Abstract
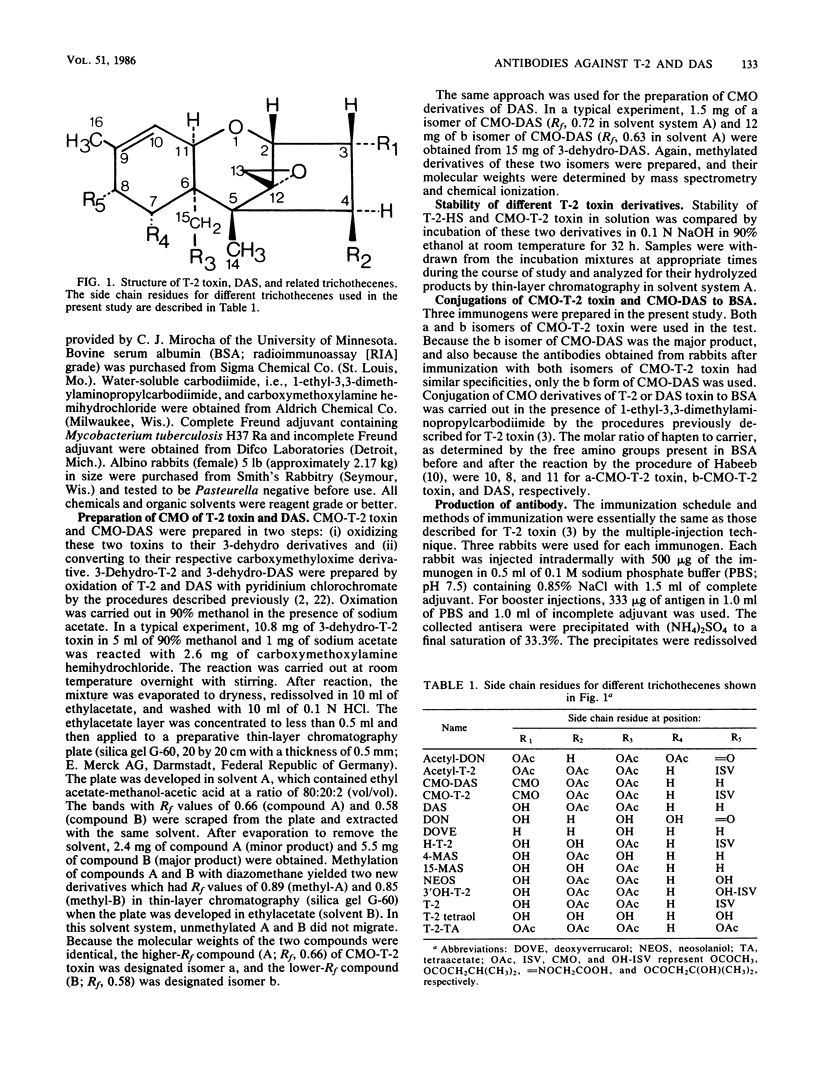
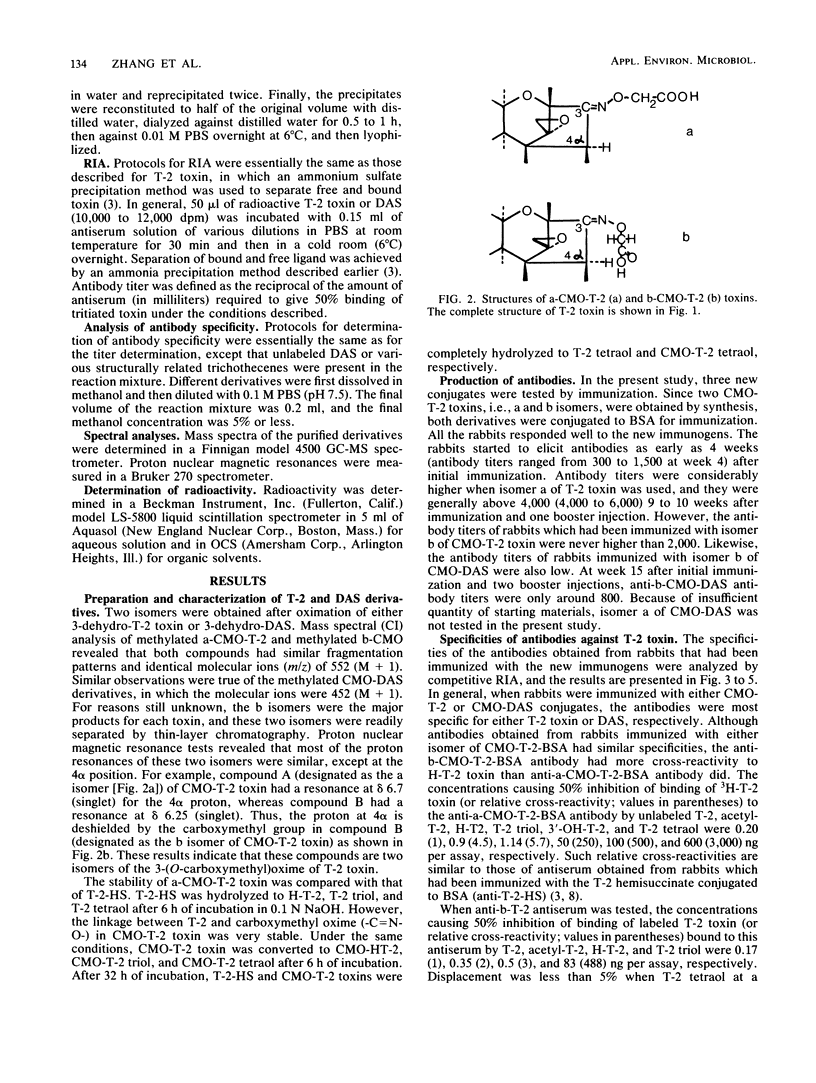
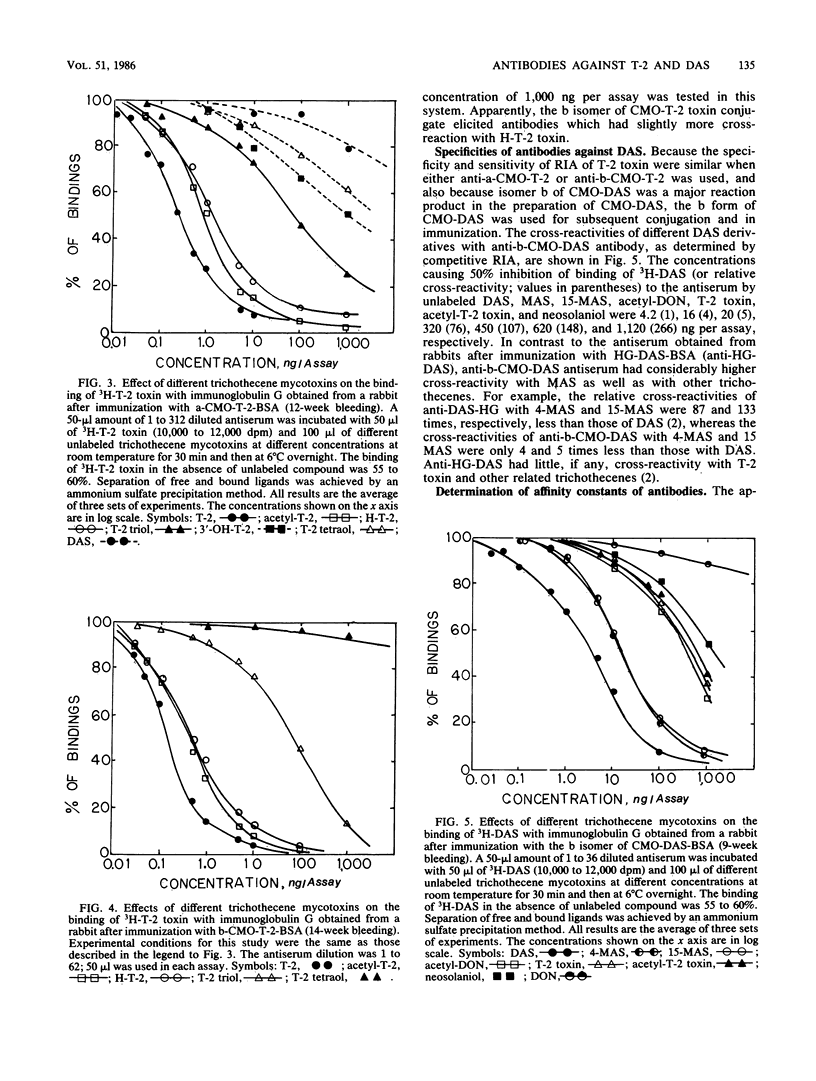
A new, improved approach for the production of antibodies against T-2 toxin and diacetoxyscirpenol (DAS) was developed. The method involves the use of immunogens which were prepared by conjugating O-carboxymethoxyl oxime (CMO) derivatives of both toxins to bovine serum albumin (BSA). Isomers a and b of CMO-T-2 toxin and isomer b of CMO-DAS were tested. Antibodies against both toxins were demonstrated as early as 4 weeks after immunization. a-CMO-T-2-BSA conjugate was a better immunogen than the b isomer, and the highest titers (6,000) were reached 14 weeks after immunization and one booster injection. Antibody titers for rabbits immunized with the b isomer of CMO-T-2 never reached more than 2,000. The specificity of antibodies obtained from rabbits after immunization with CMO-T-2-BSA was similar to that of hemisuccinate-T-2-BSA. Anti-b-T-2 antibodies had slightly higher cross-reactivity with H-T-2 toxin than did the antibody obtained from rabbits immunized with the conjugate of the a isomer. The relative cross-reactivities of anti-a-CMO-T-2 antibody with T-2, acetyl-T-2, H-T-2, T-2-triol, 3'-OH-T-2, and T-2 tetraol were 1, 4.5, 5.7, 250, 500, and 3,000, respectively. The relative cross-reactivities of anti-b-T-2 antibody with T-2, acetyl-T-2, H-T-2, and T-2 triol were 1, 2, 3, and 488, respectively. Antibodies against b-CMO-DAS showed a high degree of cross-reactivity with monoacetoxyscirpenols (MAS). The relative