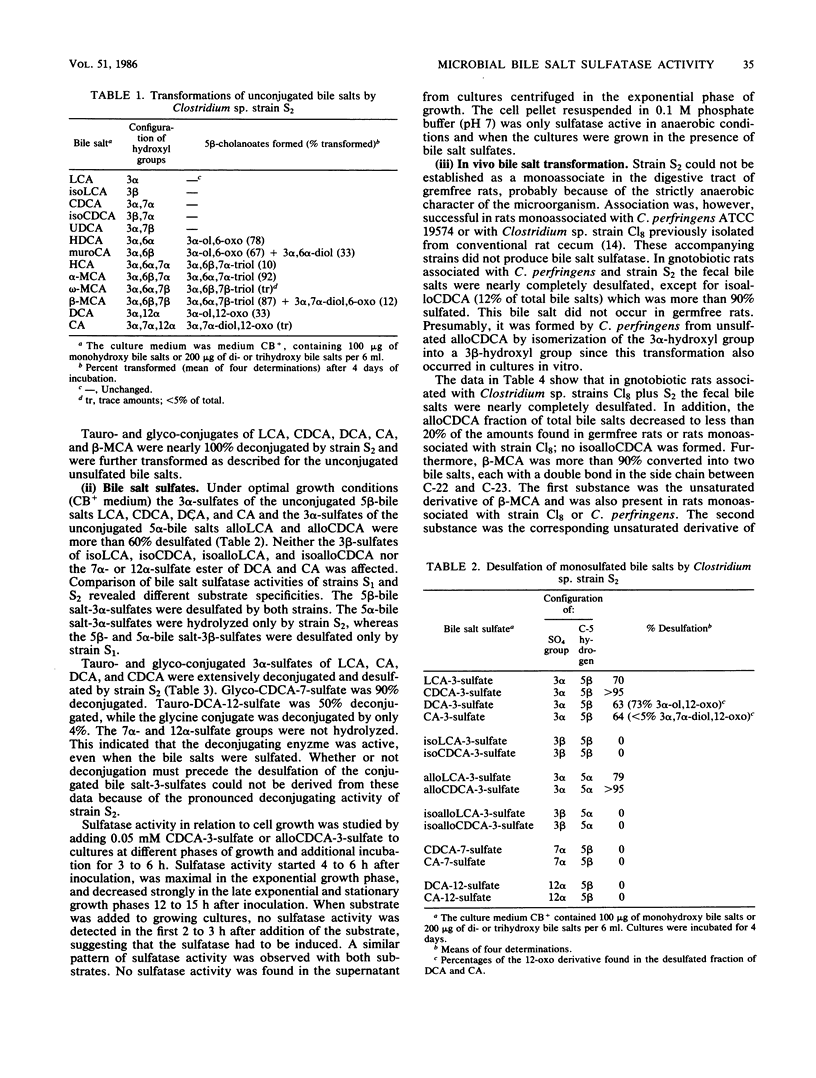
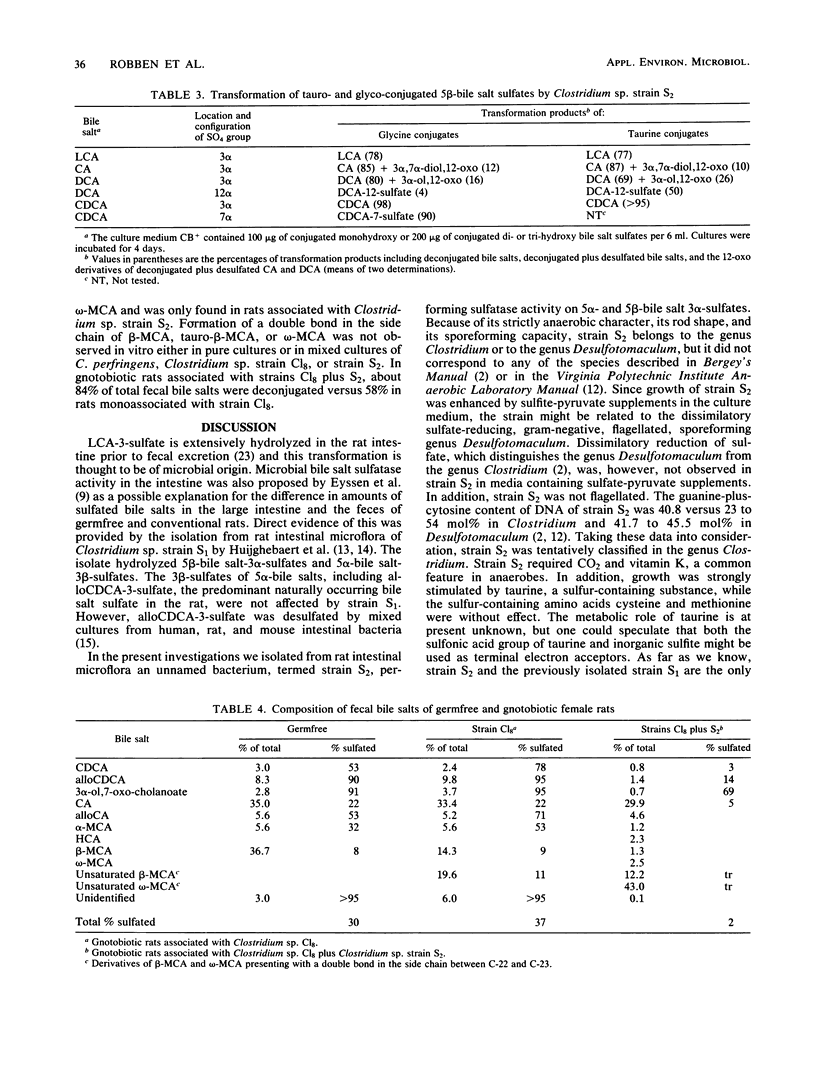
Abstract
An unnamed sporeforming microorganism, termed Clostridium sp. strain S2, possessing bile salt sulfatase activity was isolated from rat intestinal microflora. The microorganism was a strictly anaerobic, nonmotile, gram-negative, asaccharolytic, sporeforming rod requiring CO2, vitamin K, and taurine; the guanine-plus-cytosine content of the DNA was 40.8 mol% (Tm), and the strain was tentatively classified as an atypical Clostridium species. Sulfatase activity was specific for 3 alpha-sulfate esters of 5 alpha- and 5 beta-bile salts, leaving the 3 beta-, 7 alpha-, and 12 alpha-sulfates unchanged. Strain S2 also deconjugated tauro- and glyco-conjugated bile salts and partially reduced into the corresponding 6 alpha-hydroxy bile salts. By these reactions, alpha-muricholate and beta-muricholate were more than 80% converted into hyocholate and omega-muricholate, respectively. In addition, strain S2 produced 12 alpha-hydroxysteroid dehydrogenase converting deoxycholate into 3 alpha-hydroxy-12-oxo-5 beta-cholanoate. When strain S2 was associated with gnotobiotic rats, the fecal bile salts were more than 90% desulfated and the fecal excretion of allochenodeoxycholate was five times lower than in control rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. J., Bolt R. J., Admirand W. H. Enzymatic sulfation of bile salts. Partial purification and characterization of an enzyme from rat liver that catalyzes the sulfation of bile salts. Biochim Biophys Acta. 1977 Jan 11;480(1):219–227. doi: 10.1016/0005-2744(77)90335-7. [DOI] [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W., Coffin S. B. Metabolism of lithocholate in healthy man. II. Enterohepatic circulation. Gastroenterology. 1975 Jul;69(1):67–76. [PubMed] [Google Scholar]
- De Witt E. H., Lack L. Effects of sulfation patterns on intestinal transport of bile salt sulfate esters. Am J Physiol. 1980 Jan;238(1):G34–G39. doi: 10.1152/ajpgi.1980.238.1.G34. [DOI] [PubMed] [Google Scholar]
- Eyssen H. J., Parmentier G. G., Mertens J. A. Sulfate bile acids in germ-free and conventional mice. Eur J Biochem. 1976 Jul 15;66(3):507–514. doi: 10.1111/j.1432-1033.1976.tb10576.x. [DOI] [PubMed] [Google Scholar]
