Abstract
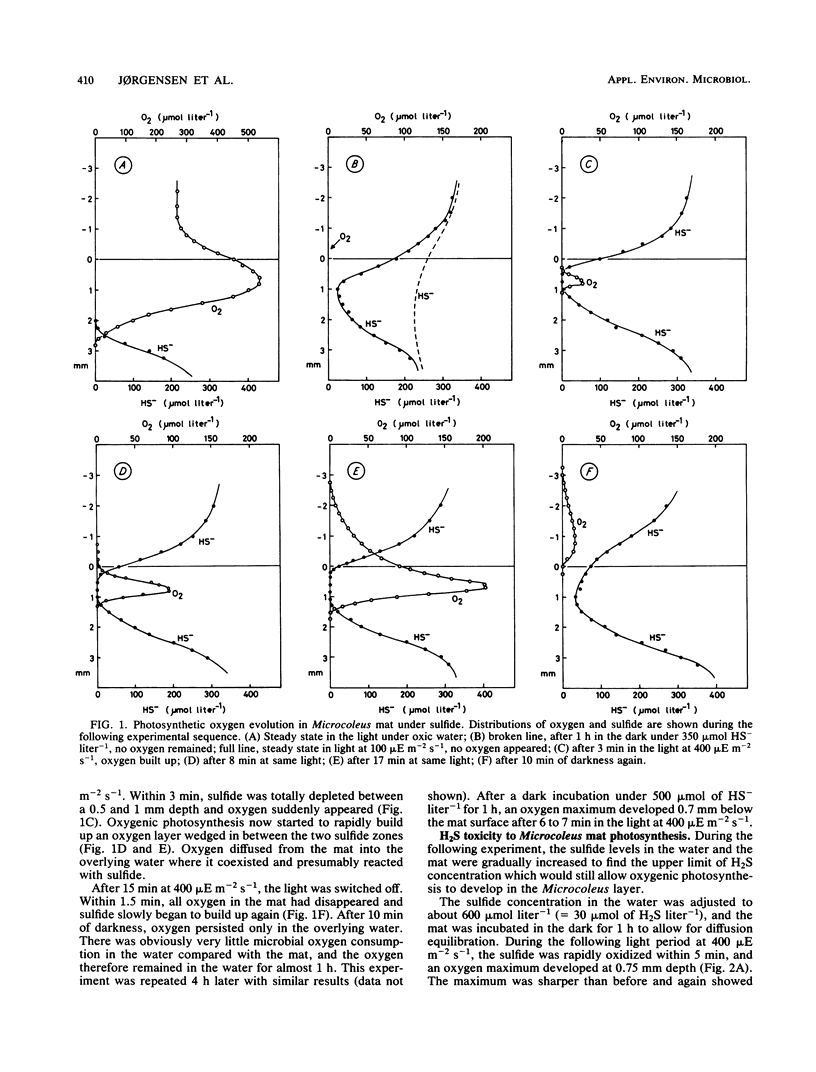
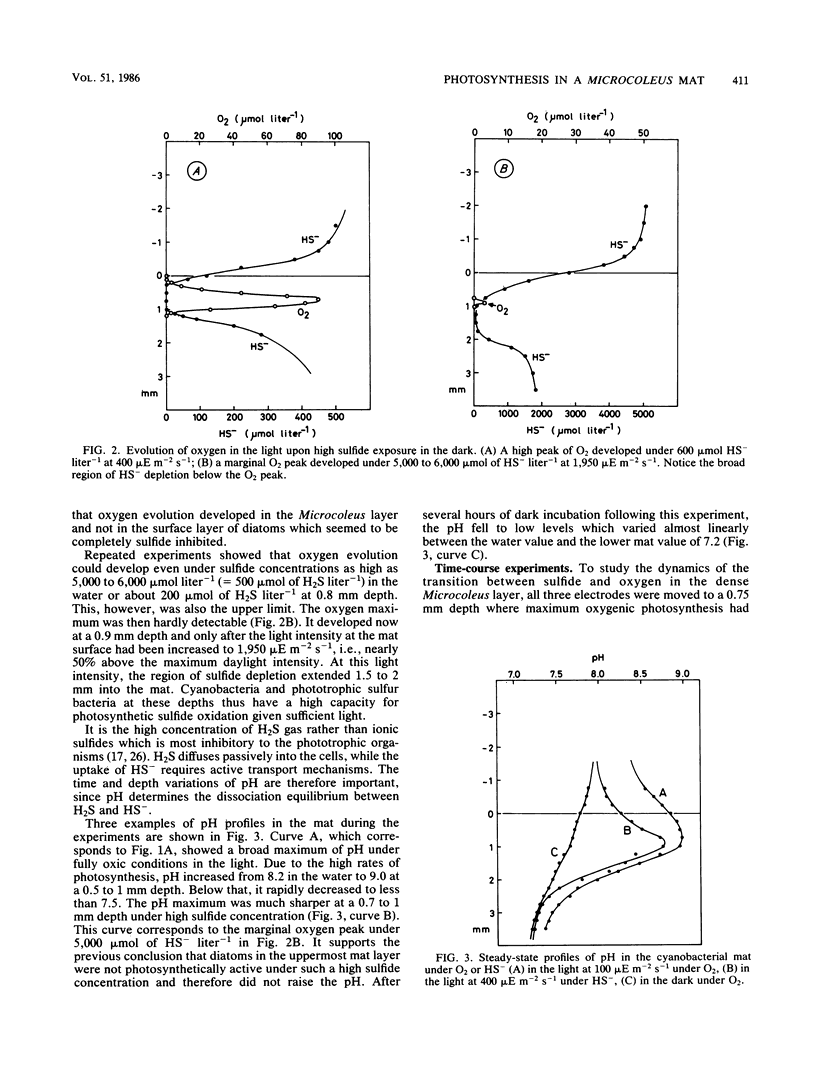
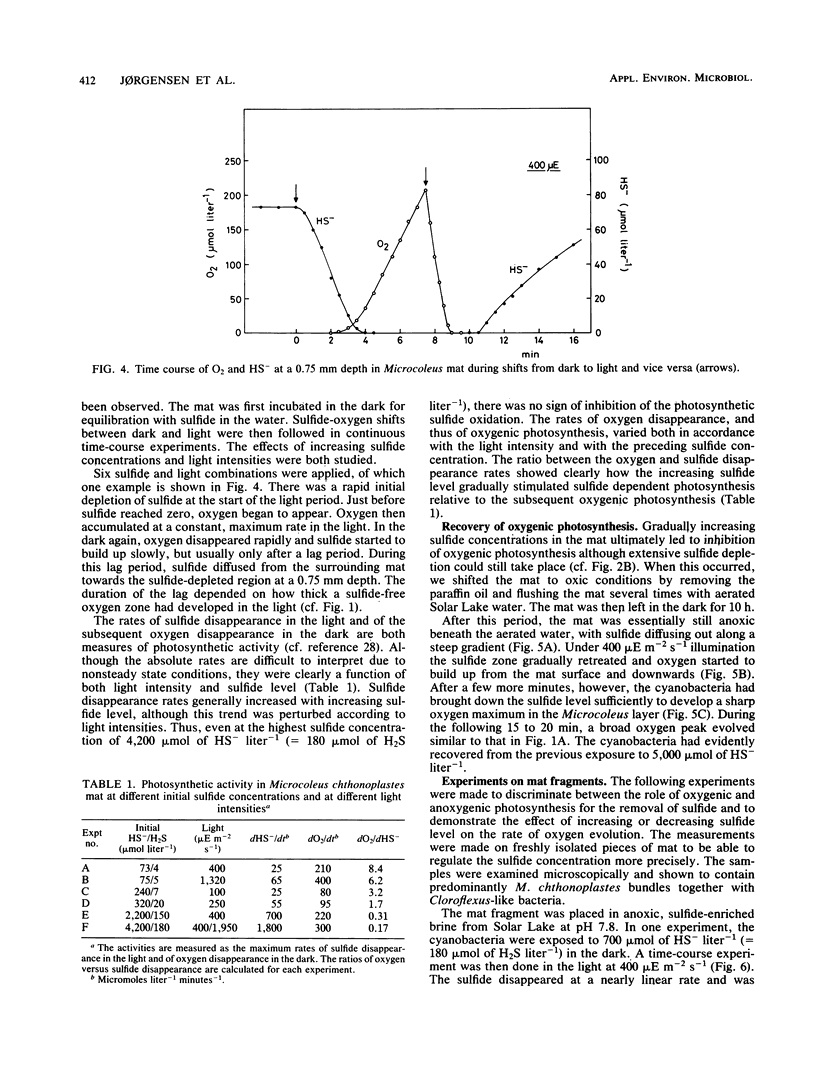
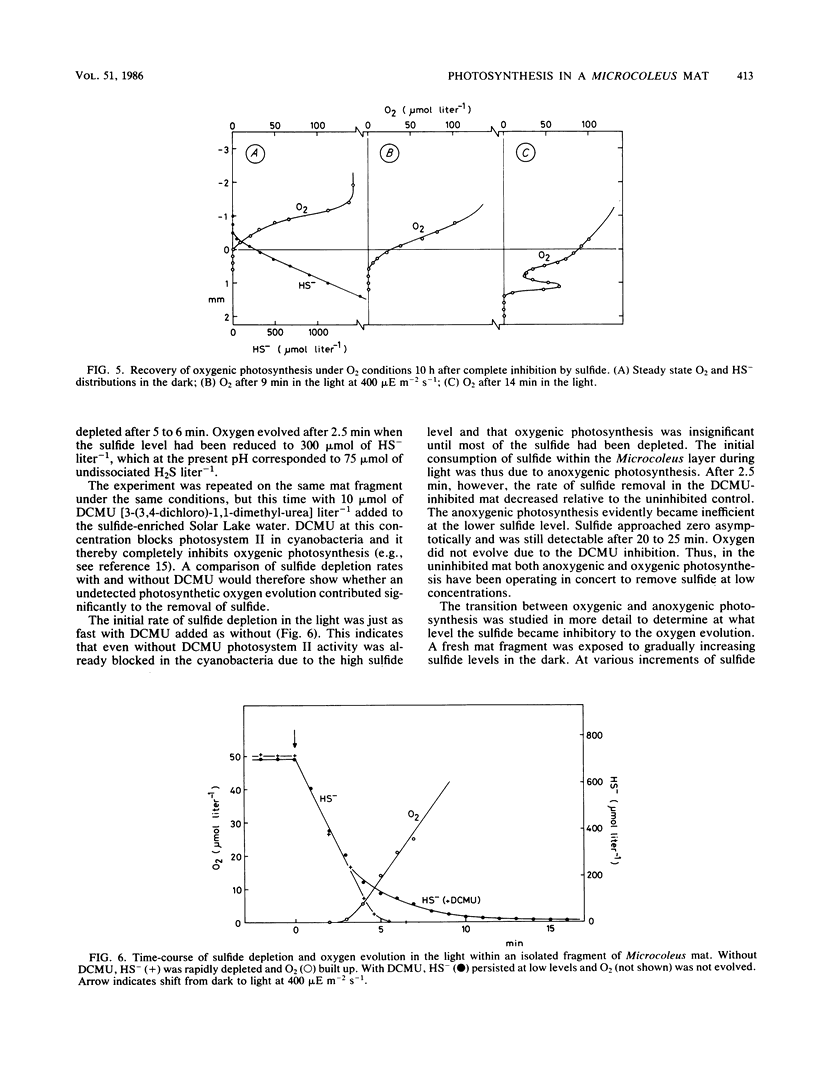
Benthic cyanobacterial mats with the filamentous Microcoleus chthonoplastes as the dominant phototroph grow in oxic hypersaline environments such as Solar Lake, Sinai. The cyanobacteria are in situ exposed to chemical variations between 200 μmol of sulfide liter−1 at night and 1 atm pO2 during the day. During experimental H2S to O2 transitions the microbial community was shown to shift from anoxygenic photosynthesis, with H2S as the electron donor, to oxygenic photosynthesis. Microcoleus filaments could carry out both types of photosynthesis concurrently. Anoxygenic photosynthesis dominated at high sulfide levels, 500 μmol liter−1, while the oxygenic reaction became dominant when the sulfide level was