Abstract
Objective
Erythropoietin is essential for red blood cell development in vivo and is also an important therapeutic agent to treat anemia resulting from kidney failure or bone marrow suppression. The erythropoietin receptor (EPOR) elicits both positive and negative regulatory signaling pathways, primarily through phosphorylated tyrosine residues in the cytoplasmic domain of the activated receptor complex. Surprisingly, however, EPOR tyrosine residues are dispensable for in vivo erythropoiesis under nonstress conditions. One of the key signaling molecules elicited by the EPOR is the Stat5 transcription factor. Stat5 activation has been mapped to tyrosines 343 and 401 in the EPOR cytoplasmic region, although non-tyrosine-containing sequences in the EPOR cytoplasmic region can also stimulate Stat5. To test the functional role of non-tyrosine-containing sequences in the EPOR, we analyzed a series of mutant EPOR isoforms in cell survival and proliferation assays.
Methods
The IL-3-dependent 32D cell line was stably transfected with cDNAs encoding the wild-type EPOR or mutant EPORs containing or lacking intracellular tyrosines, in the absence or presence of a dominant inhibitory Stat5 isoform. EPO-dependent cell signaling, survival, and proliferation were evaluated.
Results
EPOR isoforms lacking intracellular tyrosine residues elicit an important survival signal in 32D cells. Stat5 function is critical for EPO-dependent cell survival mediated by these non-tyrosine-containing receptor sequences. Interestingly, EPO-dependent survival does not require the presence of fetal calf serum (FCS) in the culture medium, yet FCS is important for 32D cell proliferation in response to EPO.
Conclusion
Our results elucidate a previously unrecognized survival pathway elicited by the EPOR. They demonstrate that this pathway requires Stat5 and is serum independent. These findings contribute significantly to our understanding of the complexity by which the EPOR functions in hematopoietic cells.
Erythropoietin (EPO) is critical for red blood cell production in vivo and is used widely in the clinic to treat anemia due to bone marrow suppression or kidney failure [1,2]. In the absence of EPO signaling during development, erythroid progenitors are generated in the fetal liver, yet they are unable to complete the terminal stages of maturation, causinglethal embryonic anemia [2,3]. Mutations in the erythropoietin receptor (EPOR) that render EPO hypersensitivity or constitutive receptor activation can lead to benign erythrocytosis or erythroleukemia, respectively [4–6]. EPOR-expressing progenitor cells are also a target for leukemic transformation by the Friend virus complex, which renders constitutive EPOR activation and EPO-independent proliferation of infected cells [7–9]. Thus, while EPOR signaling is essential for erythropoiesis, its aberrant activation can contribute to the development of hematological diseases.
The EPOR activates a number of intracellular signaling proteins that have stimulatory effects on hematopoietic growth and survival. These include the Jak2 protein tyrosine kinase, Stat 1, 3, 5a and 5b transcription factors, Lyn kinase, Erk1/2 kinases, and the phosphotidyl inositol 3-kinase/Akt pathway (PI3K/Akt) [10–16]. EPOR also activates proteins that are involved in signal termination such as the phosphatase SHP-1 and the SOCS-related protein Cis [17,18]. Stimulation of these downstream proteins is critically dependent on a ligand-induced conformational change in the EPOR/Jak2 complex, which is thought to induce Jak2 activation through a cross-phosphorylation mechanism [19–22]. Tyrosine residues on EPOR and Jak2 are phosphorylated by the activated Jak2 kinase and serve as docking sites for signaling and adaptor molecules containing phosphotyrosine-binding motifs, such as Stat5 [23,24]. The essential role of Jak2 in EPOR signaling was demonstrated by gene deletion, which results in severe embryonic anemia and lethality [25].
Previous studies have collectively indicated that a level of functional overlap exists among signaling molecules downstream of the EPOR/Jak2 complex. For example, targeted deletion of the Stat5a and Stat5b genes impairs but does not abrogate definitive erythropoiesis, indicating that both Stat5-dependent and -independent mechanisms contribute to red cell formation [24,26–28]. Studies that evaluated the role of individual EPOR tyrosines in vivo support the idea of functional redundancy among EPOR signaling pathways [29]. Surprisingly, EPOR tyrosines are dispensable for red cell formation under nonstress conditions, although mice carrying the tyrosine-null EPOR demonstrate slightly reduced hematocrits [30]. Cross-talk between EPOR signaling pathways has also been demonstrated and may contribute to receptor function in vivo [11]. Understanding the role of specific EPOR signaling pathways is important for understanding EPO function in normal erythropoiesis, as well as its therapeutic activity.
Mutant isoforms of the EPOR lacking the intracellular tyrosine residues that serve as the principal Stat5 docking sites retain the ability to stimulate Stat5, although these receptor isoforms are less efficient in activating Stat5 than the wild-type EPOR [29,31]. Whether the activation of Stat5 by non-tyrosine-containing sequences of the EPOR plays a functional role in EPO signaling has been unclear to date. To test this, we evaluated the function of full-length and mutant EPORs containing or lacking intracellular tyrosine residues. Our studies demonstrate that the primary role of the EPOR is to support hematopoietic cell survival. Functional survival signals are elicited through the membrane-proximal region of the EPOR cytoplasmic tail and their activation does not depend on receptor tyrosine residues. Furthermore, we show that the major survival pathway stimulated by this region of the EPOR requires Stat5 activity. These results are the first demonstration that a functional Stat5 signal is stimulated through tyrosine-independent sequences of the EPOR.