- Eyssen H., De Pauw G., Stragier J., Verhulst A. Cooperative formation of omega-muricholic acid by intestinal microorganisms. Appl Environ Microbiol. 1983 Jan;45(1):141–147. doi: 10.1128/aem.45.1.141-147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyssen H., Smets L., Parmentier G., Janssen G. Sex-linked differences in bile acid metabolism of germfree rats. Life Sci. 1977 Sep 1;21(5):707–712. doi: 10.1016/0024-3205(77)90079-0. [DOI] [PubMed] [Google Scholar]
- Eyssen H., Van Eldere J., Parmentier G., Huijghebaert S., Mertens J. Influence of microbial bile salt desulfation upon the fecal excretion of bile salts in gnotobiotic rats. J Steroid Biochem. 1985 Apr;22(4):547–554. doi: 10.1016/0022-4731(85)90176-1. [DOI] [PubMed] [Google Scholar]
- Hiremath S. V., Elliott W. H. Bile acids. LXIV. Synthesis of 5 alpha-cholestane-3 alpha, 7 alpha, 25-triol and esters of new 5 alpha-bile acids. Steroids. 1981 Oct;38(4):465–475. doi: 10.1016/0039-128x(81)90080-5. [DOI] [PubMed] [Google Scholar]
- Huijghebaert S. M., Eyssen H. J. Specificity of bile salt sulfatase activity from Clostridium sp. strains S1. Appl Environ Microbiol. 1982 Nov;44(5):1030–1034. doi: 10.1128/aem.44.5.1030-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijghebaert S. M., Mertens J. A., Eyssen H. J. Isolation of a bile salt sulfatase-producing Clostridium strain from rat intestinal microflora. Appl Environ Microbiol. 1982 Jan;43(1):185–192. doi: 10.1128/aem.43.1.185-192.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijghebaert S., Parmentier G., Eyssen H. Specificity of bile salt sulfatase activity in man, mouse and rat intestinal microflora. J Steroid Biochem. 1984 Apr;20(4A):907–912. doi: 10.1016/0022-4731(84)90404-7. [DOI] [PubMed] [Google Scholar]
- Kallner A. A method of synthesis of allocholanoic acids. Bile acids and steroids 182. Acta Chem Scand. 1967;21(2):322–328. doi: 10.3891/acta.chem.scand.21-0322. [DOI] [PubMed] [Google Scholar]
- Kelsey M. I., Hwang K. K., Huang S. K., Shaikh B. Characterization of microbial metabolites of sulfolithocholic acid by high-performance liquid chromatography. J Steroid Biochem. 1981 Feb;14(2):205–211. doi: 10.1016/0022-4731(81)90175-8. [DOI] [PubMed] [Google Scholar]
