Abstract
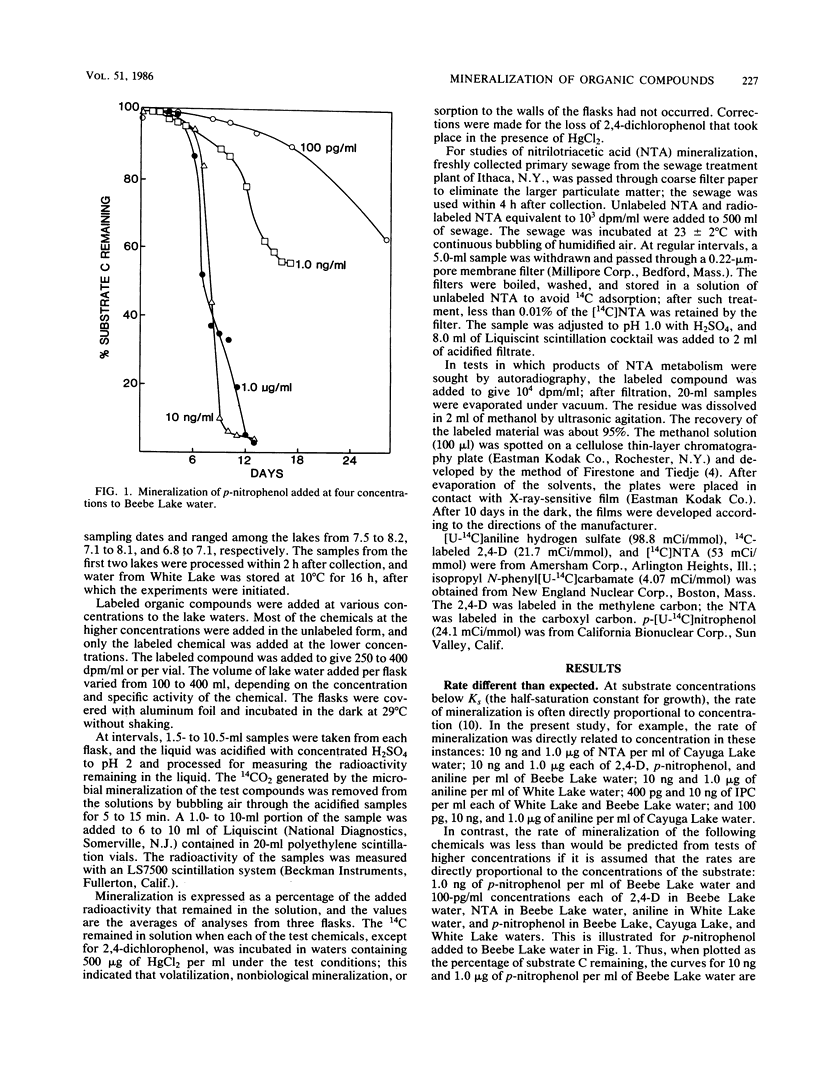
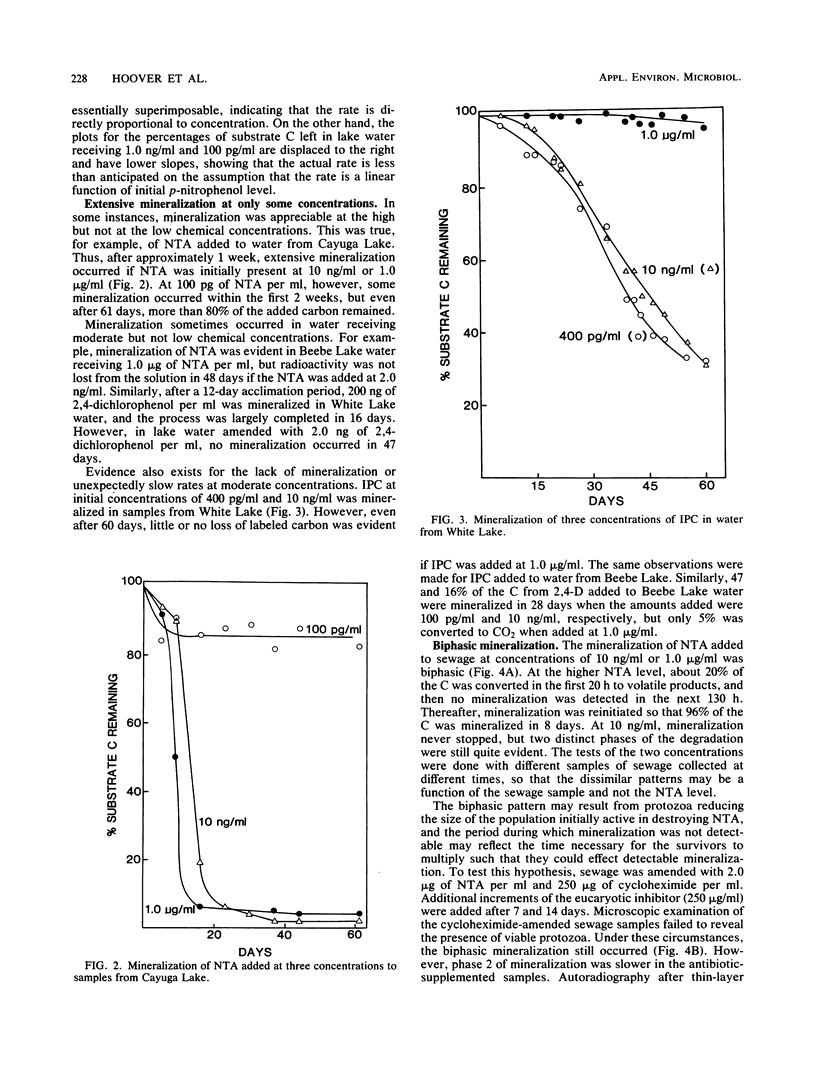
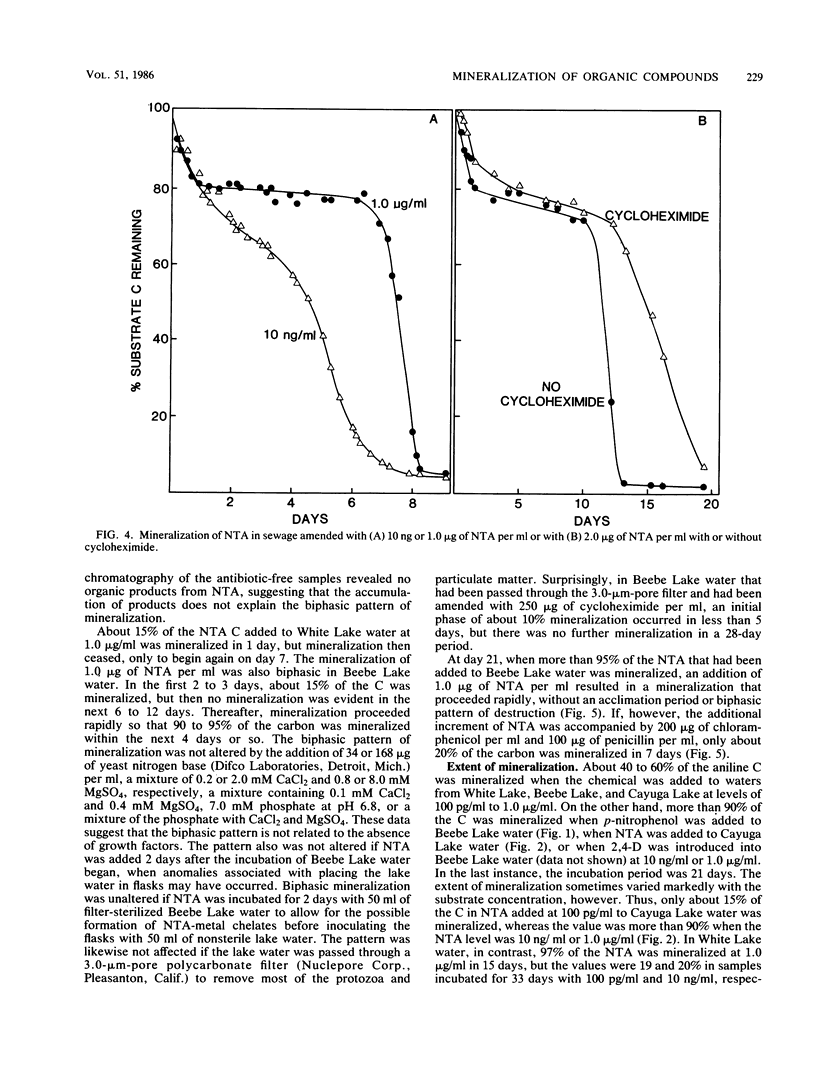
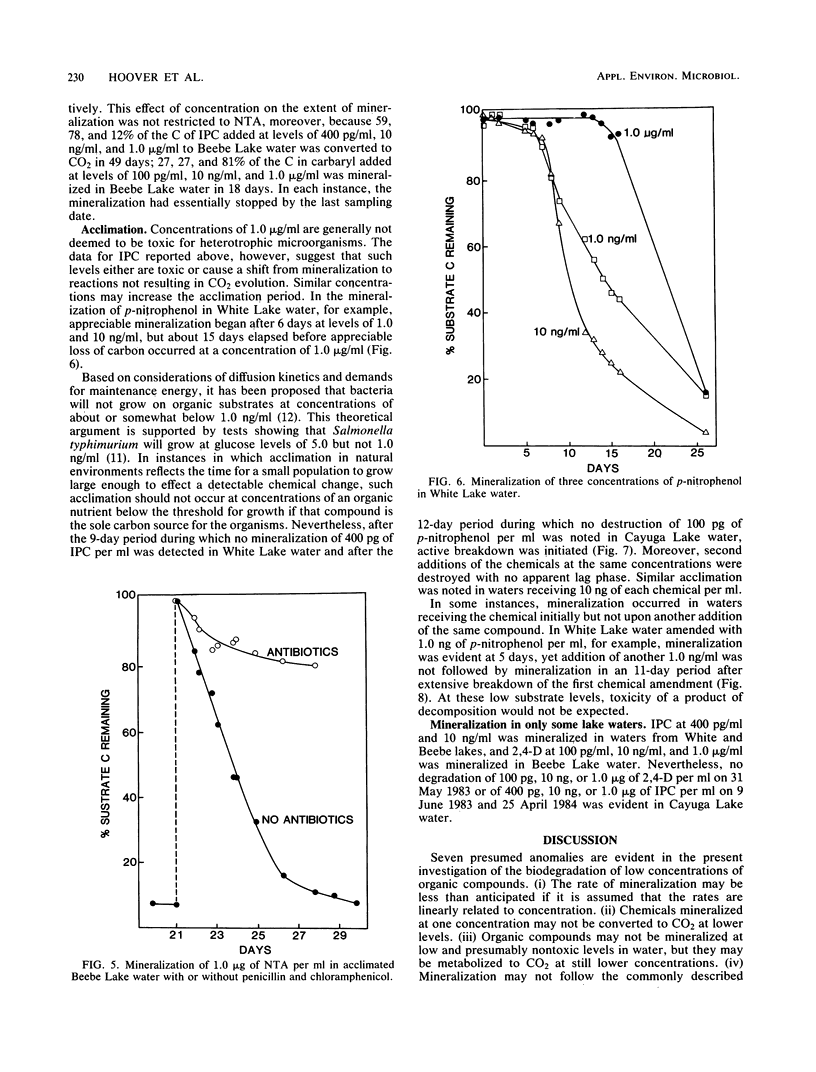
The rates of mineralization of nitrilotriacetic acid (NTA), 2,4-dichlorophenoxyacetic acid (2,4-D), p-nitrophenol, aniline, and isopropyl N-phenylcarbamate (IPC) at one or more concentrations ranging from 100 pg/ml to 1.0 microgram/ml were proportional to chemical concentrations in samples of three lakes. The rates at 100 pg of NTA, 2,4-D, p-nitrophenol, and aniline per ml in samples of one or more lakes were less than predicted, assuming the rates were linearly related to the concentration. Neither NTA nor 2,4-dichlorophenol at 2.0 ng/ml was mineralized in some lake waters, but higher levels of the two chemicals were converted to CO2 in samples of the same waters. In samples from two lakes, little or no mineralization of IPC or 2,4-D occurred at 1.0 microgram/ml, but 10 ng/ml or lower levels of the herbicides were mineralized. The mineralization in sewage of 1.0 microgram of NTA per ml was biphasic; about 