Methods
Plasmids and cDNAs
Constructs containing wild-type murine EPOR and EPOR mutants W282R, 1-321, and YF have been described previously [22,29]. To generate a dominant inhibitory isoform of Stat5b, a premature termination codon was introduced into the murine Stat5b coding sequence following Tyr683, following methods described previously [32]. Briefly, the polymerase chain reaction (PCR) method was used to amplify a DNA fragment from the Stat5b cDNA with the following primer pair: 5′-TGACCAAGGAGAACCTCGTGT-3′and 5′-TAGTTATGCGGCCGCTAGTAGTACTTAGAATATACTT-3′. This product was digested with XhoI and NotI and used to replace the XhoI-NotI fragment in the murine STAT5b cDNA. The resulting construct, Stat5b-DN, was confirmed by sequence analysis (data not shown).
Cell culture conditions and generation of cell lines
The 32D cell line is an IL-3-dependent myeloid progenitor line that lacks expression of functional EPOR [33,34] (Fig. 1). 32D cells and clonal cell lines stably transfected with EPOR isoforms were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) and 5% conditioned medium from the WEHI 3B cell line (IL-3 source) (RPMI/FCS/WEHI). Transfections with wild-type (WT) or mutant EPOR isoforms were performed as described [35]. Clonal cell lines were obtained by limiting dilution and screened for EPOR expression by immunoblot analysis with EPOR-specific antiserum [36]. Cell surface EPOR expression was analyzed by equilibrium binding assays with I-EPO, as previously described[19]. Clonal cell lines expressing Stat5b-DN were obtained in a similar manner, using cotransfection of Stat5b-DN and pBABEpuro plasmids followed by selection in 1 μg/mL puromycin. Cell lines were tested for Stat5b-DN expression by immunoblot analysis, using an antibody specific for residues 451-649 of murine Stat5 (Transduction Labs, San Diego, CA, USA).
Figure 1.
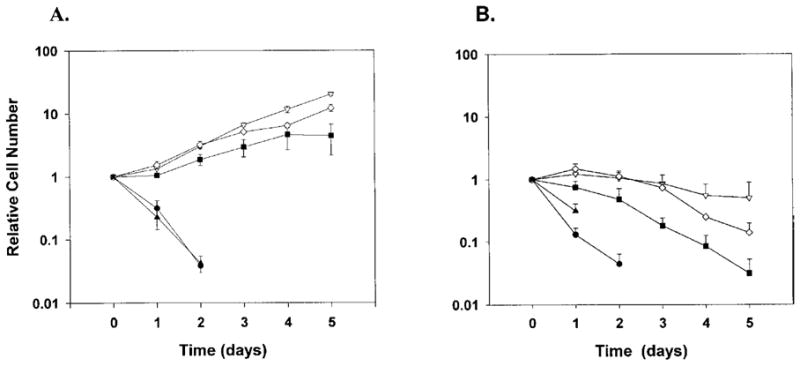
EPO-dependent cell growth in the absence or presence of FCS. Clonal 32D cells lines expressing WT EPOR (open inverted triangle), W282R (closed triangle), YF (open diamond), or 1–321 (closed square), or 32D cells lacking EPOR (closed circle), were maintained in IL-3-containing media. At the start of the assay cells were washed extensively to remove exogenous growth factors and then plated in media containing (A) 0.5 U/mL EPO and FCS or (B) 0.5 U/mL EPO and lacking FCS. Viable cell numbers were determined daily by trypan blue dye exclusion and enumeration. Cell density at individual time points was normalized to the density of the starting population, and the fold increase in cell number was plotted as a function of time in EPO-containing media. Viable cells were not detected in the W282R culture after day 2. Average results from 5 independent experiments are shown.
Cell proliferation and apoptosis assays
Cells were washed three times with RPMI to remove exogenous growth factors, then plated in RPMI containing 0.5 U/mL EPO and lacking FCS (RPMI/EPO), RPMI containing 10% FCS only (RPMI/FCS), and RPMI containing 10% FCS and 0.5 U/mL of EPO (RPMI/FCS/EPO). Control samples were maintained in RPMI/FCS/WEHI for the duration of the experiment. Cells were cultured for up to one week; the cell density was maintained below 10 cells/mL by dilution in fresh medium. Viable cells, as judged by trypan blue dye exclusion, were counted daily and total cell numbers were graphed. Doubling times were determined by linear regression analysis. All cell lines were strictly growth factor–dependent and died after 24 hours in RPMI/FCS (data not shown).
For DNA fragmentation analysis, cells were cultured for 24 hours as described above; 10 cells were then collected, resuspended in 75% ethanol, and stored at −20°C for at least 18 hours. Cells were washed twice with phosphate-buffered saline (PBS), resuspended in PBS containing 50 μg/mL propidium iodide (PI; Sigma, St. Louis, MO, USA) and 25 μg/mL RNase A, and incubated for 15 minutes at ambient temperature. Cells were analyzed by flow cytometry on a Coulter Epics Profile (Miami, FL, USA) machine. Sub-diploid percentages were generated by MultiCycle (Phoenix Flow System, San Diego, CA, USA) software.
