Abstract
The ability to ferment the trisaccharide raffinose was linked with the presence of plasmid DNA in three strains of Pediococcus pentosaceus. Parental strains showed associated inducible α-galactosidase and sucrose hydrolase activities when grown in α-galactosides and sucrose, respectively. Derivative strains of PPE1.0, PPE2.0, and PPE5.0, which had lost 30-, 28-, and 23-megadalton plasmids, respectively, had no α-galactosidase or sucrose hydrolase activity.
Full text
PDF
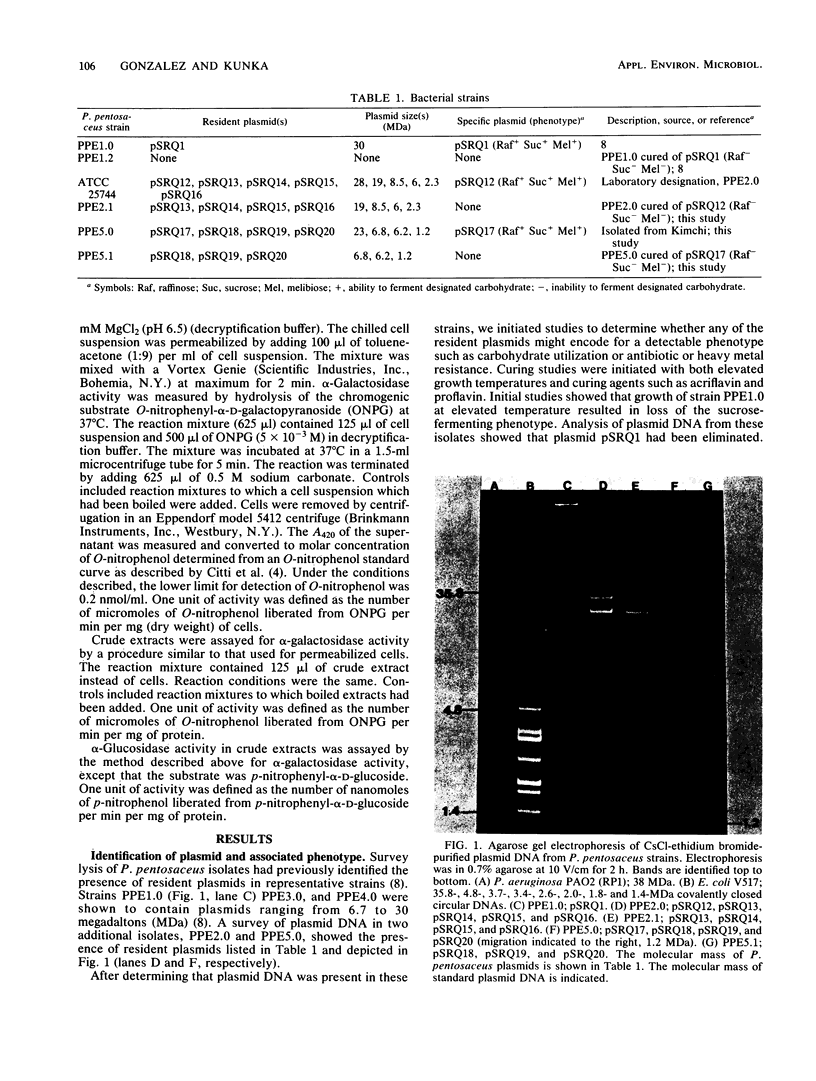

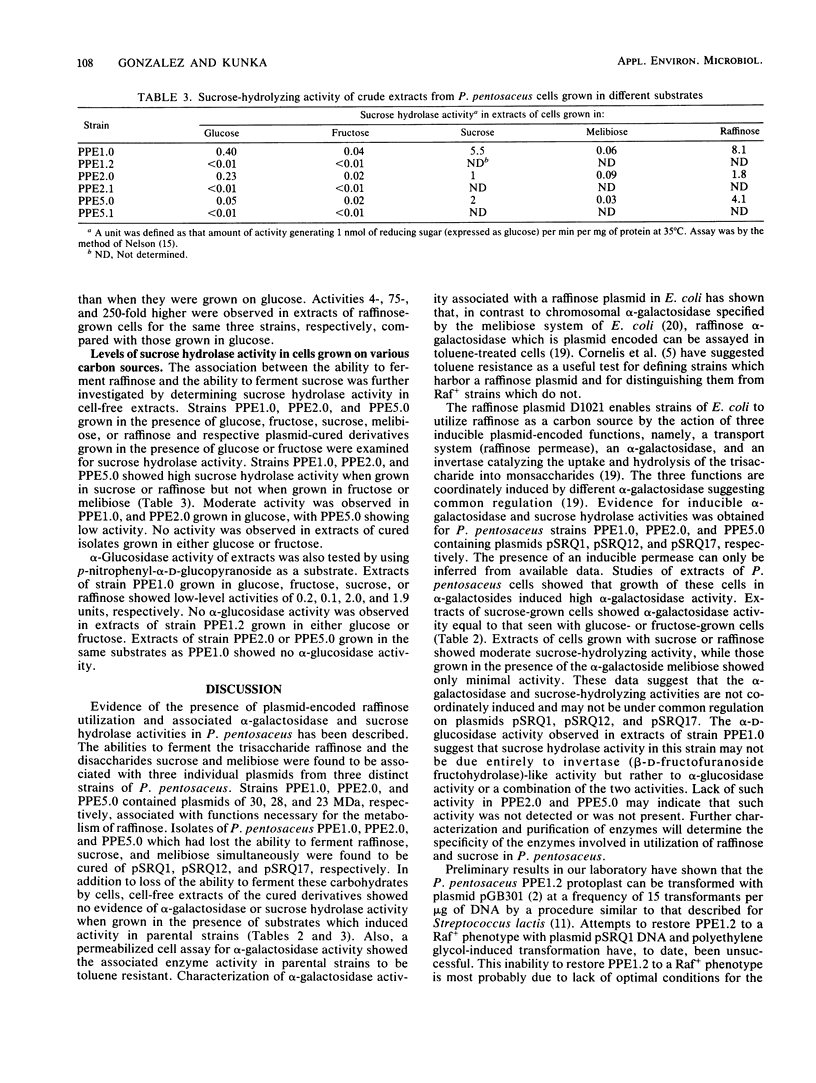

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke D., Gilmore M. S., Ferretti J. J. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol Gen Genet. 1981;182(3):414–421. doi: 10.1007/BF00293929. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Bieth G., Horaud T. Broad host range of streptococcal macrolide resistance plasmids. Antimicrob Agents Chemother. 1984 Feb;25(2):289–291. doi: 10.1128/aac.25.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Luke R. K., Richmond M. H. Fermentation of raffinose by lactose-fermenting strains of Yersinia enterocolitica and by sucrose-fermenting strains of Escherichia coli. J Clin Microbiol. 1978 Feb;7(2):180–183. doi: 10.1128/jcm.7.2.180-183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Plasmids in Streptococcus lactis: evidence that lactose metabolism and proteinase activity are plasmid linked. Appl Environ Microbiol. 1976 Jul;32(1):38–44. doi: 10.1128/aem.32.1.38-44.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Plasmid transfer in Pediococcus spp.: intergeneric and intrageneric transfer of pIP501. Appl Environ Microbiol. 1983 Jul;46(1):81–89. doi: 10.1128/aem.46.1.81-89.