20% of the substrate was mineralized in 20 h, and mineralization was only reinitiated after a period of 130 h. The biphasic transformation was not a result of the accumulation of organic products, and it was still evident if protozoan activity was inhibited. NTA also underwent a biphasic mineralization in lake waters, and the biphasic pattern was not altered by additions of growth factors and inorganic nutrients. From 40 to 60% of the carbon of aniline added to lake water at levels of 100 pg/ml to 1.0 microgram/ml was mineralized, but more than 90% of the carbon of NTA, 2,4-D, or p-nitrophenol added to lake water at 10 ng/ml or 1.0 microgram/ml was mineralized.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Pathway of degradation of nitrilotriacetate by a Pseudomonas species. Appl Environ Microbiol. 1978 May;35(5):955–961. doi: 10.1128/aem.35.5.955-961.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Wang C. H. How close to the theoretical diffusion limit do bacterial uptake systems function? Arch Microbiol. 1982 Feb;131(1):36–42. doi: 10.1007/BF00451496. [DOI] [PubMed] [Google Scholar]
- Rubin H. E., Subba-Rao R. V., Alexander M. Rates of mineralization of trace concentrations of aromatic compounds in lake water and sewage samples. Appl Environ Microbiol. 1982 May;43(5):1133–1138. doi: 10.1128/aem.43.5.1133-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Alexander M. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl Environ Microbiol. 1985 Apr;49(4):822–827. doi: 10.1128/aem.49.4.822-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A. Adaptation of natural microbial communities to degradation of xenobiotic compounds: effects of concentration, exposure time, inoculum, and chemical structure. Appl Environ Microbiol. 1983 Feb;45(2):428–435. doi: 10.1128/aem.45.2.428-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. S., Subba-Rao R. V., Alexander M. Effect of substrate concentration and organic and inorganic compounds on the occurrence and rate of mineralization and cometabolism. Appl Environ Microbiol. 1984 Jun;47(6):1195–1200. doi: 10.1128/aem.47.6.1195-1200.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


