Abstract
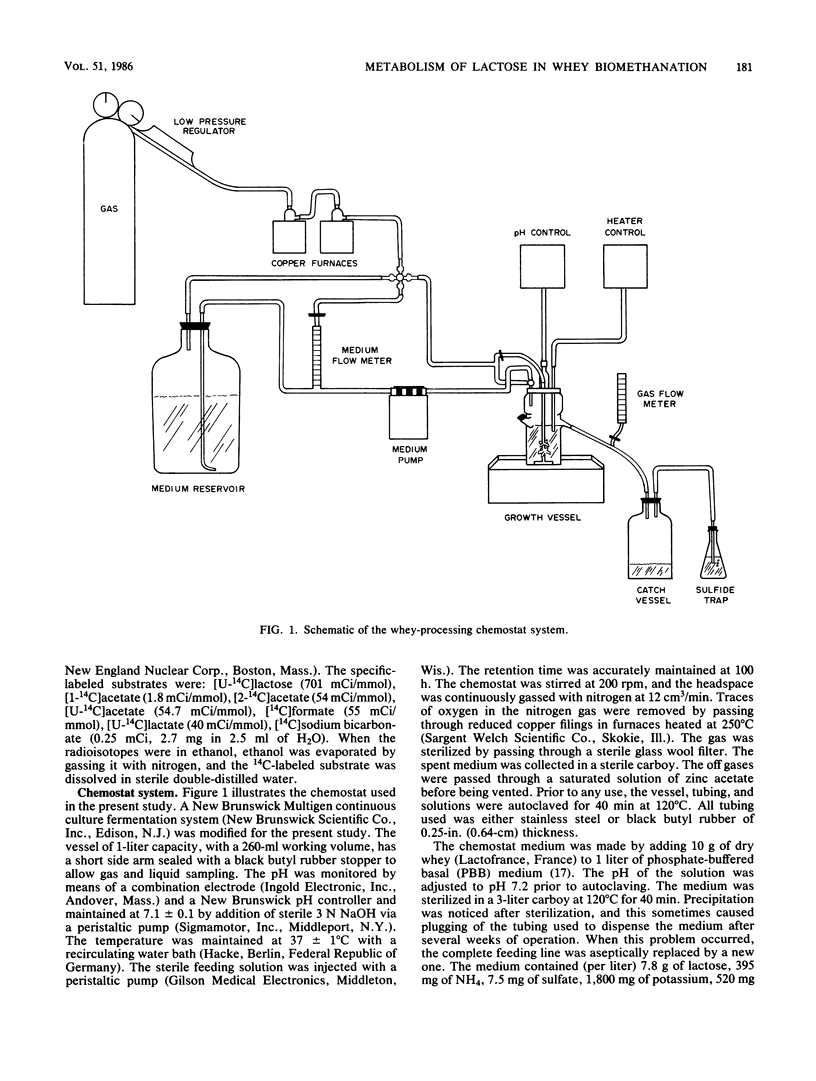
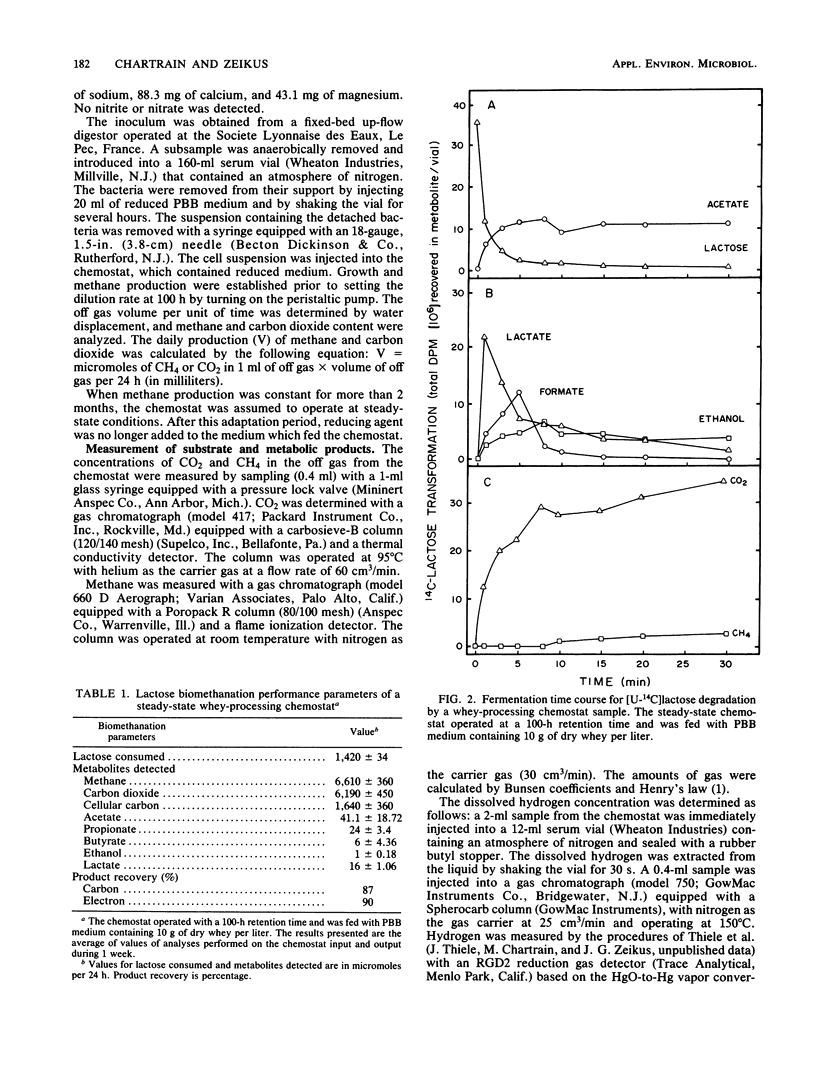
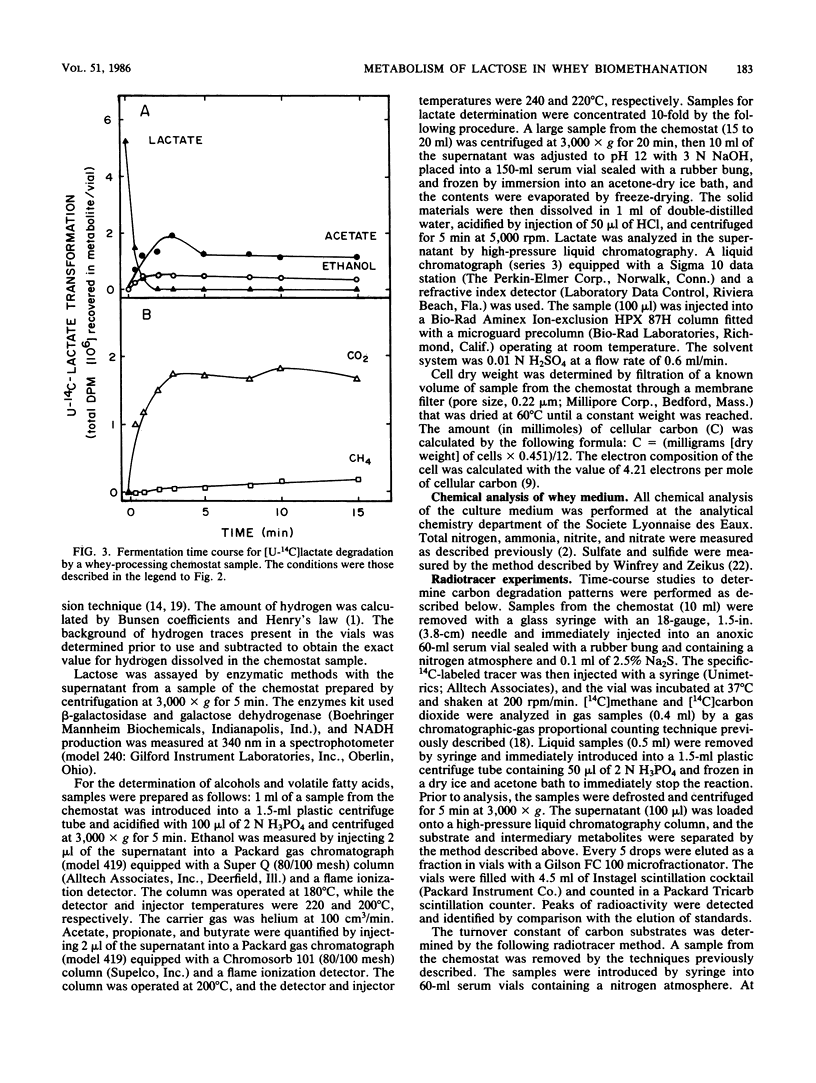
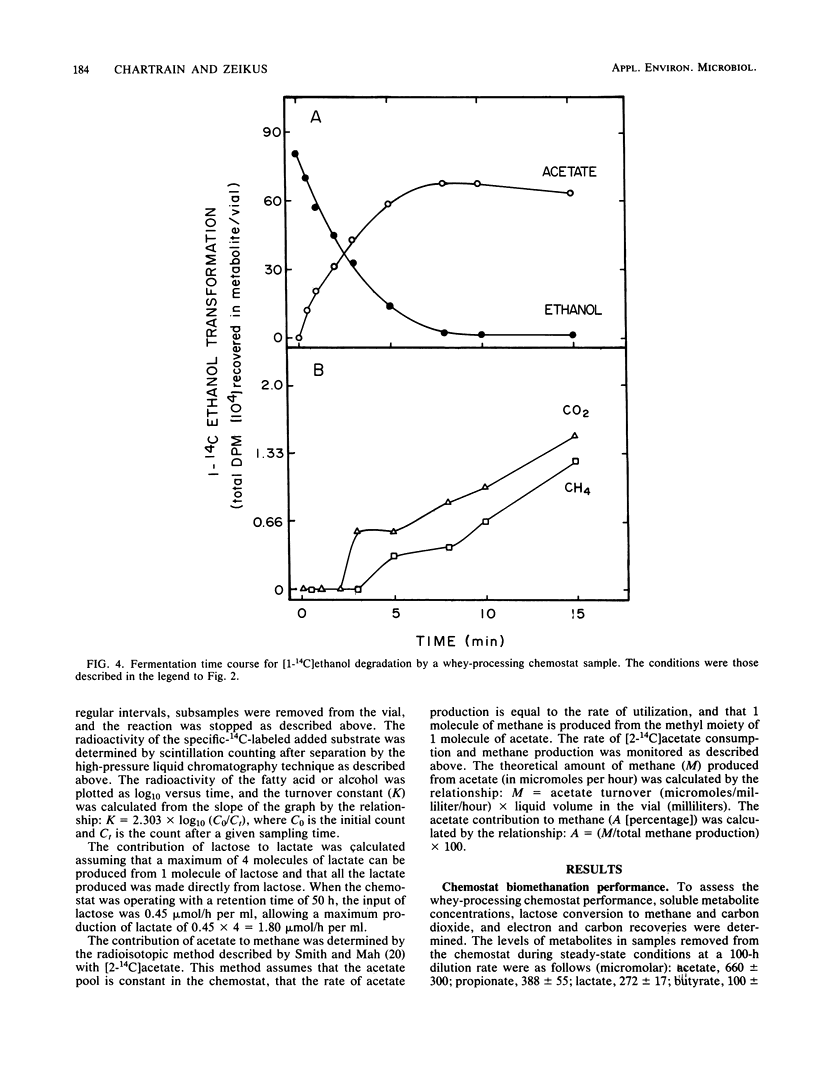
The intermediary carbon and electron flow routes for lactose degradation during whey biomethanation were studied in continuous culture. The chemostat was operated under lactose-limited conditions with a 100-h retention time. The carbon balance observed for lactose degradation was 4.65 mmol of CH4, 4.36 mmol of CO2 and 1.15 mmol of cellular carbon per mmol of lactose consumed, with other intermediary metabolites (i.e., acetate, lactate, etc.) accounting for less than 2% of the lactose consumed. The carbon and electron recoveries for this biomethanation were 87 and 90%, respectively. 14C tracer studies demonstrated that lactose biomethanation occurred in three distinct but simultaneous phases. Lactose was metabolized primarily into