Abstract
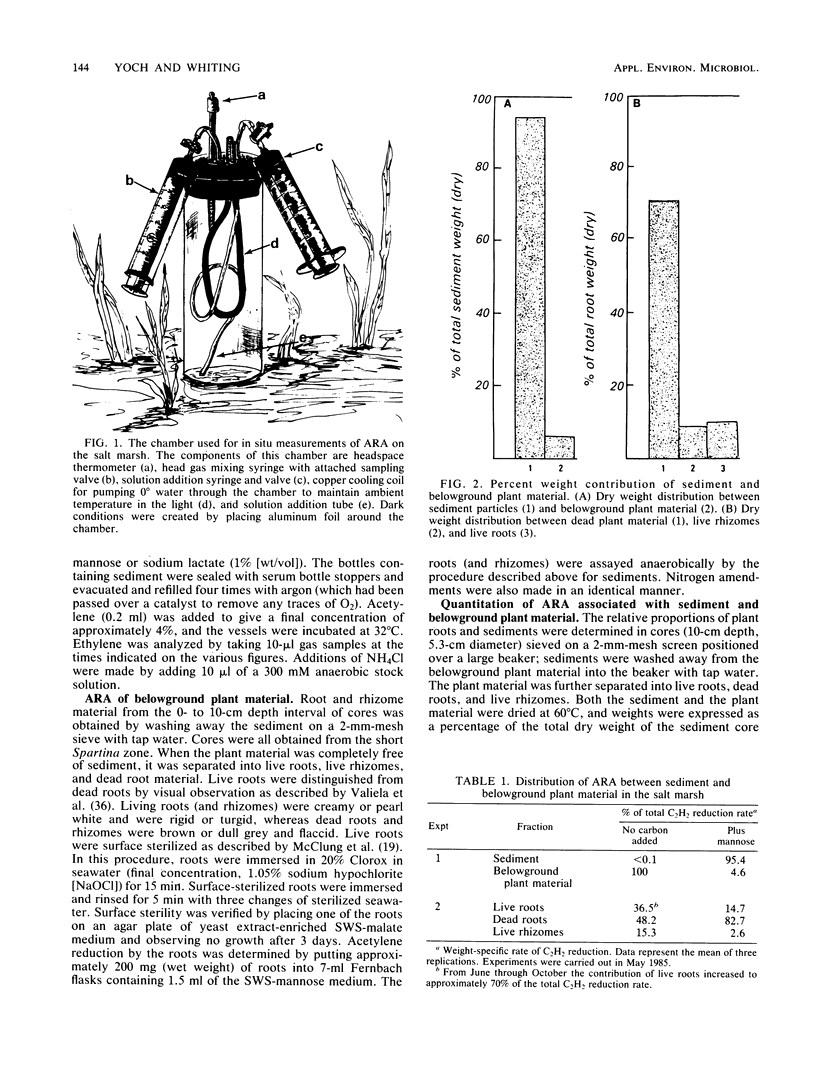
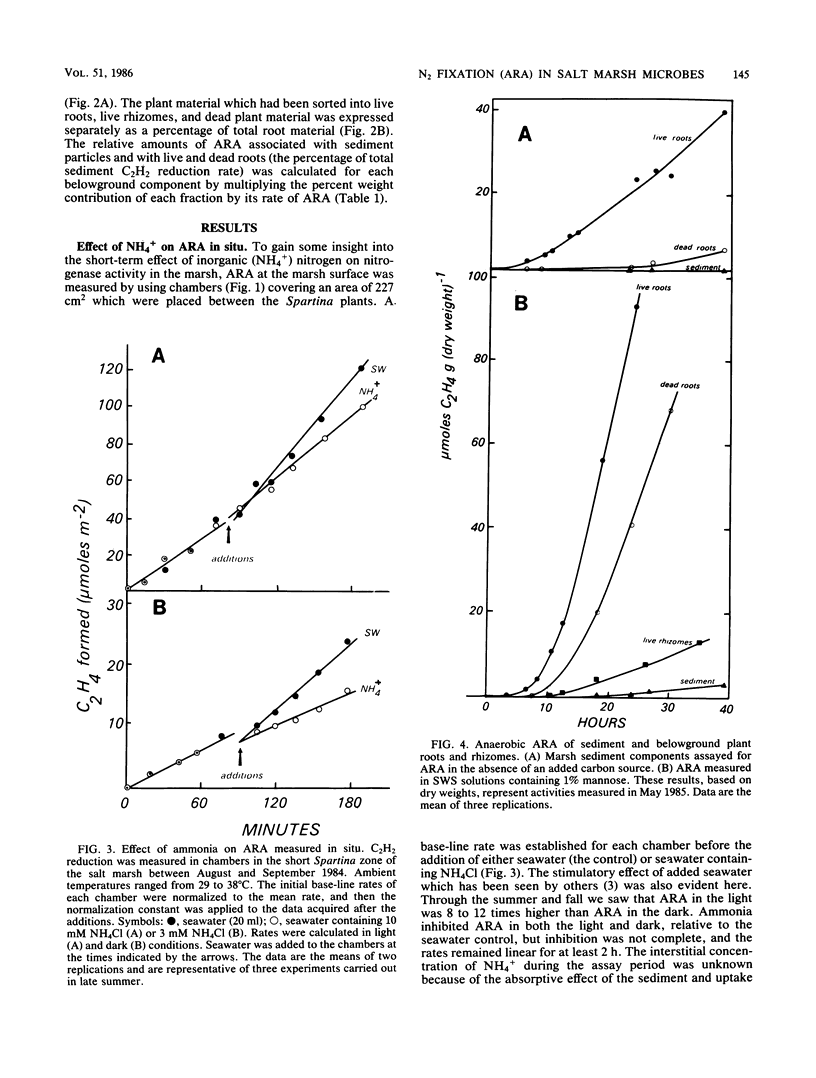
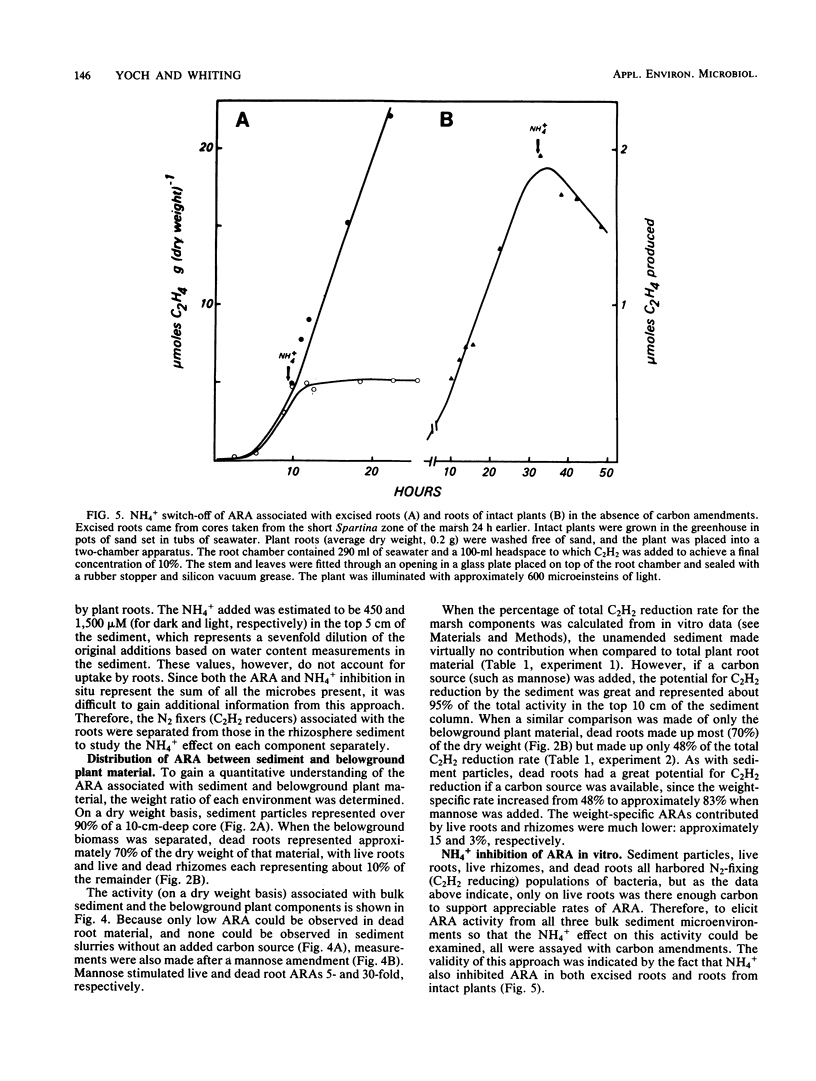
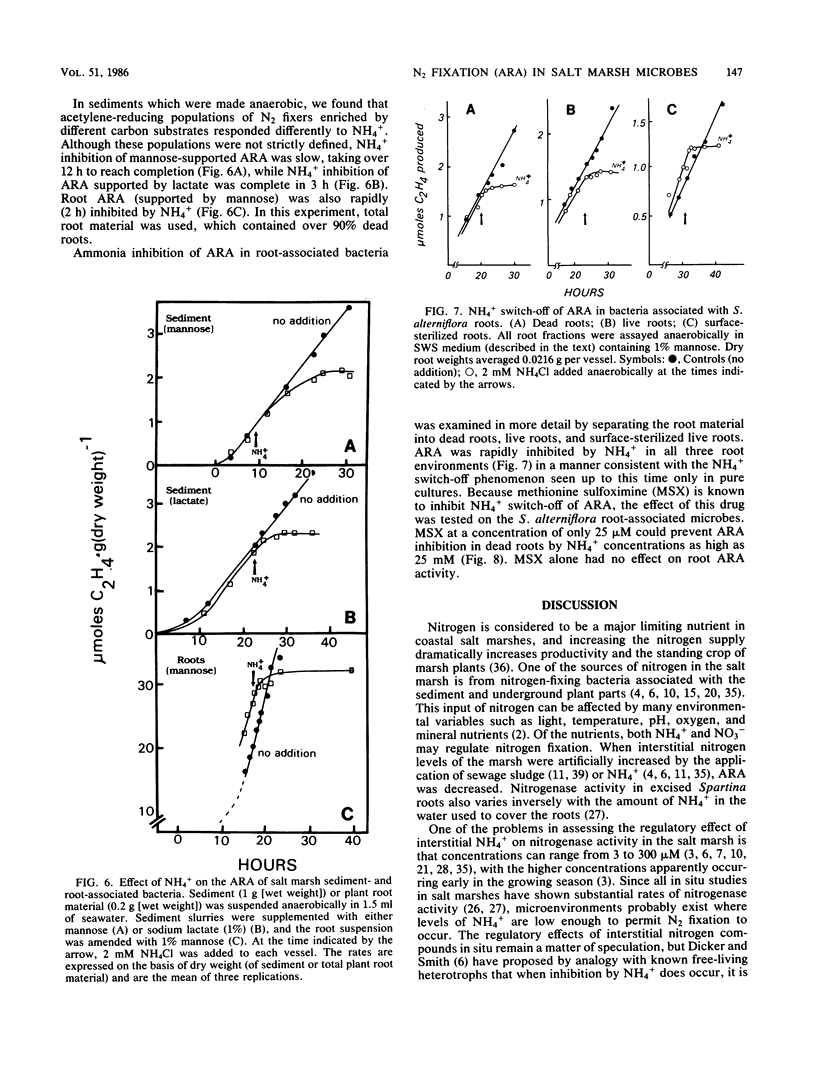
The regulatory effect of NH4+ on nitrogen fixation in a Spartina alterniflora salt marsh was examined. Acetylene reduction activity (ARA) measured in situ was only partially inhibited by NH4+ in both the light and dark after 2 h. In vitro analysis of bulk sediment divided into sediment particles, live and dead roots, and rhizomes showed that microbes associated with sediment and dead roots have a great potential for anaerobic C2H2 reduction, but only if amended with a carbon source such as mannose. Only live roots had significant rates of ARA without an added carbon source. In sediment, N2-fixing mannose enrichment cultures could be distinguished from those enriched by lactate in that only the latter were rapidly inhibited by NH4+. Ammonia also inhibited ARA in dead and live roots and in surface-sterilized roots. The rate of this inhibition appeared to be too rapid to be attributed to the repression and subsequent dilution of nitrogenase. The kinetic characteristics of this inhibition and its prevention in root-associated microbes by methionine sulfoximine are