Abstract
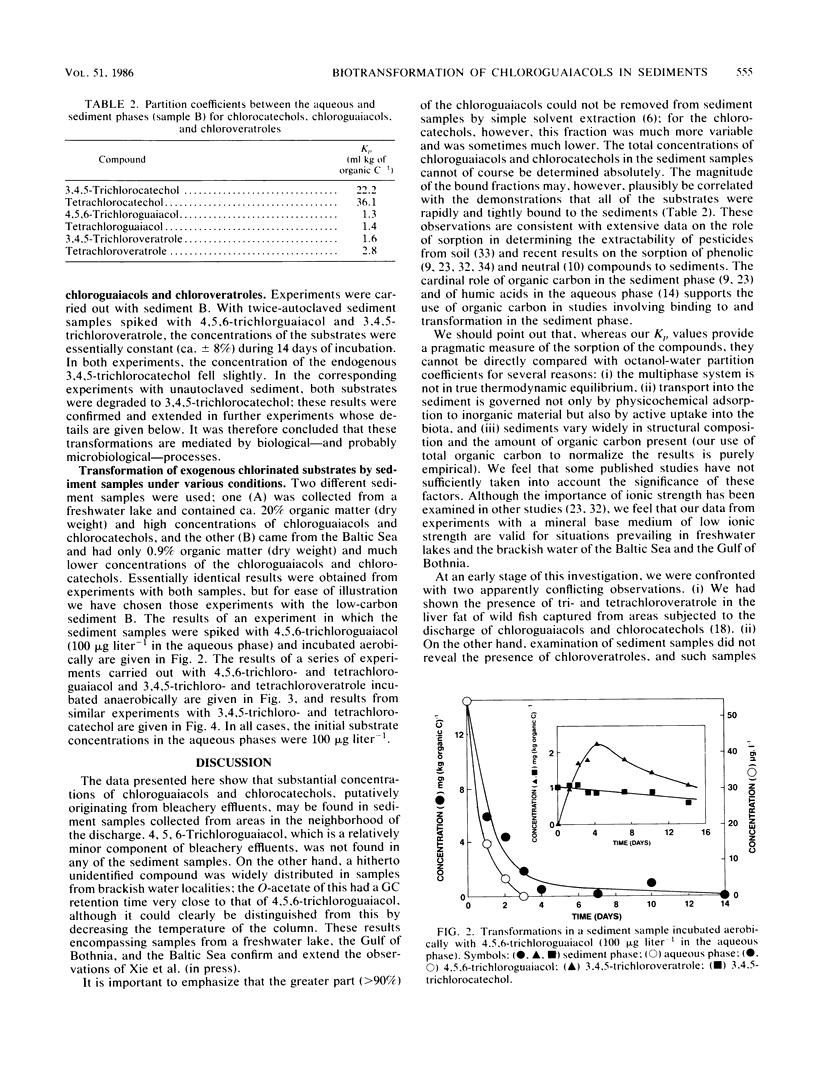
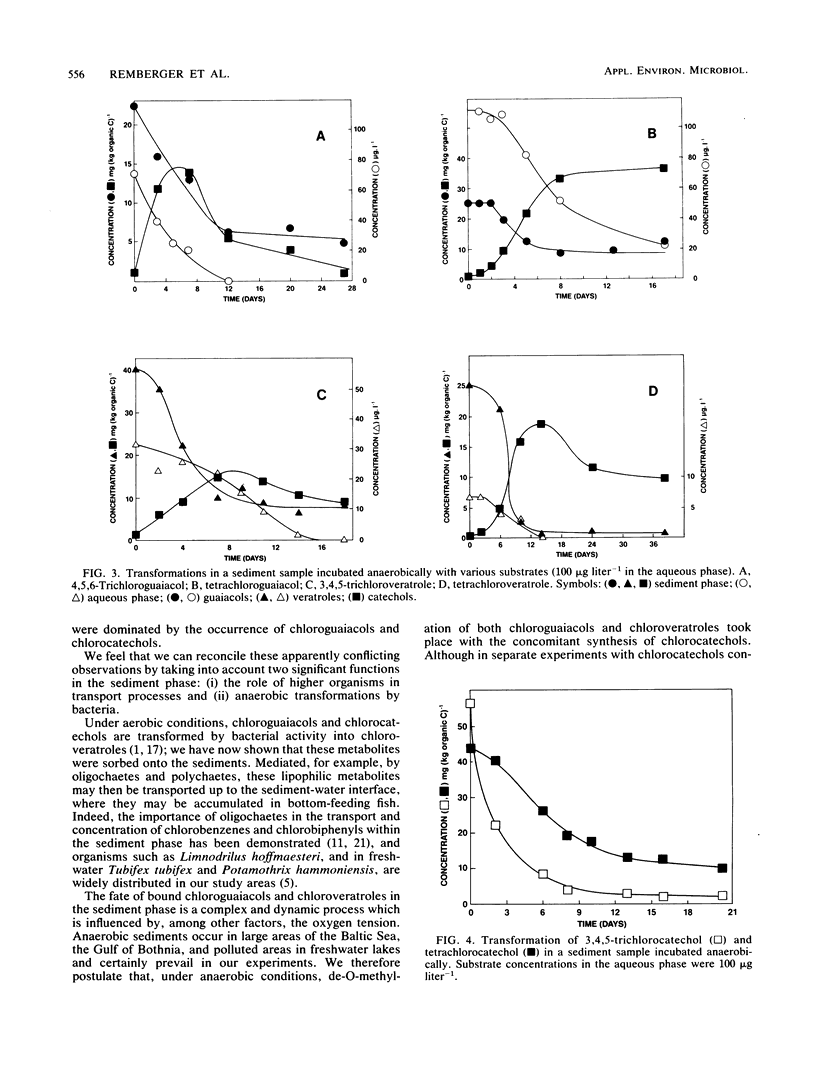
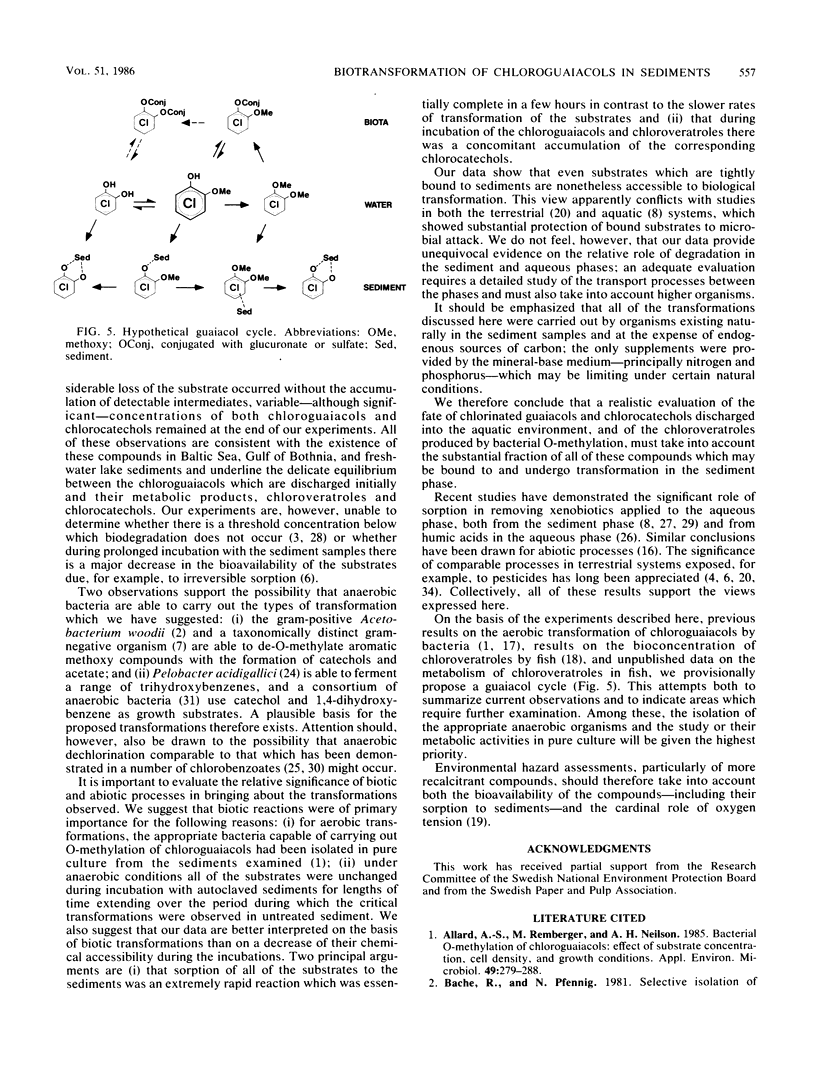
The occurrence of trichloro- and tetrachloroguaiacols, -catechols, and -veratroles and their transformation was studied in freshwater and brackish water sediments putatively exposed to bleachery discharge. The samples contained both chloroguaiacols and chlorocatechols, of which >90% could not be removed by simple extraction. The bound concentrations varied and ranged from 550 μg kg of organic C−1 for 3,4,5-trichloroguaiacol to 8,250 μg kg of organic C−1 for tetrachlorocatechol. Chlorinated substrates added to the aqueous phase were rapidly bound to the sediment with Kp values between 1.3 and 2.8 ml kg of organic C−1 for the chloroguaiacols and chloroveratroles and 22 to 36 ml kg of organic C−1 for the chlorocatechols. Sediment samples incubated aerobically brought about O-methylation of 4,5,6-trichloroguaiacol to 3,4,5-trichloroveratrole in a yield of ca. 25%. Under anaerobic conditions, however, de-O-methylation of both the chloroguaiacols and chloroveratroles took place with synthesis of the corresponding