For caspase 3 activity assays, cells were cultured for 24 hours as described in the text, then fixed with 2% paraformaldehyde and permeabilized with buffer containing 0.2% Triton X-100. The permeabilized cells were incubated with PE-conjugated active caspase 3 antibody (PharMingen, San Diego, CA, USA) under conditions described by the manufacturer. The cells were analyzed by flow cytometry on a Coulter Epics Profile machine.
EPO stimulation, immunoprecipitations, and immunoblot analysis
EPOR antisera have been described previously [36]. Antiserum specific for Stat5 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Transduction Labs (San Diego, CA, USA). Antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology (Lake Placid, NY, USA). Cells were treated with EPO as indicated in the text, and detergent cell extracts were made as described [31]. Lysates were cleared by incubation with protein A–agarose beads (30 minutes at 4°C) and used for immunoprecipitations as described [31]. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose filters. Immunoblotting assays were performed as described previously [31].
Results
Signaling through non-tyrosine-containing sequences in the EPOR cytoplasmic domain supports 32D cell survival
32D cell lines expressing wild-type or mutant EPORs were evaluated for their ability to grow in the absence or presence of FCS and EPO. A panel of EPOR isoforms was utilized, including the WT receptor, a C-terminal truncated isoform that contains only the membrane-proximal region and lacks the sequences encoding all eight intracellular tyrosine residues (1–321) [22], an isoform containing phenylalanine substitutions at all intracellular tyrosines (YF), and a Jak2 activation-deficient mutant containing a single amino acid substitution in the membrane-proximal region of the cytoplasmic domain (W282R) [37]. Clonal 32D cell lines stably expressing WT or mutant EPORs were derived; clones expressing similar levels of cell surface EPORs were used for further analysis (data not shown). All cell lines proliferated similarly in media containing FCS and IL-3, with a doubling time of 21 hours, indicating that EPOR expression did not influence IL-3 responsiveness as previously determined (data not shown) [35]. EPO-responsive proliferation was analyzed initially in media containing FCS and EPO. A subsaturating concentration of EPO (0.5 U/mL) was used in these experiments, to approximate circulating levels in vivo [38]. Cells expressing WT, 1–321, and YF demonstrated long-term proliferation in these culture conditions, although the mutant isoforms were somewhat less efficient than the full-length receptor (Fig. 1A doubling times = 26, 47, 34 hours, respectively). Cells expressing the functionally inactive isoform W282R or parental 32D cells lacking EPOR expression failed to survive or grow under these conditions, as expected (Fig. 1A) [35,37].
To evaluate EPOR function in the absence of FCS, cells were cultured up to 5 days in media containing only EPO. Viable cell numbers did not change dramatically during the first 2 to 3 days in cultures containing WT, 1–321, or YF cells (Fig. 1B). In contrast, viable cell numbers decreased rapidly in cultures containing parental 32D or W282R cells, with a reduction of approximately 50% at 24 hours and greater than 90% at 48 hours (Fig. 1B). These results suggest that the principal function of EPOR signaling in 32D cells is to support cell viability, while cell growth is dependent on the presence of both EPO and FCS. In addition, the data show that tyrosine-independent sequences in the intracellular region of the EPOR can provide this cell survival signal, although somewhat less effectively than WT EPOR.
WT, 1–321, and YF EPORs elicit anti-apoptotic signals
The capability of EPO to maintain viable cell numbers without stimulating their increase could be due to an enhanced rate of cell death during ongoing proliferation, or an ability to support cell viability but not growth. To test these possibilities, cellular DNA content was measured by propidium iodide staining and flow cytometry following 24 hours growth in media containing EPO and FCS, EPO only, or FCS only, or lacking both FCS and EPO. The 24-hour time point was chosen since it represented a point at which viability was maintained in a significant fraction of both EPO-responsive and EPO-nonresponsive cell lines (Fig. 1). After 24 hours in the absence of any exogenous growth factors (i.e., minus FCS and EPO), the majority of the cells (approximately 75–85%) were found in the sub-diploid population (Fig. 2A). The presence of only FCS in the growth medium was unable to prevent DNA fragmentation to a significant degree (Fig. 2B). DNA fragmentation was largely abrogated, however, in cells expressing functional EPORs when cultured in media containing EPO as the sole exogenous growth factor. EPO-dependent signaling through WT or YF EPORs protected over 90% of the population from undergoing DNA fragmentation, while signaling through 1–321 protected approximately 70% of the cells (Fig. 2C). The presence of FCS enhanced EPO-dependent cell survival as evidenced by a slight decrease in the sub-diploid population of WT, 1–321, and YF cells in cultures containing EPO and FCS, relative to those with EPO only (compare Fig. 2C and D). 32D cells lacking EPOR, or cells expressing the functionally inactive W282R isoform, were not protected from DNA fragmentation in the presence of EPO alone or EPO and FCS, as expected (Fig. 2 and data not shown). Similar results were obtained by analysis of DNA fragmentation levels using agarose gel electrophoresis (data not shown). Since DNA fragmentation is indicative of cellular apoptosis, these findings suggest that the principal function of EPOR signaling in 32D cells is to prevent the onset of programmed cell death. The presence of exogenous FCS modestly enhances 32D cell survival in response to a functional EPOR signal.