consistent with the NH4+ switch-off-switch-on mechanism of nitrogenase regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Zumft W. G. L-methionine-SR-sulfoximine as a probe for the role of glutamine synthetase in nitrogenase switch-off by ammonia and glutamine in Rhodopseudomonas palustris. Arch Microbiol. 1983 Jan;134(1):17–22. doi: 10.1007/BF00429400. [DOI] [PubMed] [Google Scholar]
- Capone D. G., Carpenter E. J. Perfusion method for assaying microbial activities in sediments: applicability to studies of n(2) fixation by c(2)h(2) reduction. Appl Environ Microbiol. 1982 Jun;43(6):1400–1405. doi: 10.1128/aem.43.6.1400-1405.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker H. J., Smith D. W. Enumeration and relative importance of acetylene-reducing (nitrogen-fixing) bacteria in a delaware salt marsh. Appl Environ Microbiol. 1980 May;39(5):1019–1025. doi: 10.1128/aem.39.5.1019-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Shah V. K., Brill W. J. Feedback inhibition of nitrogenase. J Bacteriol. 1981 Dec;148(3):884–888. doi: 10.1128/jb.148.3.884-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Hanson R. B. Comparison of Nitrogen Fixation Activity in Tall and Short Spartina alterniflora Salt Marsh Soils. Appl Environ Microbiol. 