Abstract
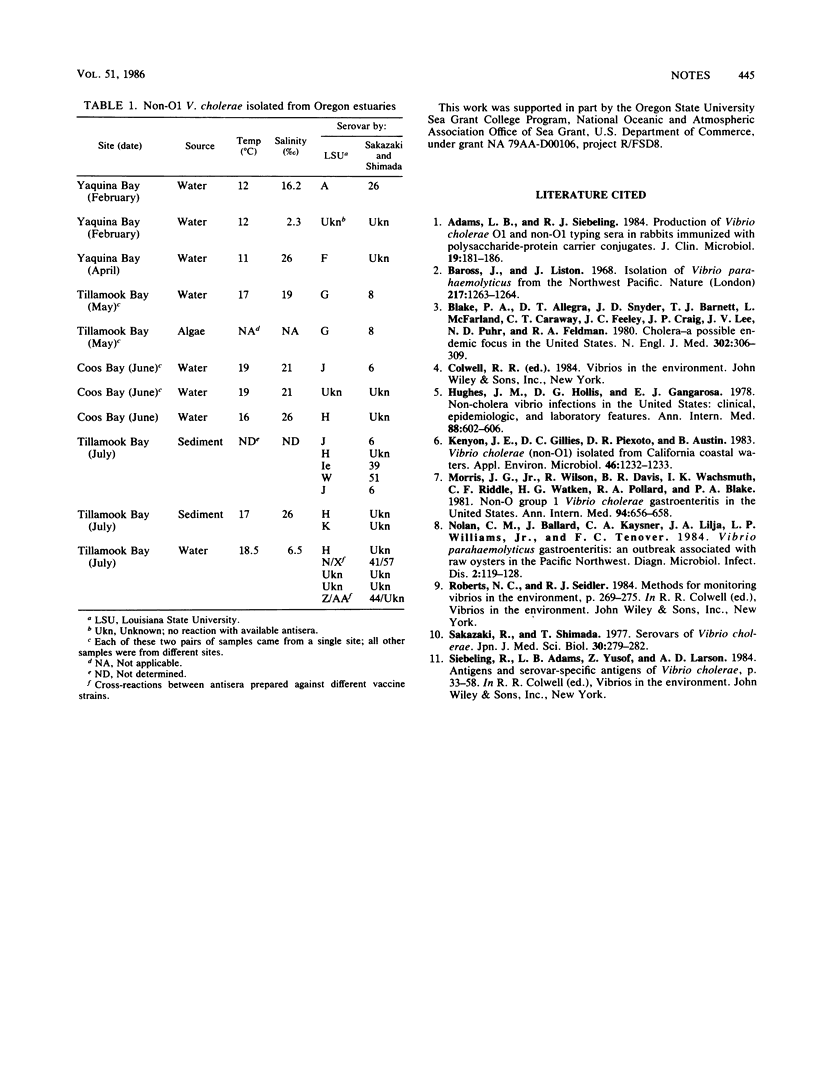
Water, sediment, and shellfish from three Oregon estuaries were cultured for pathogenic Vibrio species. Non-O1 serovars of V. cholerae were the most common pathogenic Vibrio species recovered. Non-O1 V. cholerae were isolated from all three estuaries sampled, covering an area of about 170 miles along the Oregon coast. Non-O1 V. cholerae were isolated from water and sediment, but not shellfish, at temperatures ranging from 11 to 19°C and salinities of 2.3 to 26‰. Sixteen isolates representing 12 different non-O1 serovars were identified, while four non-O1 V. cholerae isolates failed to react with any of the 54 antisera tested. These results indicate that non-O1 V. cholerae serovars can be found over a large geographic area and under a variety of environmental conditions. These organisms are apparently an autochthonous component of these estuarine microbial communities.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Siebeling R. J. Production of Vibrio cholerae O1 and non-O1 typing sera in rabbits immunized with polysaccharide-protein carrier conjugates. J Clin Microbiol. 1984 Feb;19(2):181–186. doi: 10.1128/jcm.19.2.181-186.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baross J., Liston J. Isolation of vibrio parahaemolyticus from the Northwest Pacific. Nature. 1968 Mar 30;217(5135):1263–1264. doi: 10.1038/2171263a0. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Hollis D. G., Gangarosa E. J., Weaver R. E. Non-cholera vibrio infections in the United States. Clinical, epidemiologic, and laboratory features. Ann Intern Med. 1978 May;88(5):602–606. doi: 10.7326/0003-4819-88-5-602. [DOI] [PubMed] [Google Scholar]
- Kenyon J. E., Gillies D. C., Piexoto D. R., Austin B. Vibrio cholerae (non-O1) isolated from California coastal waters. Appl Environ Microbiol. 1983 Nov;46(5):1232–1233. doi: 10.1128/aem.46.5.1232-1233.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Wilson R., Davis B. R., Wachsmuth I. K., Riddle C. F., Wathen H. G., Pollard R. A., Blake P. A. Non-O group 1 Vibrio cholerae gastroenteritis in the United States: clinical, epidemiologic, and laboratory characteristics of sporadic cases. Ann Intern Med. 1981 May;94(5):656–658. doi: 10.7326/0003-4819-94-5-656. [DOI] [PubMed] [Google Scholar]
- Nolan C. M., Ballard J., Kaysner C. A., Lilja J. L., Williams L. P., Jr, Tenover F. C. Vibrio parahaemolyticus gastroenteritis. An outbreak associated with raw oysters in the Pacific northwest. Diagn Microbiol Infect Dis. 1984 Apr;2(2):119–128. doi: 10.1016/0732-8893(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Shimada T. Serovars of Vibrio cholerae identified during 1970-1975. Jpn J Med Sci Biol. 1977 Oct;30(5):279–282. doi: 10.7883/yoken1952.30.279. [DOI] [PubMed] [Google Scholar]


