indicating normal expression and function of the reconstituted kinase.
Figure 5.
(A) Genetic deficiency of p56lck prevents cellular tyrosine phosphorylation upon treatment with 10 μM C6-ceramide, whereas retransfection of p56lck restores the tyrosine phosphorylation of cellular proteins by C6-ceramide. JCaM1.6 or JCaM1.6/Lck+ cells were stimulated with C6-ceramide and lysed, and the whole-cell lysates were analyzed for tyrosine phosphorylation as above. (B) Tyrosine phosphorylation of the n-K+ channel by C6-ceramide depends on the functional expression of p56lck in JCaM1.6/Lck+ cells and is absent in p56lck-deficient JCaM1.6 cells. JCaM1.6/Lck+ or p56lck-deficient JCaM1.6 cells were stimulated with 10 μM C6-ceramide for the indicated time, and the n-K+ channel protein was immunoprecipitated and tested for tyrosine phosphorylation by Western blotting. The strips show similar amounts of Kv1.3 protein in all lanes (small blots). (C) The tyrosine kinase p56lck is stimulated by C6-ceramide in JCaM1.6/Lck+ cells, whereas no signal could be detected in the p56lck genetically deficient JCaM1.6 cells. Kinase activity was determined by autophosphorylation as above.
DISCUSSION
The present study identifies a new target for ceramide, i.e., the n-K+ channel. Cellular stimulation with ceramides induces the activation of the Src-like tyrosine kinase p56lck, resulting in a tyrosine phosphorylation of the n-K+ channel protein. This tyrosine phosphorylation seems to inhibit the current of the n-K+ channel.
A similar inhibition of the n-K+ channel function was observed after stimulation of Jurkat T lymphocytes by the Fas receptor (40). Fas-mediated inhibition of the n-K+ channel was also prevented by genetic deficiency of p56lck or after inhibition of Src-like tyrosine kinases using the pharmacological inhibitor herbimycin A. Because Fas receptor ligation has been shown to induce the synthesis of ceramide through an activation of acidic and/or neutral sphingomyelinases (20–22), our experiments using synthetic ceramides, which mimic endogenous ceramides, point to a signaling cascade from the Fas receptor through the activation of sphingomyelinases and the synthesis of ceramide to the stimulation of Src-like tyrosine kinases and the inactivation of the n-K+ channel. The effects of synthetic ceramides on the n-K+ channel are almost identical to the effect of Fas receptor ligation pointing to the crucial role of endogenous ceramides for the regulation of n-K+ channels by the Fas receptor. The specificity of the observed inhibitory effects is indicated by the finding that the structurally similar molecules dihydro-C2-ceramide or sphingosine did not affect n-K+ channel open probability or tyrosine phosphorylation. Furthermore, dihydro-C2-ceramide or sphingosine did not significantly inhibit n-K+ channels in JCaM1.6 or herbimycin A-treated cells, excluding a direct, structural inhibition of n-K+ channels by ceramide.
The molecular mechanism of the regulation of n-K+ channel function by ceramides seems to involve Src-like tyrosine kinases, which have been recently shown to be activated by ceramides (28). An important function of Src-like tyrosine kinases for the inhibition of the n-K+ channel by ceramides is indicated by the experiments showing tyrosine phosphorylation of the n-K+ channel protein upon cellular treatment with ceramides and the almost complete abrogation of the inhibitory effect of ceramides in the genetically p56lck-deficient JCaM1.6 cells or after treatment with herbimycin A. The reconstitution experiments with p56lck-retransfected JCaM1.6 cells show that the activation of the kinase by ceramide and the inhibition of the channel by p56lck are due to the defect of kinase expression. This model permits an exact correlation between the activation of p56lck by ceramides and the inhibition of n-K+ channel currents, whereas pharmacological inhibition of tyrosine kinases is always hampered by (unknown) side effects of the inhibitor. The kinase p56lck is the major tyrosine protein kinase expressed in Jurkat and JCaM1.6 cells, whereas p59fyn is expressed at much lower levels than p56lck (45). An activation of p59fyn may explain the residual inhibition of the n-K+ channel by C6-ceramide in JCaM1.6 cells; however, the activity and/or expression level may not be high enough to induce a detectable increase of tyrosine phosphorylation of the n-K+ channel in the short experimental time of 20 min. The molecular mechanism of the activation of Src-like tyrosine kinases by ceramides has to be elucidated; however, a direct activation of p56lck by ceramides seems to be unlikely because p56lck does not contain a known lipid binding motif. It is also unknown whether p56lck directly tyrosine phosphorylates the n-K+ channel protein or whether other kinases, in particular p70zap, which has been shown to function downstream of Src-like tyrosine kinases, mediate the phosphorylation of the n-K+ channel. The molecular mechanism of tyrosine phosphorylation-mediated inhibition of n-K+ channels is unknown. Phosphorylation of a very conserved tyrosine in the H5 domain of voltage-gated K+ channels, including Kv1.3, may inhibit the current of n-K+ channels upon Fas receptor ligation or ceramide treatment (46–48). Our notion of n-K+ channel regulation by tyrosine phosphorylation is supported by recent findings of Holmes et al. (49) showing that tyrosine phosphorylation of the n-K+ channel, in particular at the tyrosine residue 449, suppresses the current.
