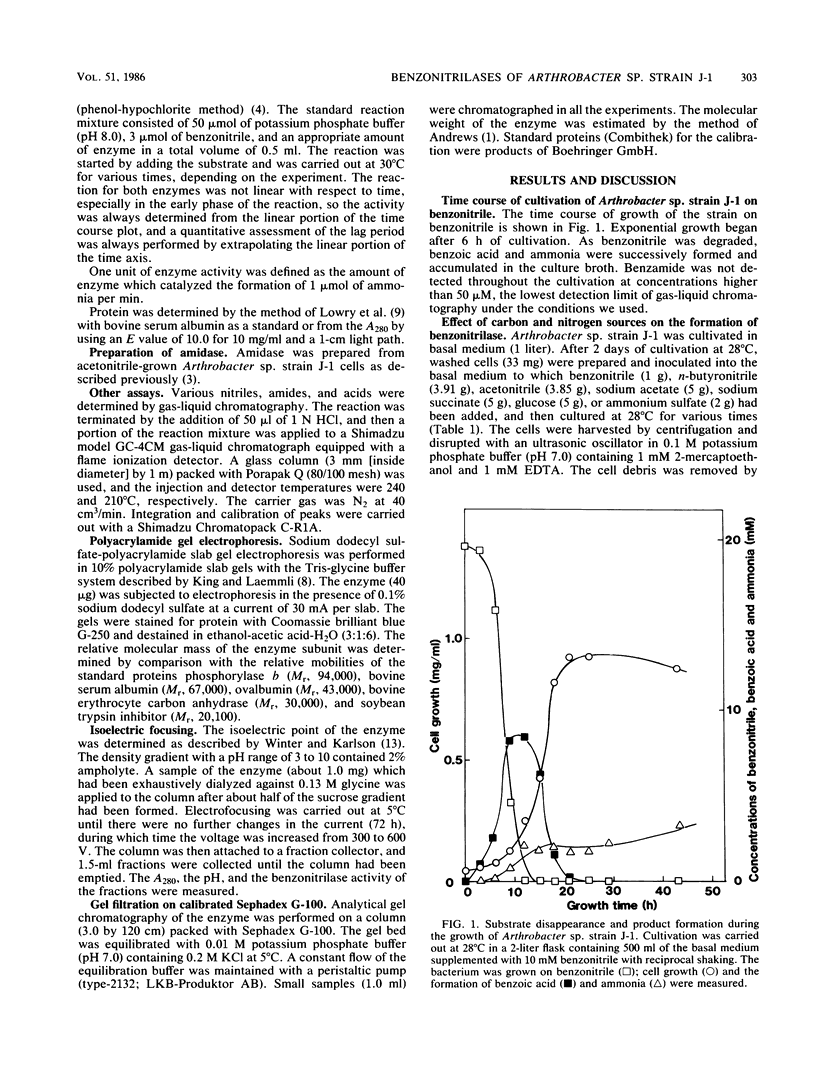
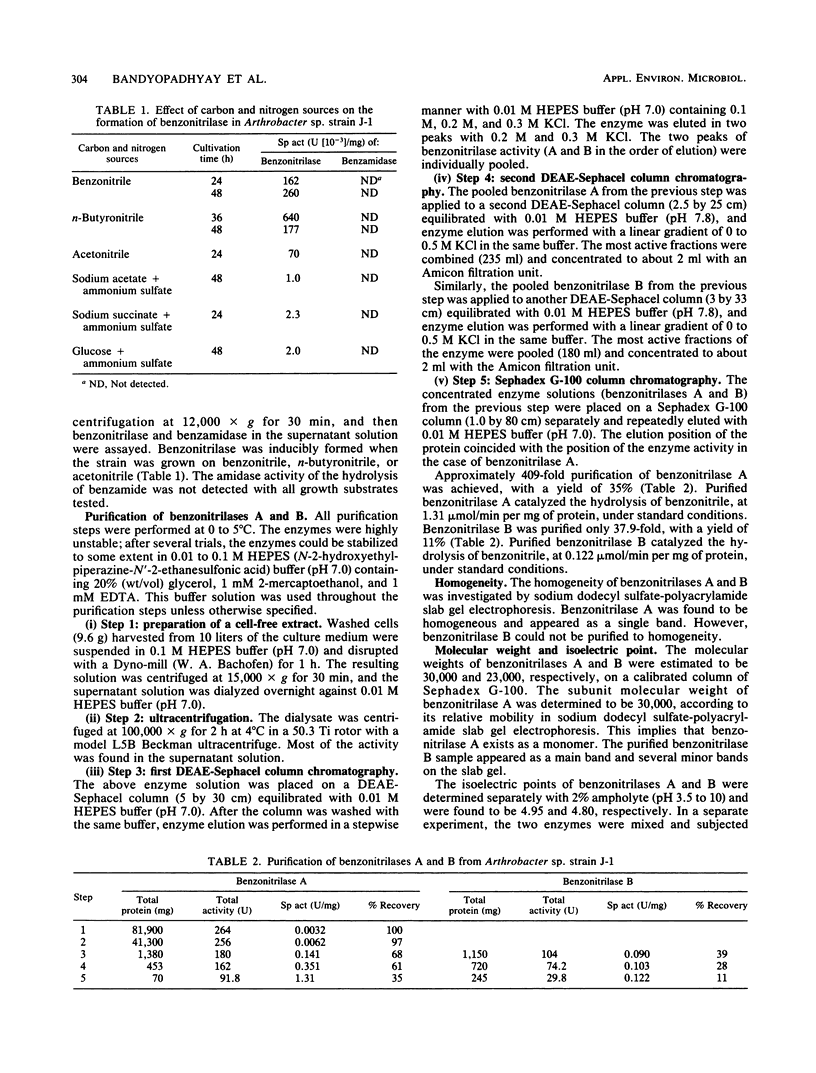
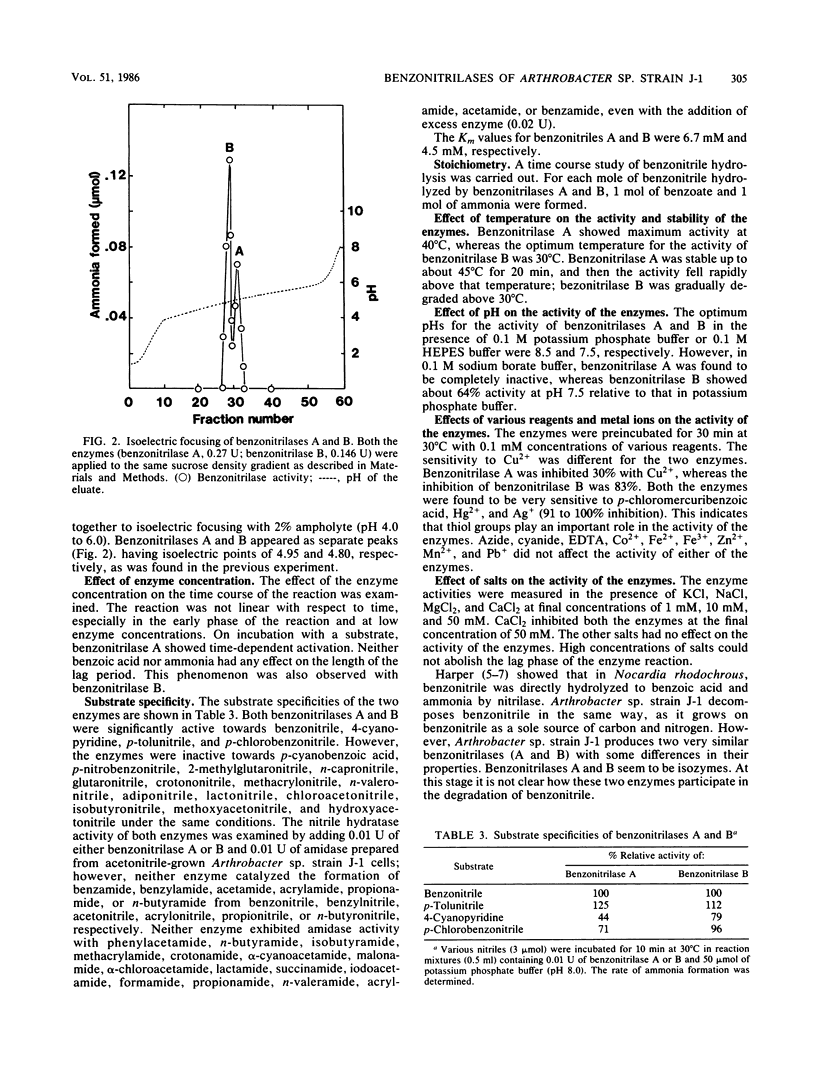
Abstract
We found two kinds of benzonitrilases, designated benzonitrilases A and B, in a cell extract of Arthrobacter sp. strain J-1 grown on benzonitrile as a sole carbon and nitrogen source. Benzonitrilases A and B were purified approximately 409-fold and 38-fold, respectively. Purified benzonitrilase A appeared to be homogeneous according to the criteria of polyacrylamide gel electrophoresis. Both the enzymes hydrolyzed benzonitrile to benzoic acid and ammonia without forming benzamide as an intermediate. The molecular weights of benzonitrilases A and B were found to be 30,000 and 23,000, respectively. The subunit molecular weight of benzonitrilase A was the same as its molecular weight. The isoelectric points of benzonitrilases A and B were 4.95 and 4.80, respectively. The optimum temperature and pH, respectively, for benzonitrilase A were 40°C and 8.5, and those for benzonitrilase B were 30°C and 7.5. The Km values for benzonitrilases A and B were 6.7 mM and 4.5 mM, respectively. Both the enzymes degraded p-tolunitrile, 4-cyanopyridine, and p-chlorobenzonitrile, but they did not attack aliphatic nitriles or amides. Both the enzymes were inhibited by thiol reagents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. B. Fungal degradation of aromatic nitriles. Enzymology of C-N cleavage by Fusarium solani. Biochem J. 1977 Dec 1;167(3):685–692. doi: 10.1042/bj1670685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. B. Microbial metabolism of aromatic nitriles. Enzymology of C-N cleavage by Nocardia sp. (Rhodochrous group) N.C.I.B. 11216. Biochem J. 1977 Aug 1;165(2):309–319. doi: 10.1042/bj1650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. B. Purification and properties of an unusual nitrilase from Nocardia N.C.I.B. 11216. Biochem Soc Trans. 1976;4(3):502–504. doi: 10.1042/bst0040502. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]