Figure 2.
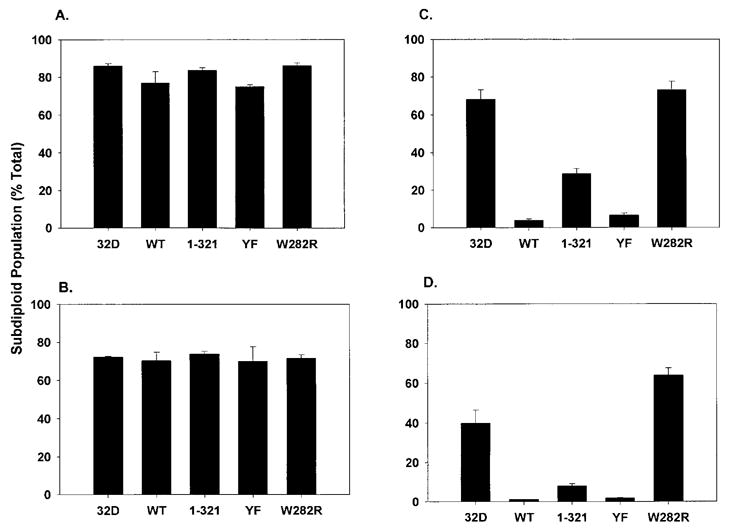
Analysis of DNA fragmentation in 32D cells lines expressing WT or mutant EPORs. Clonal 32D cell lines expressing WT, W282R, YF, or 1–321 EPORs, or 32D cells lacking EPOR, were washed extensively and then cultured in media (A) lacking FCS and exogenous cytokine, (B) containing only FCS, (C) containing only EPO, or (D) containing EPO and FCS for 24 hours. Cells were stained with propidium iodide and the fraction of sub-diploid cells in the population was determined by flow cytometry. Average results from 6 independent experiments are shown.
A hallmark of the apoptotic pathway is activation of the caspase 3 protease. Therefore, as a second method to evaluate apoptosis, we measured activation of caspase 3 in 32D cells grown in media containing FCS and IL-3 or EPO. These studies demonstrated that a significant fraction of 32D cells which lack functional EPOR (i.e., cells expressing W282R or parental 32D cells) contained activated caspase 3 after 24 hours culture in EPO-containing media, indicating they were apoptotic (Fig. 3). In contrast, signaling through WT, 1–321, or YF EPORs efficiently blocked caspase 3 activation (Fig. 3). Caspase 3 activation was also blocked in IL-3-containing media, as expected (Fig. 3). Therefore, signaling through WT EPOR abrogates apoptosis in 32D cells. Furthermore, EPO survival signals are elicited through non-tyrosine-containing sequences in the membrane-proximal region of the receptor.
Figure 3.
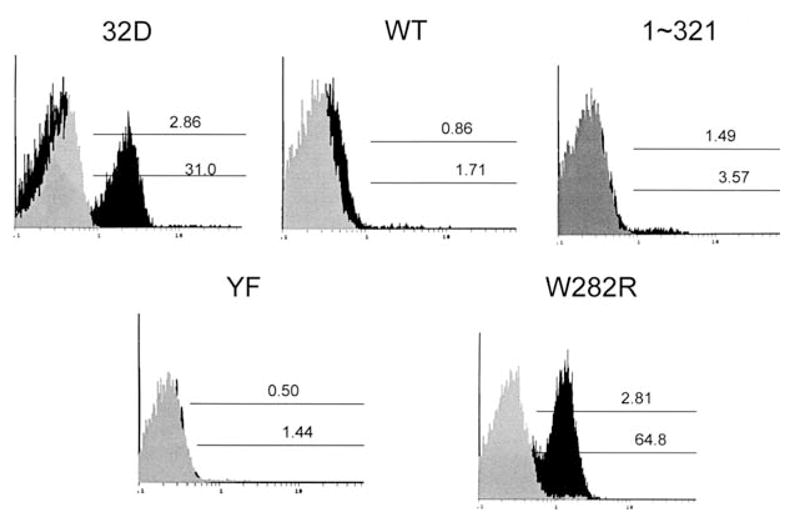
Expression of activated caspase 3 in 32D cell lines expressing WT or mutant EPORs. Clonal 32D cell lines expressing WT, W282R, YF, or 1–321 EPORs, or 32D cells lacking EPOR, were washed extensively and then cultured in media containing IL-3 and FCS (gray histograms) or 0.5 U/mL EPO and FCS (black histograms) for 24 hours. Activated caspase 3 was detected in permeabilized cells by staining with a PE-conjugated antibody specific for active caspase 3 followed by flow cytometry. Results from a representative experiment are shown.
