Abstract
Presented here are femtosecond pump-probe studies on the water-solvated 7-azaindole dimer, a model DNA base pair. In particular, studies are presented that further elucidate the nature of the reactive and nonreactive dimers and also provide new insights establishing that the excited state double-proton transfer in the dimer occurs in a stepwise rather than a concerted manner. A major question addressed is whether the incorporation of a water molecule with the dimer results in the formation of species that are unable to undergo excited state double-proton transfer, as suggested by a recent study reported in the literature [Nakajima, A., Hirano, M., Hasumi, R., Kaya, K., Watanabe, H., Carter, C. C., Williamson, J. M. & Miller, T. (1997) J. Phys. Chem. 101, 392–398]. In contrast to this earlier work, our present findings reveal that both reactive and nonreactive dimers can coexist in the molecular beam under the same experimental conditions and definitively show that the clustering of water does not induce the formation of the nonreactive dimer. Rather, when present with a species already determined to be a nonreactive dimer, the addition of water can actually facilitate the occurrence of the proton transfer reaction. Furthermore, on attaining a critical hydration number, the data for the nonreactive dimer suggest a solvation-induced conformational structure change leading to proton transfer on the photoexcited half of the 7-azaindole dimer.
Cluster science studies have long been utilized to yield unique perspectives of microscopic properties related to the bulk condensed phase (1). This approach involves the investigation of a broad spectrum of clusters ranging from isolated species to the study of fully solvated species, thus illustrating the progression from the gas phase to the condensed phase. In reference to our specific experiments as an example, we have been able to study the excited state double-proton transfer (ESDPT) of the 7-azaindole (7-Aza) dimer under conditions ranging from an isolated dimer to a state of solvation where the hydrogen-bonded dimer is clustered with as many as nine water molecules. As the number of water molecules on the nonreactive dimer increases, we begin to see evidence that the dimer molecule is behaving more as it would in a fully solvated condensed-phase environment. In support of these findings, it has been reported that clusters with as little as six waters begin to show liquid-like properties (2–4).
The model DNA base pair 7-Aza has proven to be an interesting and enlightening species for study in both the gas and condensed phases. Of utmost interest is the double-proton transfer that the 7-Aza dimer undergoes on excitation to the S1 state. This ESDPT was first observed in solution by Kasha and coworkers (5). Later, Kaya and coworkers performed extensive supersonic jet spectroscopic studies on the 7-Aza monomer, dimer, and solvated forms of these species (6–9). The first direct determination of the rates of the double-proton transfer was made in the gas phase by Zewail and coworkers (10), who provided definitive evidence for a stepwise rather than a concerted process. Subsequent studies of the proton-transfer dynamics performed in the gas phase by Folmer et al. (11), using a unique femtosecond laser Coulomb explosion technique that enabled the intermediate state in the transformation to be arrested and detected, yielded further proof of the stepwise process. Regarding the process in the condensed phase, several recent studies have been reported (12–14) of the rate of the ESDPT by using ultrafast spectroscopic techniques.
The exact nature of the mechanism for the ESDPT in 7-Aza has been vigorously debated in the literature. The major point of dispute is whether the double-proton transfer proceeds in a stepwise or a concerted fashion. Proponents of the concerted pathway point primarily to earlier condensed phase studies where, in the presence of a nonpolar liquid, the proton transfer was reported to occur in what was interpreted to be a one-step process on the order of 1.1 to 1.4 ps (12, 13, 15). Those who support the stepwise mechanism, however, look toward both gas-phase and more recent condensed-phase experiments for their most compelling arguments (10, 11, 14, 16). In their most recent paper on the subject, Zewail and coworkers performed a series of experiments in the condensed phase on the proton transfer of 7-Aza dimer in nonpolar solvents using both transient absorption and fluorescence up-conversion techniques (16). Their paper clearly shows that the double-proton transfer of 7-Aza is a stepwise process in the condensed phase as long as the excitation energy is near the 0,0 transition.
Zewail and coworkers initially reported gas-phase measurements establishing a two-step ESDPT process in the 7-Aza dimer, yielding times of 650 fs and 3.3 ps for the first and second proton transfers, respectively, for excitations near the 0,0 transition (10). Later, work in our group arrived at similar conclusions by utilizing the above-referenced Coulomb explosion technique (11). Interest in connecting the observations in the gas phase with those in the condensed phase continues. Moreover, another controversial subject has arisen in the gas-phase studies, namely the origin and nature of an unreactive dimer that has been observed to arise upon the formation of 7-Aza dimer under higher molecular beam expansion conditions than those used to produce the reactive dimer, which has been investigated most extensively.