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- Kondo J. K., McKay L. L. Plasmid transformation of Streptococcus lactis protoplasts: optimization and use in molecular cloning. Appl Environ Microbiol. 1984 Aug;48(2):252–259. doi: 10.1128/aem.48.2.252-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl Environ Microbiol. 1984 Jan;47(1):68–74. doi: 10.1128/aem.47.1.68-74.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt J. O., Beattie W. G., Wieland F. R. Pediococci residing on plants. J Bacteriol. 1969 Jun;98(3):938–942. doi: 10.1128/jb.98.3.938-942.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve H., Geis A., Teuber M. Conjugal transfer and characterization of bacteriocin plasmids in group N (lactic acid) streptococci. J Bacteriol. 1984 Mar;157(3):833–838. doi: 10.1128/jb.157.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Plasmid-determined H2S character in Escherichia coli and its relation to plasmid-carried raffinose fermentation and tetracycline resistance characters. Examination of 32 H2S-positive strains isolated during the years 1950 to 1971. J Gen Microbiol. 1973 Aug;77(2):487–499. doi: 10.1099/00221287-77-2-487. [DOI] [PubMed] [Google Scholar]
- PEDERSON C. S. The genus Pediococcus. Bacteriol Rev. 1949 Dec;13(4):225–232. doi: 10.1128/br.13.4.225-232.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Schmitt R. Raffinose metabolism in Escherichia coli K12. Purification and properties of a new alpha-galactosidase specified by a transmissible plasmid. Eur J Biochem. 1976 Aug 1;67(1):95–104. doi: 10.1111/j.1432-1033.1976.tb10637.x. [DOI] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R. J., McKay L. L. Conjugal Transfer of Lactose-Fermenting Ability Among Streptococcus cremoris and Streptococcus lactis Strains. Appl Environ Microbiol. 1981 Nov;42(5):904–911. doi: 10.1128/aem.42.5.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]