chlorocatechols. In separate experiments, the chlorocatechols were not completely stable under anaerobic conditions, but their ultimate fate has not yet been resolved. Sediment which had been autoclaved twice at 121°C for 20 min was unable to bring about any of these transformations; we therefore conclude that they were mediated by biological processes. These results emphasize that, in determining the fate of chloroguaiacols and related compounds discharged into the aquatic environment, the cardinal roles of sorption to the sediment phase and of the oxygen tension must be taken into account. We propose a hypothetical guaiacol cycle to accommodate our observations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard A. S., Remberger M., Neilson A. H. Bacterial o-methylation of chloroguaiacols: effect of substrate concentration, cell density, and growth conditions. Appl Environ Microbiol. 1985 Feb;49(2):279–288. doi: 10.1128/aem.49.2.279-288.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag J. M., Loll M. J. Incorporation of xenobiotics into soil humus. Experientia. 1983 Nov 15;39(11):1221–1231. doi: 10.1007/BF01990359. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. A gram-negative anaerobic bacterium that utilizes o-methyl substituents of aromatic acids. Appl Environ Microbiol. 1985 May;49(5):1345–1347. doi: 10.1128/aem.49.5.1345-1347.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Hynning P. A., Remberger M., Landner L. Bacterial methylation of chlorinated phenols and guaiacols: formation of veratroles from guaiacols and high-molecular-weight chlorinated lignin. Appl Environ Microbiol. 1983 Mar;45(3):774–783. doi: 10.1128/aem.45.3.774-783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogram A. V., Jessup R. E., Ou L. T., Rao P. S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol. 1985 Mar;49(3):582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R., Pfaender F. K. Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):402–407. doi: 10.1128/aem.49.2.402-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Alexander M. Effect of sorption on mineralization of low concentrations of aromatic compounds in lake water samples. Appl Environ Microbiol. 1982 Sep;44(3):659–668. doi: 10.1128/aem.44.3.659-668.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