In the course of studying the 7-Aza dimer, Kaya and coworkers observed the existence of two isomers of this species depending on the stagnation pressure used in the supersonic expansion (7). These isomers behave differently and are referred to as the reactive and nonreactive dimers. Based on Kaya’s studies, the reactive dimer appears to undergo a normal double-proton transfer, in that it is shown to emit a normal visible fluorescence spectrum on formation of the tautomer, whereas the nonreactive dimer does not show a fluorescence spectrum indicative of full tautomerization. Kaya and coworkers initially explained the behavior of the nonreactive isomer by suggesting it had a geometry differing from the planar one expected for the reactive dimer and that the two isomers arise because of different cluster beam temperatures generated through using differing stagnation pressures in the experiments. This position is also supported by Lopez-Martens et al., who suggest that spectral evidence points toward the formation of a hydrogen-bonded dimer with out-of-plane hydrogens, resulting in a dimer characterized either by a displaced biparallel geometry or by an out-of-plane geometry (17). Recently Kaya set forth another explanation (9). Invoking evidence obtained using IR depletion spectroscopy, they suggest that the nonreactive dimer actually contains an associated water molecule that, on photoexcitation, dissociates, leaving a low-energy dimer molecule that is unable to undergo the ESDPT.
The present paper begins with an overview of the femtosecond Coulomb explosion imaging method (CEIM) developed by our group and demonstrates the power of the method in directly observing intermediates in certain classes of reactions. Then, we turn our attention to recent studies of the nonreactive dimer by using both CEIM and the optical transient pump-probe technique. Finally, we address in detail the results of extensive new studies in our laboratory on the effect of hydration on the reaction dynamics.
Experimental Methods and Concepts
Arresting Intermediates in a Reaction via Coulomb Explosion.
During the course of investigating cluster dynamics by using high-intensity femtosecond laser techniques, we discovered that with proper selection of the photoionization conditions (18, 19), Coulomb explosion can be initiated in clusters at moderate fluences and in a controlled way. Using various pump-probe studies performed at selected time delays with respect to a pulsed molecular beam in which clusters were produced, and also using covariance analysis (20) to investigate the origin of observed high-charge state species, we established the critical role of clusters in effecting the Coulomb explosion phenomena (21). This led us to conceive of a way to study the course of stepwise reactions, e.g., proton transfer, by attempting to arrest the intermediates in the transfer process at selected times. Experiment, as well as theory, indicates that the formation of ions in a femtosecond laser pulse (22) and the concomitant Coulomb explosion phenomenon (21) are completed on a very short time scale (ref. 23; unpublished calculations were performed to determine the separation time between two model 7-Aza monomers yielding times less than 25 fs), lending promise that the Coulomb explosion imaging method could be used in “viewing” intermediates at selected times during the course of a reaction (11, 24). We have successfully used this method to follow the course of reactions in 7-Aza dimers and to detect the reaction intermediate as well. The concept behind the process is shown schematically in Fig. 1.
Figure 1.
Idealized rendition of the Coulomb explosion process used to directly monitor the formation of the reaction intermediate. Illustration by Lutz Poth (30).
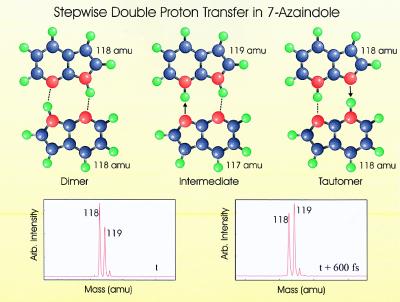
In this method, the formation and temporal evolution of the intermediate can be clearly resolved in the mass spectrum by initiating a Coulomb explosion event at varying chosen time delays with respect to the pump photon. The stepwise double-proton transfer for 7-Aza dimer is illustrated in Fig. 2, which also provides mass spectral evidence of the formation of the intermediate. The mass 119 and 118 species can be seen to vary in time with respect to pump and probe laser beam overlap. It was concluded that the rise and fall of the 119-atomic mass unit (amu) species, with respect to the 118-amu species, represented the formation of an intermediate species, followed by subsequent tautomer formation in accordance with the temporal dependence of the Coulomb explosion-generated mass fragments. Calculations suggest that the time scale during which the molecular centers separate by Coulomb explosion, and thereby prevent further transfer of the protons, is on the order of 25 fs, thus making this method a useful technique for interrogating ultrafast reaction intermediates (23). The transient behavior of the protonated 7-Aza monomer arising from the Coulomb explosion event (mass 119 amu) can be attributed only to a stepwise transfer process, with an associated minimal contribution to mass 119 due to the isotopic distributions of the constituent atoms.
Figure 2.