cross-reactivities of anti-B-DAS antibody with DAS, 4-MAS, 15-MAS, acetyl-deoxynivalenol, T-2-toxin, acetyl-T-2, and neosolaniol were 1, 4, 5, 76, 107, 147, and 266, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu F. S., Grossman S., Wei R. D., Mirocha C. J. Production of antibody against T-2 toxin. Appl Environ Microbiol. 1979 Jan;37(1):104–108. doi: 10.1128/aem.37.1.104-108.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S., Liang M. Y., Zhang G. S. Production and characterization of antibody against diacetoxyscirpenol. Appl Environ Microbiol. 1984 Oct;48(4):777–780. doi: 10.1128/aem.48.4.777-780.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S., Zhang G. S., Williams M. D., Jarvis B. B. Production and characterization of antibody against deoxyverrucarol. Appl Environ Microbiol. 1984 Oct;48(4):781–784. doi: 10.1128/aem.48.4.781-784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. C., Lillehoj E. B. Simple method for isolation of 4-deoxynivalenol from rice inoculated with Fusarium graminearum. Appl Environ Microbiol. 1984 Nov;48(5):1053–1054. doi: 10.1128/aem.48.5.1053-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontelo P. A., Beheler J., Bunner D. L., Chu F. S. Detection of T-2 toxin by an improved radioimmunoassay. Appl Environ Microbiol. 1983 Feb;45(2):640–643. doi: 10.1128/aem.45.2.640-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendloff E. H., Pestka J. J., Swanson S. P., Hart L. P. Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by enzyme-linked immunosorbent assay. Appl Environ Microbiol. 