Stat5 activation correlates with EPO survival signaling
The EPOR activates several signaling pathways that function in cell survival, including Stat1, Stat3, Stat5, Erk1/2, and PI3K/Akt. Stat5 tyrosine phosphorylation, which is a hallmark of Stat activation, was observed in 32D cells expressing WT, 1–321, and YF EPORs following EPO stimulation, while tyrosine phosphorylation of Stats 1 and 3 was not detected, even in supra-physiological concentrations of hormone (data not shown). Stat5 DNA binding activity is stimulated by YF and 1–321 EPORs [29] (data not shown), indicating that they mediate a functional Stat5 response. Stimulation of WT, 1–321, and YF EPOR cells with EPO and FCS resulted in Erk1/2 activation. In contrast, in response to EPO stimulation alone, activated Erk1/2 was detected only at very low levels in WT and YF cells and was not detectable in 1–321 cells (data not shown). Akt was activated in WT EPOR cells in response to EPO and FCS, but was not stimulated in YF or 1–321 cells. Thus, our results demonstrated that Stat5 activation was correlated with EPO survival signaling by 1–321 and YF EPORs in 32D cells.
Stat5b-DN abrogates EPO-dependent Stat5 activation
To test Stat5 function in EPO survival signaling, we generated 32D cell lines stably expressing a dominant inhibitory form of Stat5b that lacks the carboxy terminal tyrosine residue and transactivation domain (Stat5b-DN). These mutations are predicted to block Stat5 dimerizing ability and transcriptional transactivation functions, and thereby render an isoform that inhibits Stat5 function through competitive binding to the activated receptor complex [32]. Clonal cell lines were derived and cells expressing equivalent levels of Stat5b-DN were identified and used in further studies (Fig. 4A).
Figure 4.
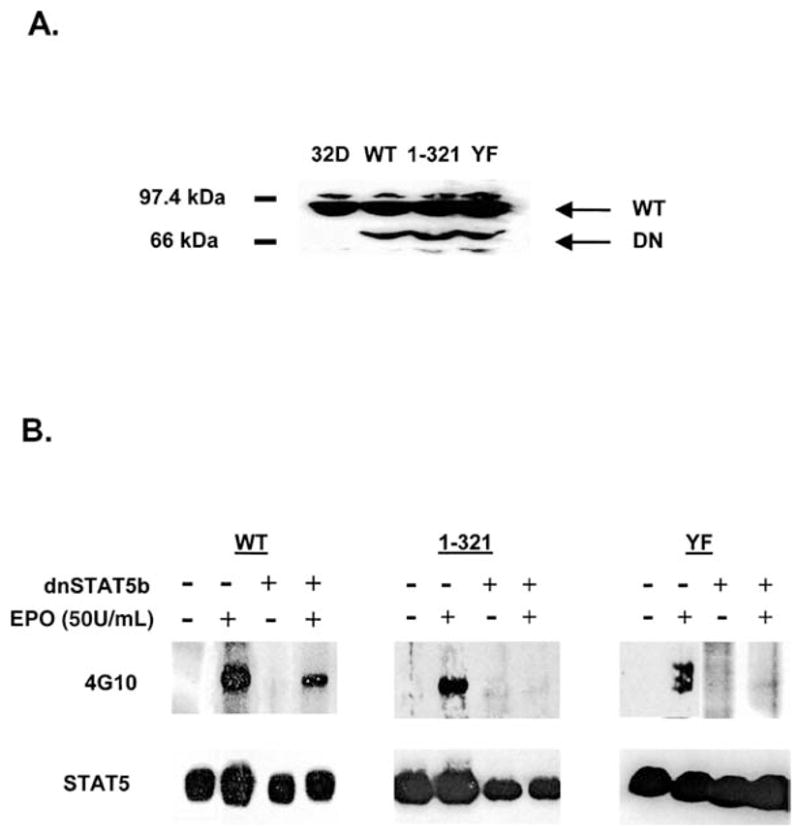
Expression of Stat5b-DN abrogates EPO-dependent Stat5 activation by WT and mutant EPOR isoforms. (A): Whole-cell lysates from parental 32D cells (32D) or 32D cells coexpressing WT, 1–321, or YF EPORs and Stat5b-DN were analyzed by immunoblotting with an antibody specific for residues 451–649 of murine Stat5. The migration positions of endogenous Stat5 (Stat5) and Stat5b-DN (Stat5b-DN) are indicated. (B): 32D cells expressing WT, 1–321, or YF EPORs and lacking Stat5b-DN expression, and cell lines expressing WT, 1–321, or YF EPORs and Stat5b-DN were starved of exogenous growth factors in serum-free medium. Cells were left unstimulated or were treated with EPO (50 U/mL) for 10 minutes at room temperature. Stat5 was immunoprecipitated from detergent cell extracts and analyzed by immunoblotting with antiphosphotyrosine antibody 4G10 (upper panel). Blots were stripped and reprobed with Stat5-specific antibody (lower panel).
Immunoprecipitation and immunoblot analyses showed that Stat5b-DN blocked EPO-dependent Stat5 activation through WT, 1–321, and YF EPORs at the subsaturating concentrations of ligand that were used for cell proliferation and viability assays (e.g., 0.5 U/mL EPO; data not shown). To confirm that Stat5b-DN abrogated EPO-dependent Stat5 activation effectively, we tested a saturating concentration of EPO (50 U/mL) that results in ligand occupancy of over 90% cell surface receptors. Importantly, even at maximal EPOR activation, Stat5b-DN blocked the ability of WT, 1 –321, or YF EPORs to stimulate Stat5 tyrosine phosphorylation (Fig. 4B). Therefore, the level of Stat5b-DN expression in these cell lines is sufficient to effectively abrogate EPOR-induced Stat5 activation.
