Abstract
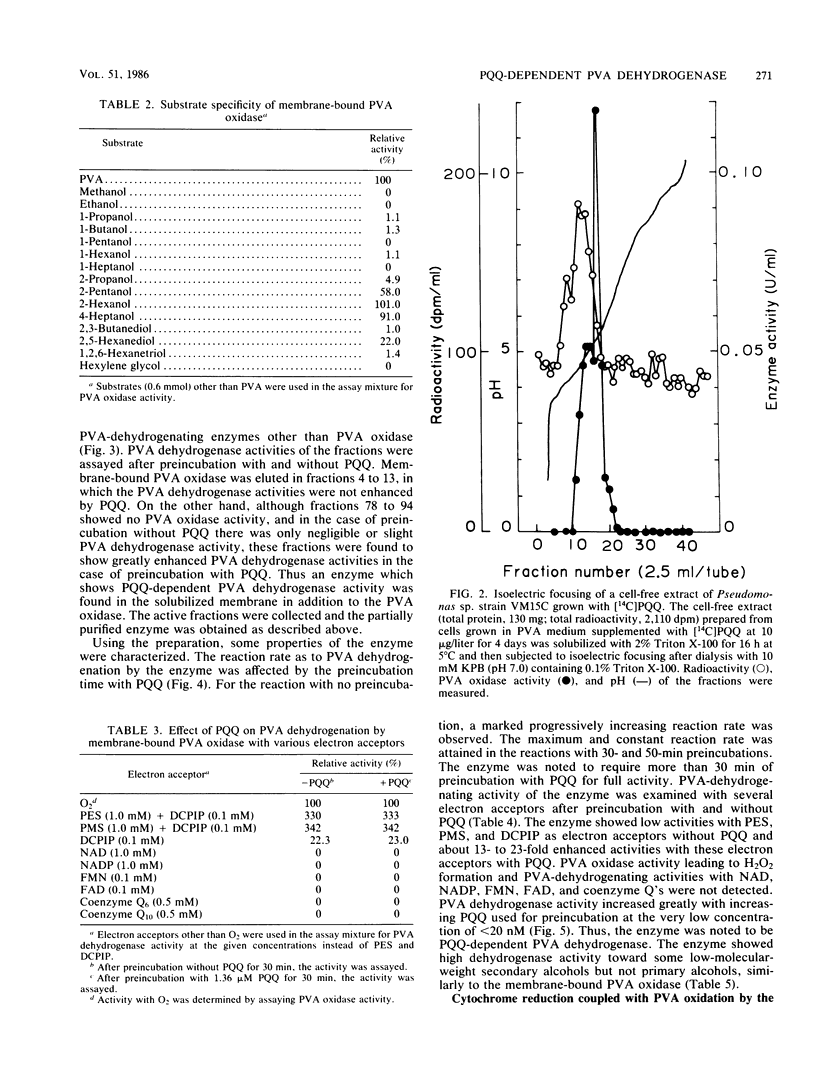
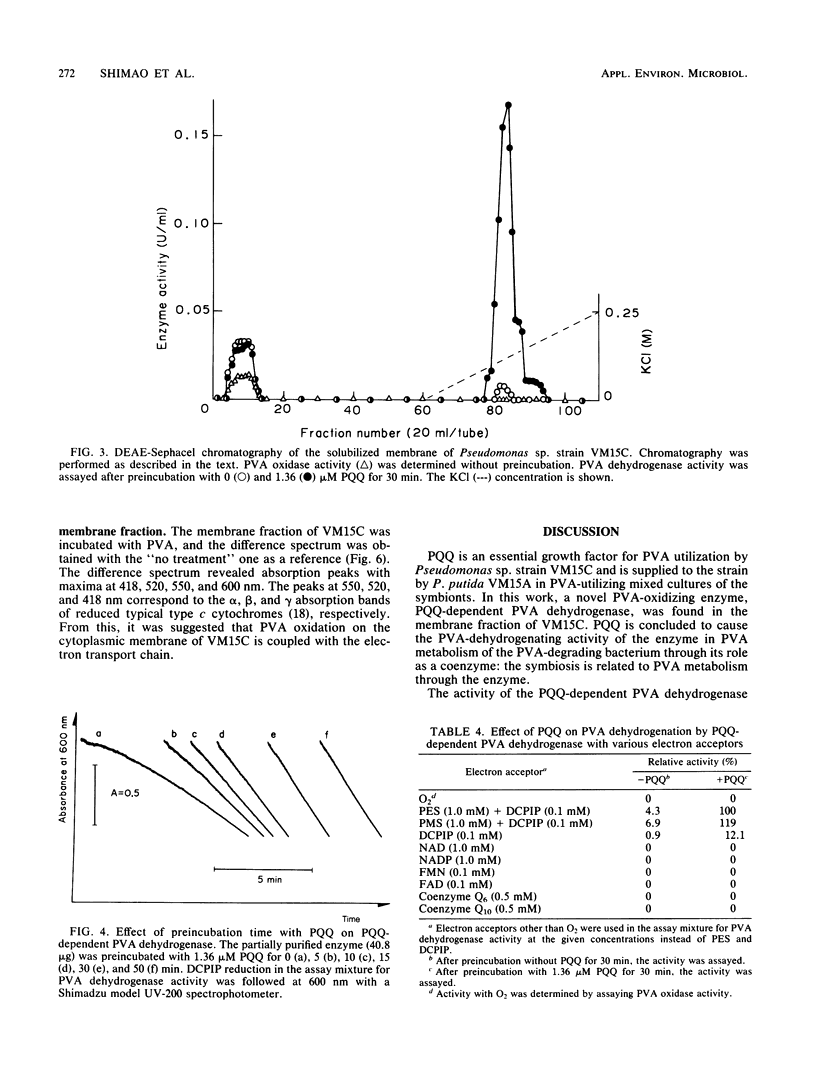
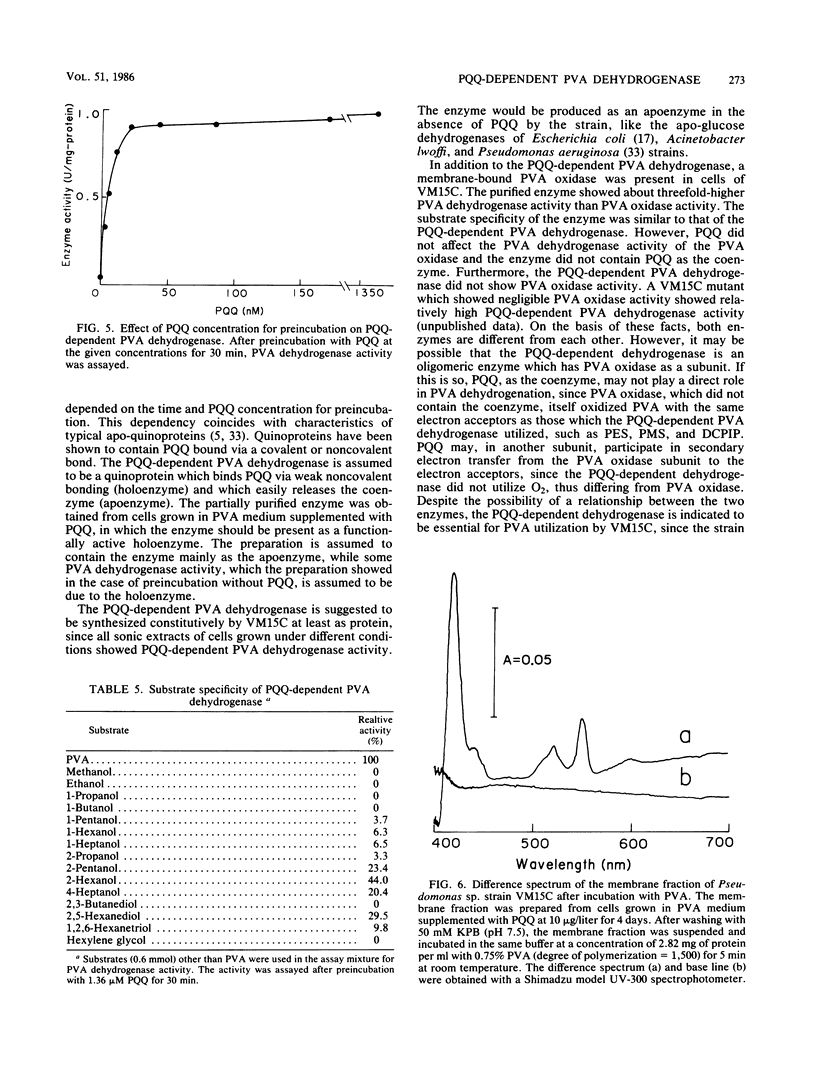
A novel enzyme, pyrroloquinoline quinone (PQQ)-dependent polyvinyl alcohol (PVA) dehydrogenase, was found in and partially purified from the membrane fraction of a PVA-degrading symbiont, Pseudomonas sp. strain VM15C. The enzyme required PQQ for PVA dehydrogenation with phenazine methosulfate, phenazine ethosulfate, and 2,6-dichlorophenolindophenol as electron acceptors and did not show PVA oxidase activity leading to H2O2 formation. The enzyme was active toward low-molecular-weight secondary alcohols rather than primary alcohols. A membrane-bound PVA oxidase was also present in cells of VM15C. Although the purified oxidase showed a substrate specificity similar to that of PQQ-dependent PVA dehydrogenase and about threefold-higher PVA-dehydrogenating activity with phenazine methosulfate or phenazine ethosulfate than PVA oxidase activity with H2O2 formation, it was shown that the enzyme does not contain PQQ as the coenzyme, and PQQ did not affect its activity. Incubation of the membrane fraction of cells with PVA caused a reduction in the cytochrome(s) of the fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Singer T. P. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Ameyama M., Matsushita K., Ohno Y., Shinagawa E., Adachi O. Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria. FEBS Lett. 1981 Aug 3;130(2):179–183. doi: 10.1016/0014-5793(81)81114-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Berkhout M. P. NAD-dependent, PQQ-containing methanol dehydrogenase: a bacterial dehydrogenase in a multienzyme complex. FEBS Lett. 1984 Mar 26;168(2):217–221. doi: 10.1016/0014-5793(84)80249-5. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J. Quinoprotein alcohol dehydrogenase from a non-methylotroph, Acinetobacter calcoaceticus. J Gen Microbiol. 1981 Feb;122(2):201–209. doi: 10.1099/00221287-122-2-201. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Groen B., Frank J., Jr, Duine J. A. Quinoprotein alcohol dehydrogenase from ethanol-grown Pseudomonas aeruginosa. Biochem J. 1984 Nov 1;223(3):921–924. doi: 10.1042/bj2230921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., SASAGAWA M., KUSAI K., NAKAI M., OKUNUKI K. Preparation of crystalline Pseudomonas cvtochrome c-551 and its general properties. Biochem J. 1960 Oct;77:194–201. doi: 10.1042/bj0770194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobenstein-Verbeek C. L., Jongejan J. A., Frank J., Duine J. A. Bovine serum amine oxidase: a mammalian enzyme having covalently bound PQQ as prosthetic group. FEBS Lett. 1984 May 21;170(2):305–309. doi: 10.1016/0014-5793(84)81333-2. [DOI] [PubMed] [Google Scholar]
- Sakazawa C., Shimao M., Taniguchi Y., Kato N. Symbiotic utilization of polyvinyl alcohol by mixed cultures. Appl Environ Microbiol. 1981 Jan;41(1):261–267. doi: 10.1128/aem.41.1.261-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury S. A., Forrest H. S., Cruse W. B., Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979 Aug 30;280(5725):843–844. doi: 10.1038/280843a0. [DOI] [PubMed] [Google Scholar]