1984 May;47(5):1161–1163. doi: 10.1128/aem.47.5.1161-1163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Brimfield A. A., Miller M., Finkelman F. D., Chu S. F. Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl Environ Microbiol. 1985 Jan;49(1):168–172. doi: 10.1128/aem.49.1.168-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chu F. S. Radioimmunoassay of T-2 toxin in biological fluids. J Assoc Off Anal Chem. 1981 May;64(3):684–688. [PubMed] [Google Scholar]
- Lee S., Chu F. S. Radioimmunoassay of T-2 toxin in corn and wheat. J Assoc Off Anal Chem. 1981 Jan;64(1):156–161. [PubMed] [Google Scholar]
- Müller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- Peters H., Dierich M. P., Dose K. Enzyme-linked immunosorbent assay for detection of T-2 toxin. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1437–1441. doi: 10.1515/bchm2.1982.363.2.1437. [DOI] [PubMed] [Google Scholar]
- Wallace E. M., Pathre S. V., Mirocha C. J., Robison T. S., Fenton S. W. Synthesis of radiolabeled T-2 toxin. J Agric Food Chem. 1977 Jul-Aug;25(4):836–838. doi: 10.1021/jf60212a058. [DOI] [PubMed] [Google Scholar]
- Wei R. D., Chu F. S. Modification of in vitro metabolism of T-2 toxin by esterase inhibitors. Appl Environ Microbiol. 1985 Jul;50(1):115–119. doi: 10.1128/aem.50.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Strong F. M., Smalley E. B., Schnoes H. K. Chemical interconversion of T-2 and HT-2 toxins and related compounds. Biochem Biophys Res Commun. 1971 Oct 15;45(2):396–401. doi: 10.1016/0006-291x(71)90832-1. [DOI] [PubMed] [Google Scholar]