This study used a concentration of 10 μM C6- or C2-ceramide, which results in an intracellular concentration of 10–100 pmol/nmol lipid (50). These intracellular concentrations are physiologically relevant because they are obtained upon Fas receptor ligation or serum deprivation (50).
Several studies suggested an important function of tyrosine phosphorylation and activation of Src-kinases in Fas-induced apoptosis (29–33). In particular, a reduction of Fas-triggered apoptosis in p59fyn- or p58lyn-deficient mouse lymphocytes could be recently demonstrated (32, 33). Further, incubation of lymphocytes with the tyrosine kinase inhibitors herbimycin A or tyrphostin B66 almost completely prevented Fas-mediated programmed cell death, pointing to an essential function of Src-like tyrosine kinases and tyrosine phosphorylation for Fas-induced apoptosis (48). Atkinson et al. showed an association of the Src-kinase p59fyn with the Fas receptor (31). However, p56lck-deficient JCaM1.6 cells show a slower but almost normal rate of apoptosis after stimulation by the Fas receptor (51). JCaM1.6 cells express predominantly p56lck; however, they also express p59fyn (45). Thus, p59fyn may replace at least partially p56lck, and the remaining signal via p59fyn may be strong enough to signal apoptosis after long-term incubation with anti-Fas receptor antibodies. However, p59fyn may not be able to fully compensate for p56lck deficiency during the short experimental period used for the patch-clamp experiments and analysis of phosphorylation, a finding that explains the failure of JCaM1.6 cells to respond with inactivation and tyrosine phosphorylation of the n-K+ channel in the first few minutes after Fas receptor ligation or ceramide treatment. This notion is supported by the inhibitor studies blocking all Src-like tyrosine kinases, which showed a clear inhibition of Fas-induced programmed cell death (29, 51).
The voltage-gated n-K+ channels have been shown to be responsible for the maintenance of the resting membrane potential in lymphocytes because their inhibition by the specific inhibitors margatoxin and noxiustoxin leads to marked depolarization (52). Changes in membrane potential seem to be an early and important event involved in activation of lymphocytes (53, 54). Because the margatoxin-induced depolarization of Jurkat cells correlates with a block of proliferation (55), an inhibition of lymphokine synthesis (55), and a slight increase of Fas-induced DNA fragmentation (40), it might be possible that the regulation of the membrane potential has a crucial function for Fas-triggered programmed cell death. However, the exact molecular role of n-K+ channels for Fas-induced apoptosis has to be determined; in particular it has to be determined whether n-K+ channel inhibition is directly involved in apoptosis or is secondary to the generalized process of cell death.
The data of the present study provide evidence for a signaling cascade from the Fas receptor through sphingomyelinases and ceramides to the stimulation of Src-like tyrosine kinases and the inactivation of the n-K+ channel protein. They provide evidence for a complete new function of ceramides, i.e., the (indirect) regulation of ion channels during programmed cell death.
Acknowledgments
We thank Dr. A. Weiss for valuable reagents and A. Beyhl for expert technical help. The study was supported by Deutsche Forschungsgemeinschaft Grants Gu 335/2-1 and La 315/6-1 to E.G. and F.L., Association International Cancer Research Grant 96-18, Scheel Grant 10-0983, and Sandoz Grant 95-1-005 (to E.G). I.S. is a recipient of an EMBO long-term fellowship.