Depiction of the 7-Aza dimer as it undergoes a stepwise ESDPT. Masses are shown for the species that would arise at each step if Coulomb explosion were to break the dimer apart. Mass spectra are also shown at a time t and t + 600 fs depicting the rise seen in the mass 119 signal as the intermediate is formed and broken apart by Coulomb explosion. For the case of the reactive dimer, the mass 119 signal drops back to its original intensity, whereas in the case of the nonreactive dimer, the mass 119 species remains dominant over the mass 118 throughout the duration of the experiment.
One question that might be raised, however, is whether it is possible that the protonated 7-Aza monomer detected in the experiment arises merely from collisions between a 7-Aza monomer and a proton formed during the excitation process. An analysis of the density within the molecular beam and the possible number of collisions definitively establishes that this process could not account for the observed protonated 7-Aza monomer intensity. Assuming an ion–molecule reaction with an upper limit Langevin rate constant of 8 × 10−9 sec−1, we determined that under our experimental conditions, only a maximum of 0.8% of the mass 119 could result from an ion–molecule reaction. Moreover, if this were the origin of the species of mass 119, it would not show the temporal evolution with the femtosecond pump-probe delay times, as is observed in our Coulomb explosion experiments.
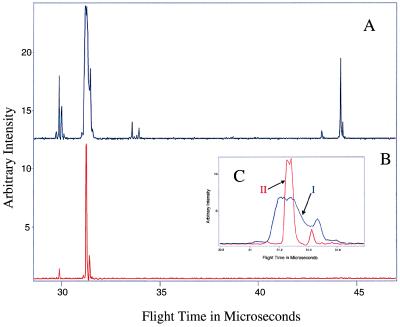
In addition, it was necessary to establish that Coulomb explosion was actually operative during the interrogation of the reaction via the intense probe pulse. The fact that Coulomb explosion is actually operative is demonstrated in Fig. 3. As can be seen, when the dimer is not present, the nascent 119-amu peak that is present is only because of the isotopic distribution of 7-Aza. When 7-Aza dimer is present, however, the intensity of the 119-amu species far exceeds the isotopic composition, thus proving that the 119-amu species is arising from the dimer. Furthermore, peak broadening of the 118- and 119-amu species illustrates that Coulomb explosion is the operative separation mechanism for the dimer (19, 21).
Figure 3.
(A) The spectrum shows that Coulomb explosion is evident in the mass 118- and 119-amu fragments (I) when 7-Aza dimer is present in the molecular beam. (B) Spectrum showing the absence of Coulomb explosion in the mass 118- and 119-amu species when the dimer is not present in the molecular beam. The residual mass 119 seen is primarily caused by the C, N, and H isotopic composition of the monomer. (C) Overlapped expanded view of the mass 118- and 119-amu region showing the difference between Coulomb exploded (I) and non-Coulomb exploded (II) fragments. Reprinted from ref. 11, Copyright 1998, with permission from Elsevier Science.
Cluster Production and Experimental Procedures.
A cluster beam of 7-Aza was formed by heating the compound (Sigma) to about 150°C, followed by supersonic expansion into a vacuum with a helium backing gas. The helium pressure used depended on the desired characteristics of the generated cluster beam. A lower backing pressure [≈300 torr (1 torr = 133 Pa)] resulted in the almost exclusive formation of the reactive dimer, whereas higher pressures (≈3,000 torr) resulted in the near-exclusive formation of nonreactive dimer. Backing pressures between these values led to cluster beams containing both reactive and nonreactive dimer species. Subsequent experiments were conducted at 1,200 torr, where both reactive and nonreactive dimers were detectable, depending on which portion of the molecular beam was investigated.
Experiments to produce a dimer purposely solvated with a water molecule were accomplished by bubbling the helium backing gas through a water cell before introduction into the sample holder. Once formed, the desired cluster species were interrogated by using pump-probe transient spectroscopy. The ionized species were then analyzed with a reflectron time-of-flight mass spectrometer. The femtosecond laser pulses were generated by using a colliding pulse mode-locked ring dye laser. The laser pulses had a pulse width of ≈200 fs, were centered at 620 nm, and had an energy after amplification of around 1.5 mJ per pulse. Pump-probe experiments were performed by using a 310-nm pump beam and a 620-nm probe beam. The 310-nm pump beam was prepared by separating 50% of the fundamental and focusing it through a beta barium borate crystal to generate the second harmonic. The 310-nm pump and the 620-nm probe beam were then colinearly recombined and focused (40-cm lens) into the mass spectrometer to intercept the molecular beam.
Results and Discussion
Studies of the Nonreactive Dimer.