Anti-apoptotic signaling through non-tyrosine-containing sequences in the EPOR is dependent on functional Stat5
Cells containing or lacking Stat5b-DN were cultured for 24 hours in the presence or absence of EPO (0.5 U/mL) and FCS, to test the function of Stat5 in EPO-dependent anti-apoptotic signaling. As shown previously, the presence of EPO as the sole exogenous growth factor was able to prevent apoptosis in the majority of cells expressing WT, 1–321, and YF EPORs (Figs. 2 and 5A). In contrast, cells expressing Stat5b-DN were highly sensitive to apoptosis in cultures containing only EPO. After 24 hours, approximately 45% of cells expressing WT EPOR and Stat5b-DN were sub-diploid while only 10% of WT cells lacking Stat5b-DN were in the sub-diploid population (Fig. 5A). A functional Stat5 signal is also important in the prevention of apoptosis by EPOR isoforms lacking intracellular tyrosine residues. Approximately 70 to 80% of YF or 1–321 cells coexpressing Stat5b-DN were in the sub-diploid population, while only 10% of YF cells and ~45% of 1–321 cells were sub-diploid. Therefore, EPO-dependent cell survival is mediated primarily by a functional Stat5 signal in cultures lacking exogenous FCS.
Figure 5.
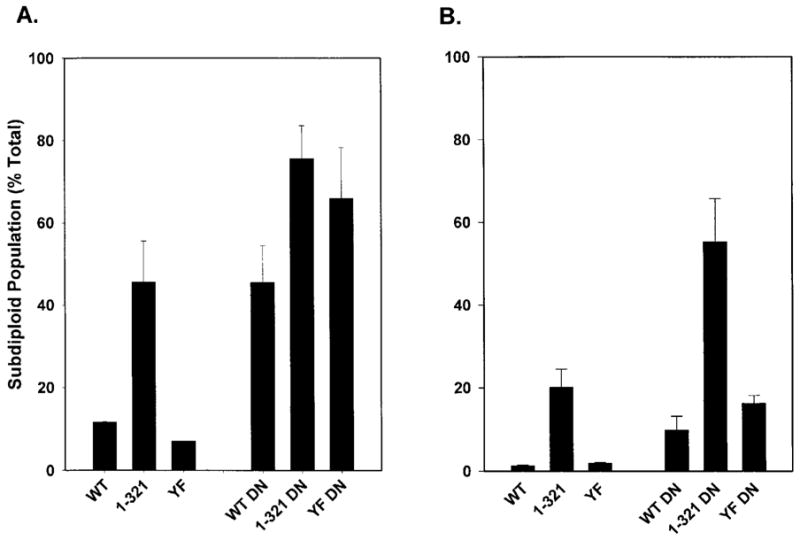
Analysis of DNA fragmentation in 32D cells lines expressing Stat5b-DN. 32D cells expressing WT, 1–321, or YF EPORs and lacking Stat5b-DN expression, and cell lines expressing WT, 1–321, or YF EPORs and Stat5b-DN were washed extensively and then cultured in media (A) containing only EPO or (B) containing EPO and FCS for 24 hours. Cells were stained with propidium iodide and the fraction of sub-diploid cells in the population was determined by flow cytometry. Average results from 7 independent experiments are shown.
The presence of FCS modestly enhanced cell survival signaling by WT, 1–321, and YF EPORs as evidenced by a decrease in the respective sub-diploid populations in media containing EPO and FCS relative to cultures containing only EPO (compare Fig. 5A and B; Fig. 2). The presence of FCS also reversed the sensitivity of WT or YF cells expressing Stat5b-DN to apoptosis (compare Fig. 5A and B), suggesting that serum factors may compensate in part for the loss of Stat5 function. This protective effect of FCS was less apparent in cells expressing the truncated EPOR isoform 1–321 and Stat5b-DN as high levels of sub-diploid cells were found in these cultures (compare Fig. 5A and B). Collectively, these results show that Stat5 is a principal survival signal elicited by non-tyrosine-containing sequences in the EPOR cytoplasmic region.
Discussion
EPO precisely regulates circulating red blood cell levels through its control of erythroid progenitor survival in the bone marrow. Under nonstress conditions, only a fraction of erythroid progenitors complete terminal stages of development to the erythrocyte while others undergo apoptosis. A rapid rise in serum EPO levels, for example in response to hypoxia or anemia, supports increased erythroid progenitor survival and differentiation. In response to abnormally low EPO levels, erythroid progenitors demonstrate enhanced apoptosis [39–41]. The specific EPO-dependent signaling pathways that support cell survival have not been completely characterized. Definition of these pathways will be significant for understanding the clinical functions of EPO, as well as the normal development of red blood cell progenitors and their aberrant regulation in diseases such as polycythemia and leukemia.