reduced below 100 to 300 μmol liter−1 (25 to 75 μmol of H2S liter−1). An increasing inhibition of the oxygenic photosynthesis was observed upon transition to oxic conditions from increasing sulfide concentrations. Oxygen built up within the Microcoleus layer of the mat even under 5 mmol of sulfide liter−1 (500 μmol of H2S liter−1) in the overlying water. The implications of such a localized O2 production in a highly reducing environment are discussed in relation to the evolution of oxygenic photosynthesis during the Proterozoic era.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen Y., Jørgensen B. B., Revsbech N. P., Poplawski R. Adaptation to Hydrogen Sulfide of Oxygenic and Anoxygenic Photosynthesis among Cyanobacteria. Appl Environ Microbiol. 1986 Feb;51(2):398–407. doi: 10.1128/aem.51.2.398-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y., Padan E., Shilo M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1975 Sep;123(3):855–861. doi: 10.1128/jb.123.3.855-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P., Blackburn T. H., Cohen Y. Diurnal cycle of oxygen and sulfide microgradients and microbial photosynthesis in a cyanobacterial mat sediment. Appl Environ Microbiol. 1979 Jul;38(1):46–58. doi: 10.1128/aem.38.1.46-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Padan E. Induction of anaerobic, photoautotrophic growth in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1978 Feb;133(2):558–563. doi: 10.1128/jb.133.2.558-563.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D., Doemel W., Brock T. D. Requirement of low oxidation-reduction potential for photosynthesis in a blue-green alga (Phormidium sp.). Arch Microbiol. 1975 Jun 20;104(1):7–13. doi: 10.1007/BF00447293. [DOI] [PubMed] [Google Scholar]