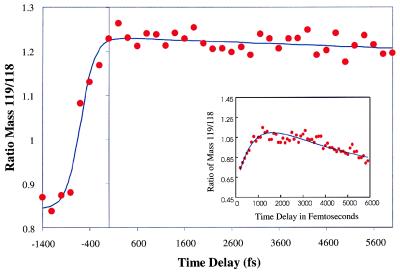
In recent experiments reported here, we have used our Coulomb explosion technique to interrogate the nature of the nonreactive dimer. The method was performed in an identical manner to the technique used to study the reactive dimer, i.e., with a weak 310-nm pump pulse and a strong Coulomb explosion inducing 620-nm probe pulse (11). We observed a Coulomb exploded mass 119 species that grew in around the laser overlap time and then remained constant for the remainder of the experiment (unlike the reactive dimer). The temporal behavior of the nonreactive dimer is shown in Fig. 4, where the ratio of the mass 119 to the mass 118 species generated for this cluster is plotted with respect to the delay between the pump and probe laser beam. Fig. 4 Inset shows the Coulomb explosion transient for the reactive dimer where, relative to the species of mass 118, the mass 119 species exhibits a rise and fall corresponding to two proton transfers. The rise of the 119 species in the nonreactive dimer transient is suggestive of the formation of an intermediate species by means of a single-proton transfer, but of a species that is unable to complete the second proton transfer to form the tautomer. This result is consistent with the suggestion that the nonreactive dimer is a conformational isomer that is conducive only to a single-proton transfer, but that does not facilitate the completion of the two-step process. Such a result could be imagined from the suggestions put forth by Lopez-Martens et al. (17) of a displaced biparallel or out-of-plane geometry that is not favorably aligned to allow for a double-proton transfer on excitation.
Figure 4.
Coulomb explosion transient for the nonreactive dimer depicting the ratio of mass 119- to 118-amu with respect to time delay. The rise time as determined by the fit for the nonreactive dimer is less than 600 fs. (Inset) Coulomb explosion transient for the reactive dimer τ1 = 660 fs and τ2 = 5 ps.
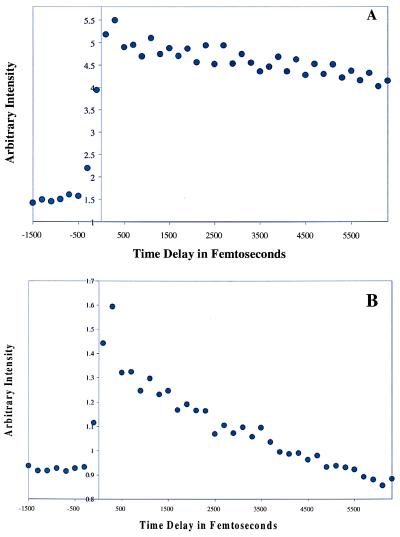
If one proton were being transferred, as the Coulomb explosion data suggest, then the optical transient for the nonreactive dimer (Fig. 5A) would also be expected to show this in the form of a fast decay. Hence, experiments were conducted of the ionization yield as a function of pump-probe delay at low fluences for the bare singly and doubly hydrated nonreactive dimer in a matter similar to that used by Zewail (see Fig. 5). The expected optical transient for the bare nonreactive dimer is not seen in Fig. 5A. The seemingly conflicting results that these two techniques show could be explained if the ionization cross section for the nonreactive dimer with one transferred proton were the same as, or nearly identical to, the cross section for the normal nonreactive dimer. If the cross sections were the same, then one would not be able to see the single-proton transfer in the optical transient experiment. In view of the differences in molecular symmetry of the species studied under reactive vs. nonreactive conditions, differences in the cross section of the intermediate formed in the two situations could be expected.
Figure 5.
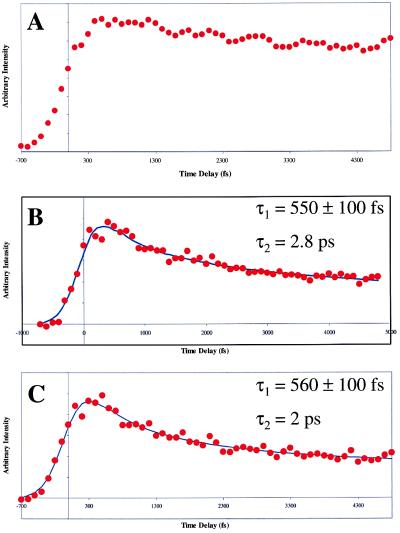
(A) Pump-probe transient of the nonreactive dimer at 1,200 torr. It can be seen from the lack of a fast decay that proton transfer is not occurring in this species. (B) Pump-probe transient of the nonreactive dimer with one water clustered to it. It can be seen here that an attached water molecule actually facilitates the occurrence of the proton transfer in an otherwise nonreactive species. (C) Pump-probe transient of the nonreactive dimer with two waters clustered to it. The second step of the proton transfer can be seen to occur even more quickly with two waters present.