Our results show that EPO signaling through non-tyrosine-containing sequences in the membrane-proximal region of the EPOR supports 32D cell survival. We demonstrated that EPO survival signaling does not require the presence of FCS in the medium, indicating that signals activated by the EPOR are sufficient. The combination of EPO and FCS is necessary for 32D cell growth, however, since FCS alone is unable to prevent apoptosis or stimulate growth. Thus, EPO elicits a survival signal in 32D cells and this signal may be required for EPO-dependent 32D cell proliferation in combination with serum factors. Previous work in IL-3-dependent BA/F3 cells expressing a C-terminal EPOR truncation mutant that retains only tyrosine 343 showed that serum insulin-like growth factor-1 (IGF-1) provides a crucial mitogenic signal [42]. IGF-1 may be important in EPO-dependent 32D cell proliferation and additional work is required to test this. Primary red cell progenitors are also likely to require additional growth factors in combination with EPO for efficient proliferation and survival in vivo; stem cell factor and its receptor c-kit provide one example [43].
EPORs YF and 1–321 activate Stat5 despite the absence of the major Stat5 recruitment sites at tyrosines 343 and 401. EPO dose/response assays have shown, however, that these receptor isoforms are less efficient than the WT EPOR in activation of Stat5, particularly at low doses of EPO [29] (data not shown). In contrast, EPORs YF and 1–321 demonstrate similar or slightly elevated levels of Jak2 activation in comparison to the WT EPOR in dose/response assays [29] (data not shown). Since direct interaction has been found between Jak2 and Stat5 [44,45], we suggest that Stat5 may be recruited directly to activated Jak2 in the YF and 1–321 receptor complexes.
The function of Stat5 activation through tyrosine-independent sequences of the EPOR was analyzed in experiments that employed a dominant inhibitory Stat5 isoform, Stat5b-DN. Stat5b-DN abrogated EPO-dependent Stat5 activation through the WT, YF, and 1-321 EPOR isoforms. Stat5b-DN also inhibited the ability of these EPOR isoforms to support 32D cell survival. Thus, Stat5 signaling elicited through non-tyrosine-containing sequences in the membrane-proximal region of the EPOR plays an important role in mediating cell survival. bcl-XL has been implicated as an anti-apoptotic Stat5 target gene in EPO signaling [27]. 32D cells, however, express constitutively high bcl-XL levels that are not further induced by EPO (data not shown), suggesting that alternative target genes mediate EPO-dependent survival in this cell line.
Our findings are significant in light of previous work indicating that imbalanced or constitutive EPOR signaling contributes to diseases such as familial erythrocytosis/polycythemia or erythroleukemia [4,5,46]. Stat5 activation through non-tyrosine-containing sequences in the membrane-proximal region of the EPOR may play a role in these diseases, particularly polycythemias resulting from truncations in the receptor cytoplasmic region [4,46]. Furthermore, an EPOR truncation mutant lacking the distal portion of the cytoplasmic region and containing a phenylalanine substitution at the sole remaining tyrosine (residue 343 in the cytoplasmic domain) supports erythropoiesis in vivo under nonstress conditions [30]. The critical signaling molecules elicited by this receptor to support red cell production have not been elucidated, although our results suggest that Stat5 plays an essential role.
In summary, our work has shown for the first time that a Stat5-dependent pathway elicited through tyrosine-independent sequences in the EPOR cytoplasmic domain plays an important role in hematopoietic cell survival signaling. Additional work is required to identify the critical Stat5 target genes that regulate cell survival, as well as to evaluate the significance of this pathway in normal and aberrant red blood cell growth and development in vivo.
Acknowledgments
We thank Ling Zhang for excellent technical support, Greg Longmore for the EPOR YF cDNA, and Joan Egrie and Steve Elliot (Amgen, Thousand Oaks, CA, USA) for the generous gift of recombinant EPO. In addition, we thank Gary Gallick, David McConkey, Brad McIntyre, and members of the Watowich lab for their suggestions throughout the course of this work and for generous use of their reagents. We thank Donna Badgwell and Athanasia Panopoulos for their critical review of the manuscript. This work was supported grants from the National Cancer Institute, National Institutes of Health (CA 77447), the Gillson Longenbaugh Foundation, and the University of Texas MD Anderson Cancer Center Institutional Grants Program to SSW.