1977 Mar;33(3):596–602. doi: 10.1128/aem.33.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. B. Nitrogen fixation (acetylene reduction) in a salt marsh amended with sewage sludge and organic carbon and nitrogen compounds. Appl Environ Microbiol. 1977 Apr;33(4):846–852. doi: 10.1128/aem.33.4.846-852.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Okon Y., Burris R. H. The nitrogenase system of Spirillum lipoferum. Biochem J. 1978 Sep 1;173(3):1001–1003. doi: 10.1042/bj1731001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C. R., Patriquin D. G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980 Aug;26(8):881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- McClung C. R., van Berkum P., Davis R. E., Sloger C. Enumeration and Localization of N(2)-Fixing Bacteria Associated with Roots of Spartina alterniflora Loisel. Appl Environ Microbiol. 1983 Jun;45(6):1914–1920. doi: 10.1128/aem.45.6.1914-1920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Neyra C. A., Van Berkum P. Nitrate reduction nitrogenase activity in Spirillum lipoferum1. Can J Microbiol. 1977 Mar;23(3):306–310. doi: 10.1139/m77-045. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U. Nitrogenase from Rhodospirillum rubrum. Relation between 'switch-off' effect and the membrane component. Hydrogen production and acetylene reduction with different nitrogenase component ratios. Biochim Biophys Acta. 1979 Sep 11;547(3):429–437. doi: 10.1016/0005-2728(79)90023-9. [DOI] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. G., Ludden P. W. Change in subunit composition of the iron protein of nitrogenase from Rhodospirillum rubrum during activation and inactivation of iron protein. Biochem J. 1982 Sep 1;205(3):489–494. doi: 10.1042/bj2050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Zhang Z. M., Claybrook D. L. Methylamine metabolism and its role in nitrogenase "switch off" in Rhodopseudomonas capsulata. Arch Microbiol. 1983 Jan;134(1):45–48. doi: 10.1007/BF00429405. [DOI] [PubMed] [Google Scholar]
- van Berkum P., Sloger C. Comparing time course profiles of immediate acetylene reduction by grasses and legumes. Appl Environ Microbiol. 1981 Jan;41(1):184–189. doi: 10.1128/aem.41.1.184-189.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Sloger C. Immediate acetylene reduction by excised grass roots not previously preincubated at low oxygen tensions. Plant Physiol. 1979 Nov;64(5):739–743. doi: 10.1104/pp.64.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]