Another possible explanation is that, in the case of the nonreactive dimer, the Coulomb explosion technique is not yielding observation of a complete proton transfer event. Instead, it may be sampling a hydrogen atom arising from the hydrogen-bonding proton located between the nitrogen atoms on opposite dimer species that becomes displaced upon photoexcitation. If this were the case, the formation of the temporal dependent mass 119 species would result from forced separation of the dimer species upon Coulomb explosion. The optical transient experiment would not be able to detect an event of this nature.
Next, we address the question whether the presence of a water molecule bound to the 7-Aza dimer could be responsible for the nonreactive dimer, as suggested by one study in the literature (9). In the present communication, we present evidence from femtosecond pump-probe studies on the water-solvated dimer that establishes that the presence of a water molecule is not responsible for the formation of what Kaya termed a nonreactive dimer. In fact, our studies show that the presence of a water molecule clustered with the nonreactive dimer actually facilitates proton transfer in this species.
As one would expect, the reactive and nonreactive dimers were observed to exhibit very different pump-probe optical transients. As mentioned above, although only the reactive dimer is seen at low backing pressures and the nonreactive, at high pressures, both can be observed at intermediate pressures such as 1,200 torr. Fig. 6A depicts the pump-probe transient for the nonreactive dimer, whereas Fig. 6B shows data for the reactive dimer. Both transients were taken under identical conditions, except that the femtosecond ionization transient for the nonreactive dimer was taken with the laser intercepting the molecular beam after a delay of 20 μs, with respect to when the reactive dimer was interrogated. The transient of the reactive dimer was best fit with a biexponential function with time constants nearly identical to those obtained by Zewail (10) and deduced via our Coulomb explosion investigations (11). The transient of the nonreactive dimer, on the other hand, shows a long-lived decay with respect to the reactive dimer, which indicates that the double-proton transfer has not occurred. The fact that both species are present under the same experimental conditions points toward the original suggestion (7) that different structural isomers are responsible for the different reactive nature of the two experimental situations. This observation leads one to conclude that the temperature of the molecular beam is responsible for the formation of different isomers, rather than the bonding of a water molecule being responsible for the change in the ESDPT behavior.
Figure 6.
(A) Pump-probe transient of nonreactive dimer showing long decay. Backing pressure is ≈1,200 torr. (B) Pump-probe transient of reactive dimer showing characteristic decay caused by ESDPT. Backing pressure is ≈1,200 torr.
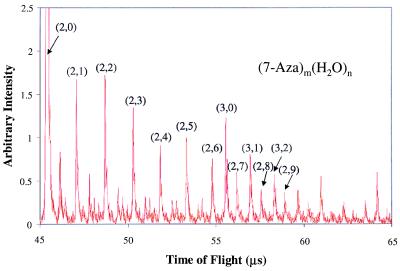
To further address this point, we subsequently investigated hydrated 7-Aza to examine whether water molecules incorporated with the dimer would promote the formation of a nonreactive dimer. A characteristic solvation mass spectrum is shown in Fig. 7. Typically, species of (7-Aza)2(H2O)n, where n = 0–9, were produced at concentrations sufficiently intense to be interrogated with pump-probe techniques. Again, both reactive and nonreactive dimer species could be produced in conjunction with higher-order clusters that have varying degrees of solvation. As mentioned above, species are considered reactive if they exhibit a fast decay in their pump-probe trace that can be fit best by using a biexponential function, whereas species are considered nonreactive if their pump-probe transients show a long-lived lifetime with respect to the reactive dimer.
Figure 7.
Water solvation mass spectra of 7-Aza expanded to show water clustering on the dimer. Clusters of (7-Aza)2(H2O)n, where n = 1–9, can readily be seen.
A significant finding is seen from the data displayed in Fig. 5 B and C. Here, solvation studies were carried out on a typical nonreactive dimer species obtained at intermediate backing pressures yielding the femtosecond ionization transient data displayed in Fig. 5A. With the addition of one water molecule to the dimer, the transient shows a pump-probe trace that is indicative of the occurrence of the double-proton transfer (see Fig. 5B). Transfer times correspond to 550 ± 100 fs for the first transfer and to about 2.8 ps for the second step. The same situation is seen in Fig. 5C, where two waters are attached to the dimer. Here proton transfer rates occur at 560 ± 100 fs and 2 ps, respectively. The above-mentioned transients show that a water molecule cannot be responsible for the formation of the nonreactive dimer and provides conclusive evidence that the conformational isomers resulting from temperature differences in the molecular beam are responsible for the changing reactivity of the 7-Aza dimer.