- Lack L., Dorrity F. O., Jr, Walker T., Singletary G. D. Synthesis of conjugated bile acids by means of a peptide coupling reagent. J Lipid Res. 1973 May;14(3):367–370. [PubMed] [Google Scholar]
- Macdonald I. A., Bokkenheuser V. D., Winter J., McLernon A. M., Mosbach E. H. Degradation of steroids in the human gut. J Lipid Res. 1983 Jun;24(6):675–700. [PubMed] [Google Scholar]
- Palmer R. H. Bile acid sulfates. II. Formation, metabolism, and excretion of lithocholic acid sulfates in the rat. J Lipid Res. 1971 Nov;12(6):680–687. [PubMed] [Google Scholar]
- Parmentier G. G., Smets L. M., Jannsen G. A., Eyssen H. J. Effects of cholesterol feeding on the bile acids of male and female germ-free rats. Eur J Biochem. 1981 May 15;116(2):365–372. doi: 10.1111/j.1432-1033.1981.tb05344.x. [DOI] [PubMed] [Google Scholar]
- Parmentier G., Eyssen H. Synthesis and characteristics of the specific monosulfates of chenodeoxycholate, deoxycholate and their taurine or glycine conjugates. Steroids. 1977 Nov;30(5):583–590. doi: 10.1016/0039-128x(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Sacquet E. C., Raibaud P. M., Mejean C., Riottot M. J., Leprince C., Leglise P. C. Bacterial formation of omega-muricholic acid in rats. Appl Environ Microbiol. 1979 Jun;37(6):1127–1131. doi: 10.1128/aem.37.6.1127-1131.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D., Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982 Oct 27;125(2):135–144. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y. A convenient synthesis of 3-keto bile acids by selective oxidation of bile acids with silver carbonate-Celite. J Lipid Res. 1978 May;19(4):501–504. [PubMed] [Google Scholar]
- Tserng K. Y., Klein P. D. Formylated bile acids: improved synthesis, properties, and partial deformylation. Steroids. 1977 May;29(5):635–648. doi: 10.1016/0039-128x(77)90015-0. [DOI] [PubMed] [Google Scholar]
- Wensinck F., Ruseler-van Embden J. G. The intestinal flora of colonization-resistant mice. J Hyg (Lond) 1971 Sep;69(3):413–421. doi: 10.1017/s0022172400021665. [DOI] [PMC free article] [PubMed] [Google Scholar]