References
- 1.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989;321:158–163. doi: 10.1056/NEJM198907203210305. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 3.Lin CS, Lim SK, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 4.Divoky V, Liu Z, Ryan TM, et al. Mouse model of congenital polycythemia: Homologous replacement of murine gene by mutant human erythropoietin receptor gene. Blood. 2001;98:986–991. doi: 10.1073/pnas.98.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longmore GD, Lodish HF. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: A cytokine receptor superfamily oncogene. Cell. 1991;67:1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- 6.de la Chapelle A, Traskelin A-L, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993;90:4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friend C. Cell-free transmission in adult swiss mice of a disease having the character of a leukemia. J Exp Med. 1957;105:307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankins WD, Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980;22:693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- 9.Kabat D. Molecular biology of friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 10.Kirito K, Nakajima K, Watanabe T, et al. Identification of the human erythropoietin receptor region required for Stat1 and Stat3 activation. Blood. 2002;99:102–110. doi: 10.1182/blood.v99.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Haq R, Halupa A, Beattie BK, et al. Regulation of erythropoietin-induced Stat serine phosphorylation by distinct mitogen-activated protein kinases. J Biol Chem. 2002;277:17359–17366. doi: 10.1074/jbc.M201842200. [DOI] [PubMed] [Google Scholar]
- 12.Damen JE, Cutler RL, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (Epr) is essential for binding the p85 subunit of phosphatidylinositol (PI) 3-kinase and for Epr-associated PI 3-kinase activity. J Biol Chem. 1995;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- 13.Damen JE, Wakao H, Miyajima A, et al. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura Y, Miura O, Ihle JN, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 15.Tilbrook PA, Ingley E, Williams JH, Hibbs ML, Klinken SP. Lyn tyrosine kinase is essential for erythropoietin-induced differentiation of J2E erythroid cells. EMBO J. 1997;16:1610–1619. doi: 10.1093/emboj/16.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witthuhn BA, Quelle FW, Silvennoinen O, et al. Jak2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 17.Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of Jak2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto A, Masuhara M, Mitsui K, et al. Cis, a cytokine-inducible SH2 protein, is a target of the Jak-Stat5 pathway and modulates Stat5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 19.Watowich SS, Yoshimura A, Longmore GD, et al. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantinescu SN, Huang LJ, Nam H, Lodish HF. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol Cell. 2001;7:377–385. doi: 10.1016/s1097-2765(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 21.Syed RS, Reid SW, Li C, et al. Efficiency of signaling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 22.Watowich SS, Liu KD, Xie X, et al. Oligomerization and scaffolding functions of the erythropoietin receptor cytoplasmic tail. J Biol Chem. 1999;274:5415–5421. doi: 10.1074/jbc.274.9.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dusanter-Fourt I, Casadevall N, Lacombe C, et al. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992;267:10670–10675. [PubMed] [Google Scholar]
- 24.Klingmuller U, Wu H, Hsiao JG, et al. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci U S A. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 26.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 27.Socolovsky M, Fallon AEJ, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a5b mice: A direct role for Stat5 in bcl-x induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 28.Snow JW, Abraham N, Ma MC, et al. Stat5 promotes multilineage hematolymphoid development in vivo through effects on early hematopoietic progenitor cells. Blood. 2002;99:95–101. doi: 10.1182/blood.v99.1.95. [DOI] [PubMed] [Google Scholar]
- 29.Longmore GD, You Y, Molden J, et al. Redundant and selective roles for erythropoietin receptor tyrosines in erythropoiesis in vivo. Blood. 1998;91:870–878. [PubMed] [Google Scholar]
- 30.Zang H, Sato K, Nakajima H, et al. The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis. EMBO J. 2001;20:3156–3166. doi: 10.1093/emboj/20.12.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooten DK, Xie X, Bartos D, et al. Cytokine signaling through Stat3 activates integrins, promotes adhesion, and induces growth arrest in the myeloid cell line 32D. J Biol Chem. 2000;275:26566–26575. doi: 10.1074/jbc.M003495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mui AL-F, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a c-terminal truncated Stat5: Role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberger JS, Eckner R, Ostertag W, et al. Release of spleen focus-forming virus (SFFV) from differentiation inducible promyelocytic leukemia cell lines transformed in vitro by friend leukemia virus. Virology. 1980;105:425–435. doi: 10.1016/0042-6822(80)90043-4. [DOI] [PubMed] [Google Scholar]
- 34.Greenberger JS, Sakakeeny MA, Humphries RK, Eaves CJ, Eckner RJ. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watowich SS, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function: Role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura A, D’Andrea AD, Lodish HF. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990;87:4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura O, Cleveland JL, Ihle JN. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993;13:1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 39.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 40.Kelley LL, Koury MJ, Bondurant MC, et al. Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: A mechanism for controlled rates of erythrocyte production. Blood. 1993;82:2340–2352. [PubMed] [Google Scholar]
- 41.Yu H, Bauer B, Lipke GK, Phillips RL, Van Zant G. Apoptosis and hematopoiesis in murine fetal liver. Blood. 1993;81:373–384. [PubMed] [Google Scholar]
- 42.Damen JE, Krosl J, Morrison D, Pelech S, Krystal G. The hyperresponsiveness of cells expressing truncated erythropoietin receptors is contingent on insulin-like growth factor-1 in fetal calf serum. Blood. 1998;92:425–433. [PubMed] [Google Scholar]
- 43.Russell ES. Hereditary anemias of the mouse: A review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 44.Fujitani Y, Hibi M, Fukada T, et al. An alternative pathway for Stat activation that is mediated by direct interaction between Jak and Stat. Oncogene. 1997;14:751–761. doi: 10.1038/sj.onc.1200907. [DOI] [PubMed] [Google Scholar]
- 45.Barahmand-Pour F, Meinke A, Groner B, Decker T. Jak2-Stat5 interactions analyzed in yeast. J Biol Chem. 1998;273:12567–12575. doi: 10.1074/jbc.273.20.12567. [DOI] [PubMed] [Google Scholar]
- 46.Watowich SS, Xie X, Klingmuller U, et al. Erythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous state. Blood. 1999;94:2530–2532. [PubMed] [Google Scholar]