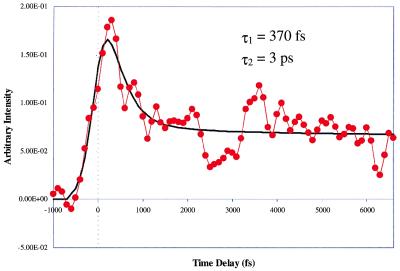
For comparison, Fig. 8 shows the case where a water molecule was attached to a species that exhibited reactive dimer characteristics in the unsolvated state; the species were produced at an intermediate backing pressure. If a water molecule was responsible for the formation of the nonreactive dimer, the species (7Aza)2H2O should show a long-lived transient. A long-lived species is not observed. Fig. 6A displays the transient for the dimer with no water attached, and it is readily seen that the trace is indicative of a reactive dimer. Fig. 8 exhibits the trace for the monohydrated reactive dimer. This trace also shows a pattern that can be fit with a biexponential function having transfer times, τ1 and τ2, of 370 fs and 3 ps, respectively. Our findings establish conclusively that double-proton transfer also occurs for the monohydrated dimer species.
Figure 8.
Pump-probe transient of the reactive dimer with one water clustered to it. The transient clearly shows that the presence of a water molecule does not inhibit the proton transfer from occurring.
The Effects of Progressive Hydration.
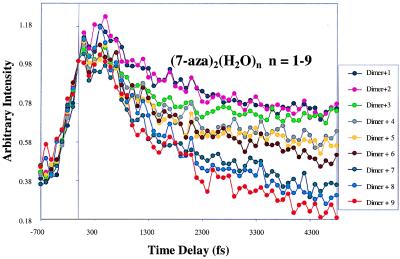
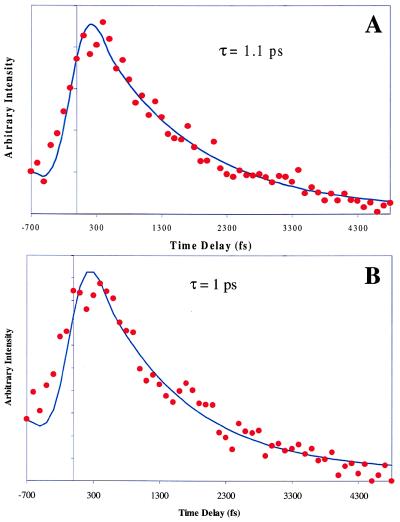
Pump-probe traces were obtained for (7-Aza)2(H2O)n, n = 0–9, as displayed in Fig. 9. For the experimental conditions used in conducting the experiments reported in this section, the unsolvated dimer species was found to exhibit a nonreactive pump-probe trace, whereas all solvated species show a fast decay illustrating the occurrence of double-proton transfer. An interesting phenomenon in this data set is the progression from a biexponential fit to a single exponential decay fit at progressively higher orders of solvation. Table 1 shows the proton transfer times obtained by fitting the data. The data for one, two, and three waters fit well to a biexponential function; however, the data from four to nine waters do not. A single exponential fitting function was found to successfully model the data for five, six, seven, eight, and nine waters. However, the data for four waters did not fit well with either a single or double exponential function. Fig. 10 depicts the single exponential decay fits for the dimer solvated with eight and nine waters. A compilation of both single and biexponential fit values is shown in Table 1.
Figure 9.
Data set showing normalized pump-probe transients for (7-Aza)2(H2O)n, where n = 1 to 9.
Table 1.
Proton transfer times for solvated 7-Aza dimers
| Number of waters | First transfer, fs | Second transfer, fs | Transfer time, fs |
|---|---|---|---|
| 1 | 550 ± 100 | 2,800 | |
| 2 | 565 ± 100 | 2,000 | |
| 3 | 560 ± 100 | 2,000 | |
| 4 | |||
| 5 | 1,800 | ||
| 6 | 1,600 | ||
| 7 | 1,300 | ||
| 8 | 1,100 | ||
| 9 | 1,000 |
Figure 10.
(A) Single exponential fit of the pump-probe transient for 7-Aza dimer solvated with eight water molecules. (B) Single exponential fit of the pump-probe transient for 7-Aza dimer solvated with nine water molecules.
Initially, it seemed that the transition from a biexponential to a single exponential process signified a shift from stepwise to concerted double-proton transfer. In light of Zewail and coworkers’ recent work (16), however, this seems unlikely. Alternately, instead of a transition from a two-step process to a concerted mechanism as the number of solvent molecules increases, it might be thought that the initial proton transfer could be occurring on a time scale too fast to be resolved with our current pulse width. But, in view of the trends in lifetimes with the degree of solvation that we have measured for one to three waters, this explanation seems unlikely, and we consider other possible reasons.