lactate, ethanol, acetate, formate, and carbon dioxide. During this hydrolytic phase, 82% of the lactose was transformed into lactate. These metabolites were transformed into acetate and H2-CO2 in a second, acetogenic, phase. Finally, the direct methane precursors were transformed during the methanogenic phase, with acetate accounting for 81% of the methane formed. A general scheme is proposed for the exact carbon and electron flow route during lactose biomethanation, which predicts the prevalent microbial populations in this ecosystem.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone D. R. Terminal reactions in the anaerobic digestion of animal waste. Appl Environ Microbiol. 1982 Jan;43(1):57–64. doi: 10.1128/aem.43.1.57-64.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Harris R. F., Adams S. S. Determination of the carbon-bound electron composition of microbial cells and metabolites by dichromate oxidation. Appl Environ Microbiol. 1979 Feb;37(2):237–243. doi: 10.1128/aem.37.2.237-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Dolfing J., Wuhrmann K., Zehnder A. J. Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol. 1983 Apr;45(4):1411–1414. doi: 10.1128/aem.45.4.1411-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton F. S., Conrad R., Zeikus J. G. Physiological function of hydrogen metabolism during growth of sulfidogenic bacteria on organic substrates. J Bacteriol. 1984 Sep;159(3):843–849. doi: 10.1128/jb.159.3.843-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Cardwell S. C., Anguish T., Lee M., Koch M. Methanogenesis in a Thermophilic (58 degrees C) Anaerobic Digestor: Methanothrix sp. as an Important Aceticlastic Methanogen. Appl Environ Microbiol. 1984 Apr;47(4):796–807. doi: 10.1128/aem.47.4.796-807.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