ABBREVIATION
- n-K+ channel
n-type K+ channel
References
- 1.Wyllie A H, Kerr J F R, Currie A R. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 2.Barres B A, Burne J F, Coles H S, Ishizaka Y, Jacobson M D. Philos Trans R Soc London B. 1994;345:265–268. doi: 10.1098/rstb.1994.0104. [DOI] [PubMed] [Google Scholar]
- 3.Raff M C. Nature (London) 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 4.Singer G G, Abbas A K. Immunity. 1994;1:365–372. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 5.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, van Huffel C. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 8.Oehm A, Behrmann I, Weih F, Pawlita M, Maier G, Klas C, Li W M, Richards S, Dhein J, Trauth B C, Ponstingl H, Krammer P H. J Biol Chem. 1992;267:10709–10715. [PubMed] [Google Scholar]
- 9.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Nature (London) 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson M A, Pollock S S, Coleman C N, Calderwood S K. Cancer Res. 1994;54:12–15. [PubMed] [Google Scholar]
- 11.Hockenberry D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 12.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 13.Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 14.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 15.Collins M. Am J Respir Crit Care Med. 1995;152:S20–S24. doi: 10.1164/ajrccm/152.4_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- 16.Cowling G J, Dexter T M. Philos Trans R Soc London B. 1994;345:257–263. doi: 10.1098/rstb.1994.0103. [DOI] [PubMed] [Google Scholar]
- 17.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 20.Cifone M G, DeMaria R, Roncaioli P, Rippo M R, Azuma M, Lanier L L, Santoni A, Testi R. J Exp Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulbins E, Bissonette R, Mahboubi A, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, Kolesnick R, Altman A, Green D. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 22.Cifone M G, Roncaioli P, de Maria R, Camarda G, Santoni A, Ruberti G, Testi R. EMBO J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pronk G J, Ramer K, Amiri P, Williams L T. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 24.Dobrowsky R T, Kamibayashi C, Mumby M C, Hannun Y A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 25.Joseph C K, Byun H S, Bittman R, Kolesnick R N. J Biol Chem. 1993;268:20002–20006. [PubMed] [Google Scholar]
- 26.Westwick J K, Bielawska A E, Dbaibo G, Hannun Y A, Brenner D A. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 27.Huwiler A, Brunner J, Hummel R, Vervoordelonk M, Stabel S, van den Bosch H, Pfeilschifter J. Proc Natl Acad Sci USA. 1996;93:6959–6993. doi: 10.1073/pnas.93.14.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji L, Zhang G, Hirabayashi Y. Biochem Biophys Res Commun. 1995;215:489–496. doi: 10.1006/bbrc.1995.2491. [DOI] [PubMed] [Google Scholar]
- 29.Eischen C M, Dick C J, Leibson P J. J Immunol. 1994;153:1947–1953. [PubMed] [Google Scholar]
- 30.Sato T, Irie S, Kitada S, Reed J C. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson E A, Ostergaard H, Kane K, Pinkowski M J, Caputo A, Olszowi M W, Bleakley R C. J Biol Chem. 1996;271:5968–5971. doi: 10.1074/jbc.271.11.5968. [DOI] [PubMed] [Google Scholar]
- 32.Hane M, Lowin B, Peitsch M, Becker K, Tschopp J. FEBS Lett. 1995;373:265–268. doi: 10.1016/0014-5793(95)01051-f. [DOI] [PubMed] [Google Scholar]
- 33.Wag J, Koizumi T, Watanabe T. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. J Exp Med. 1995;182:865–874. doi: 10.1084/jem.182.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latinis K M, Koretzky G A. Blood. 1996;87:871–875. [PubMed] [Google Scholar]
- 36.Los M, van de Craen M, Penning L C, Schenk H, Westendorp M, Baeuerle P A, Dröge W, Krammer P H, Fiers W, Schultze-Osthoff K. Nature (London) 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 37.Enari M, Hug H, Nagata S. Nature (London) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 38.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 39.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 40.Szabo I, Gulbins E, Apfel H, Zhang X, Barth P, Busch A E, Schlottmann K, Pongs O, Lang F. J Biol Chem. 1996;271:20465–20469. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- 41.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 42.Sands S B, Lewis R S, Cahalan M D. J Gen Physiol. 1989;93:1061–1074. doi: 10.1085/jgp.93.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Calvo M, Leonard R J, Novick J, Stevens S P, Schmalhofer W, Kaczorowski G J, Garcia M L. J Biol Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- 44.Petrou S, Ordway R W, Hamilton J A, Walsh J V, Singer J J. J Gen Physiol. 1994;103:471–486. doi: 10.1085/jgp.103.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straus D B, Weiss A. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 46.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 47.Huang X Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 48.Pongs O. Physiol Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- 49.Holmes T C, Fadool D A, Levitan I B. J Neurosci. 1995;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hannun Y A. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 51.Schlottmann K, Gulbins E, Lau S M, Coggeshall K M. J Leukocyte Biol. 1996;60:546–554. doi: 10.1002/jlb.60.4.546. [DOI] [PubMed] [Google Scholar]
- 52.Leonard R J, Garcia M L, Slaughter R S, Reuben J P. Proc Natl Acad Sci USA. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsien R Y, Pozzan T, Rink T J. Nature (London) 1982;295:68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- 54.Grinstein S, Dixon S J. Physiol Rev. 1989;69:417–581. doi: 10.1152/physrev.1989.69.2.417. [DOI] [PubMed] [Google Scholar]
- 55.Lin C S, Boltz R C, Blake J T, Nguyen M, Talento A, Fischer P A, Springer M S, Sigal N H, Slaughter R S, Garcia M L, Kaczorowski J G, Koo G C. J Exp Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]