The anomaly shown in our data on the addition of four waters to the dimer, as shown in Table 1, is a clue; it might be explainable by conformational structure changes if they were to occur at a critical number of water molecules. In fact, preliminary calculations suggest that this may be the case (P. Hobza, personal communication). In calculations of the structure of a number of hydrogen-bonded base pairs, Hobza has found that hydration causes significant structural rearrangement and, with sufficient degrees, structural arrangements that can be described as card-stacked dimers. This argument would suggest the change from a hydrogen-bonded dimer to a stacked dimer once enough water molecules become associated with the complex. The stacked dimer would no longer be hydrogen bonded and essentially would consist of two nearly isolated solvated monomer species. It is expected that only one of the two moieties in the dimer becomes excited by the pump laser pulse (25). If this were the case, the single exponential decay times observed in our experiments for five to nine waters could result from self-tautomerization of the excited 7-Aza moiety by means of a water molecule (26, 27) acting as a catalytic proton bridge (28). Further experiments are under way to further elucidate this behavior.
Conclusions
On the basis of the results presented in the previous section, it is clear that the clustering of a water molecule with the 7-Aza dimer is not responsible for the formation of the nonreactive dimer. Instead, we conclude that Kaya’s original assumption is correct that the temperature of the molecular beam results in the formation of two different structural isomers, one that can undergo ESDPT, and another that cannot (7). Even though the structure of the reactive dimer is well known and documented, currently we can only speculate about the structure of the nonreactive dimer despite the information presented in this study. Kaya suggested three possible structures for the nonreactive dimer: T shaped, single bonded, and out of plane (8). High-resolution laser-induced fluorescence (LIF) studies in conjunction with simulated LIF structures for each species led Kaya and coworkers to conclude that the T-shaped structure is favored (9). However, in considering our findings that a water molecule actually enhances the reaction of the nonreactive dimer originally discussed by Kaya, we believe that Lopez-Martens et al.’s suggestion (17) that a biparallel dimer with out-of-plane hydrogen atoms is a reasonable possibility for the structure of the nonreactive dimer.
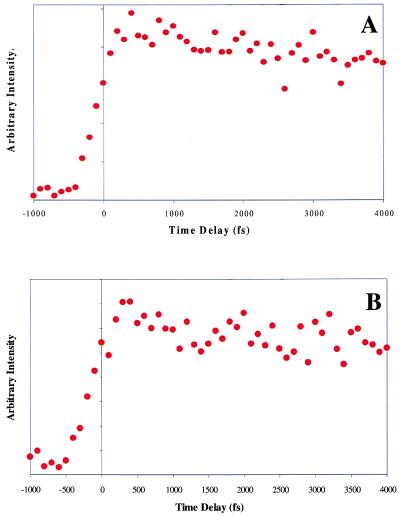
We report herein further evidence toward the behavior of the nonreactive dimer, with studies performed at much higher backing pressures. Under these expansion conditions, the resulting clusters are much cooler because of an increased number of collisions, resulting in the formation of a more rigid nonreactive conformational isomer. This nonreactive behavior is illustrated with optical transients presented in Fig. 11, where it is shown that the addition of water to the nonreactive dimer formed under the most intense expansion conditions does not result in reactive behavior. However, at warmer clustering conditions produced with an intermediate backing pressure, the incorporation of a water molecule allows the nonreactive dimer to undergo proton transfer as in Fig. 5B. If the water molecule were located above such a displaced biparallel structure, one could imagine that the first step in the double-proton transfer occurs as a result of interactions with the water. The water molecule might thereby accept a proton from the five-membered ring nitrogen of the 7-Aza, which could result in a transfer of a proton, initially bonded to the water, to the other 7-Aza’s six-membered ring nitrogen and could therefore influence the temporal characteristics. This situation could be envisioned to take place for species having one, two, and three waters, thus explaining the biexponential behavior of the transients before stacking occurs at higher solvation levels.
Figure 11.
High backing pressure (3,000 torr) 7-Aza pump-probe transients. (A) Nonreactive 7-Aza dimer. (B) Pump-probe transient maintaining nonreactive behavior even with the addition of one water molecule.
The question remains why the nonreactive dimer created at higher backing pressures does not exhibit reactive behavior on addition of water. A likely explanation is that at higher backing pressures, a different nonreactive conformational isomer is formed that does not allow water to act as a bridge for the proton-transfer reactions. This behavior has been exhibited in the benzene dimer, where it has been shown that conformational isomers in addition to the T-shaped structure are formed at higher backing pressures (29). In conjunction with a theoretical study, the information presented here may yield additional clues regarding the true structure of the nonreactive dimer.
Acknowledgments
The authors thank Drs. R. Bernheim (Pennsylvania State University), P. Hobza (Academy of Sciences of the Czech Republic), J. Hynes (University of Colorado), J. Syage (Syagen Technology, Inc.), and A. Zewail (California Institute of Technology) for fruitful discussions. Funding by the Air Force Office of Scientific Research, Grant No. F49620–94-1–0162, is gratefully acknowledged.
Abbreviations
- ESDPT
excited state double-proton transfer
- amu
atomic mass unit
- 7-Aza
7-azaindole
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 28, 1998.