- Shimao M., Fujita I., Kato N., Sakazawa C. Enhancement of Pyrroloquinoline Quinone Production and Polyvinyl Alcohol Degradation in Mixed Continuous Cultures of Pseudomonas putida VM15A and Pseudomonas sp. Strain VM15C with Mixed Carbon Sources. Appl Environ Microbiol. 1985 Jun;49(6):1389–1391. doi: 10.1128/aem.49.6.1389-1391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Fukuta I., Kato N., Sakazawa C. Mixed Continuous Cultures of Polyvinyl Alcohol-Utilizing Symbionts Pseudomonas putida VM15A and Pseudomonas sp. Strain VM15C. Appl Environ Microbiol. 1984 Oct;48(4):751–754. doi: 10.1128/aem.48.4.751-754.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Nishimura Y., Kato N., Sakazawa C. Localization of Polyvinyl Alcohol Oxidase Produced by a Bacterial Symbiont, Pseudomonas sp. Strain VM15C. Appl Environ Microbiol. 1985 Jan;49(1):8–10. doi: 10.1128/aem.49.1.8-10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Saimoto H., Kato N., Sakazawa C. Properties and roles of bacterial symbionts of polyvinyl alcohol-utilizing mixed cultures. Appl Environ Microbiol. 1983 Sep;46(3):605–610. doi: 10.1128/aem.46.3.605-610.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Taniguchi Y., Shikata S., Kato N., Sakazawa C. Production of polyvinyl alcohol oxidase by a symbiotic mixed culture. Appl Environ Microbiol. 1982 Jul;44(1):28–32. doi: 10.1128/aem.44.1.28-32.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer R., Duine J. A., Frank J., Large P. J. The prosthetic group of methylamine dehydrogenase from Pseudomonas AM1: evidence for a quinone structure. Biochim Biophys Acta. 1980 Apr 25;622(2):370–374. doi: 10.1016/0005-2795(80)90050-1. [DOI] [PubMed] [Google Scholar]