References
- 1.Castleman A W, Jr, Bowen K H., Jr J Chem Phys. 1996;100:12911–12944. [Google Scholar]
- 2.Liu K, Brown M G, Carter C, Saykally R J, Gregory J K, Clary D C. Nature (London) 1996;381:501–503. [Google Scholar]
- 3.Lee N, Keesee R G, Castleman A W., Jr J Colloid Interface Sci. 1980;75:555–565. [Google Scholar]
- 4.Holland P M, Castleman A W., Jr J Phys Chem. 1982;86:4181–4188. [Google Scholar]
- 5.Taylor C A, El Bayoumi M A, Kasha M. Proc Natl Acad Sci USA. 1969;63:253–260. doi: 10.1073/pnas.63.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuke K, Yoshiuchi H, Kaya K. J Phys Chem. 1984;88:5840–5844. [Google Scholar]
- 7.Fuke K, Kaya K. J Phys Chem. 1989;93:614–621. [Google Scholar]
- 8.Nakajima A, Ono F, Kihara Y, Ogawa A, Matsurbara K, Ishikawa K, Baba M, Kaya K. Laser Chem. 1995;15:167–181. [Google Scholar]
- 9.Nakajima A, Hirano M, Hasumi R, Kaya K, Watanabe H, Carter C C, Williamson J M, Miller T. J Phys Chem. 1997;101:392–398. [Google Scholar]
- 10.Douhal A, Kim S K, Zewail A H. Nature (London) 1995;378:260–263. doi: 10.1038/378260a0. [DOI] [PubMed] [Google Scholar]
- 11.Folmer D E, Poth L, Wisniewski E S, Castleman A W., Jr Chem Phys Lett. 1998;287:1–7. [Google Scholar]
- 12.Takeuchi S, Tahara T. Chem Phys Lett. 1997;277:340–346. [Google Scholar]
- 13.Takeuchi S, Tahara T. J Phys Chem A. 1998;102:7740–7753. [Google Scholar]
- 14.Chachisvilis M, Fiebig T, Douhal A, Zewail A H. J Phys Chem A. 1998;102:669–673. [Google Scholar]
- 15.Share P, Pereira M, Sarisky M, Repinec S, Hochstrasser R M. J. Lumin. 1991;48/49:204–208. [Google Scholar]
- 16.Fiebig, T., Chachisvilis, M., Manger, M. M., Zewail, A. H., Douhal, A., Garcia-Ochoa, I. & de La Hoz Ayuso, A. (1999) J. Phys. Chem., in press.
- 17.Lopez-Martens R, Long P, Solgadi D, Soep B, Syage J, Millie P. Chem. Phys Lett. 1997;273:219–226. [Google Scholar]
- 18.Purnell J, Snyder E M, Wei S, Castleman A W., Jr Chem Phys Lett. 1994;229:333–339. [Google Scholar]
- 19.Snyder E M, Wei S, Purnell J, Buzza S A, Castleman A W., Jr Chem. Phys Lett. 1996;248:1–7. [Google Scholar]
- 20.Card D A, Folmer D E, Sato S, Buzza S, Castleman A W., Jr J Phys Chem A. 1997;101:3417–3423. [Google Scholar]
- 21.Snyder E M, Buzza S A, Castleman A W., Jr Phys Rev Lett. 1996;77:3347–3349. doi: 10.1103/PhysRevLett.77.3347. [DOI] [PubMed] [Google Scholar]
- 22.Rose-Petruck C, Schafer K J, Barty C P J. Appl. Laser Plasma Radiat. II, Proc. SPIE-Int. Soc. Opt. Eng., v. 2523, 1995. pp. 272–283. [Google Scholar]
- 23.Poth L, Castleman A W., Jr J Phys Chem A. 1998;102:4075–4081. [Google Scholar]
- 24.Trushin S A, Fuss W, Schikarski T, Schmid W E, Kompa K L. J Chem Phys. 1997;106:9386–9389. [Google Scholar]
- 25.Douhal A, Guallar V, Moreno M, Lluch J M. Chem Phys Lett. 1996;256:370–376. [Google Scholar]
- 26.Chou P T, Martinez M L, Cooper W C, McMorrow D, Collins S T, Kasha M. J Phys Chem. 1992;96:5203–5205. [Google Scholar]
- 27.Chaban G, Gordon M S. J Phys Chem A. 1999;103:185–189. [Google Scholar]
- 28.Ando K, Hynes J T. J Mol Liq. 1995;64:25–37. [Google Scholar]
- 29.Scherzer W, Kratzschmar O, Selzle H L, Schlag E W. Z Naturforsch A. 1992;47:1248–1252. [Google Scholar]
- 30.Rouhi M. Chem Eng News. 1998;76(8):17–18. [Google Scholar]