Abstract
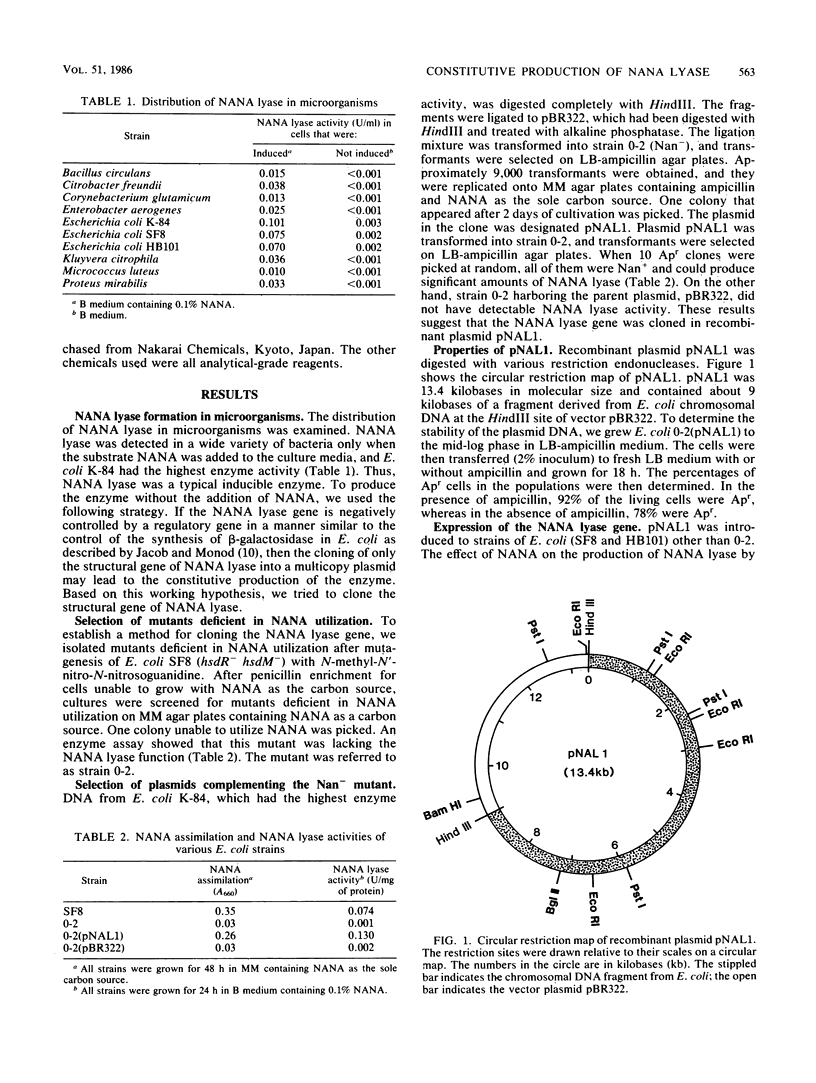
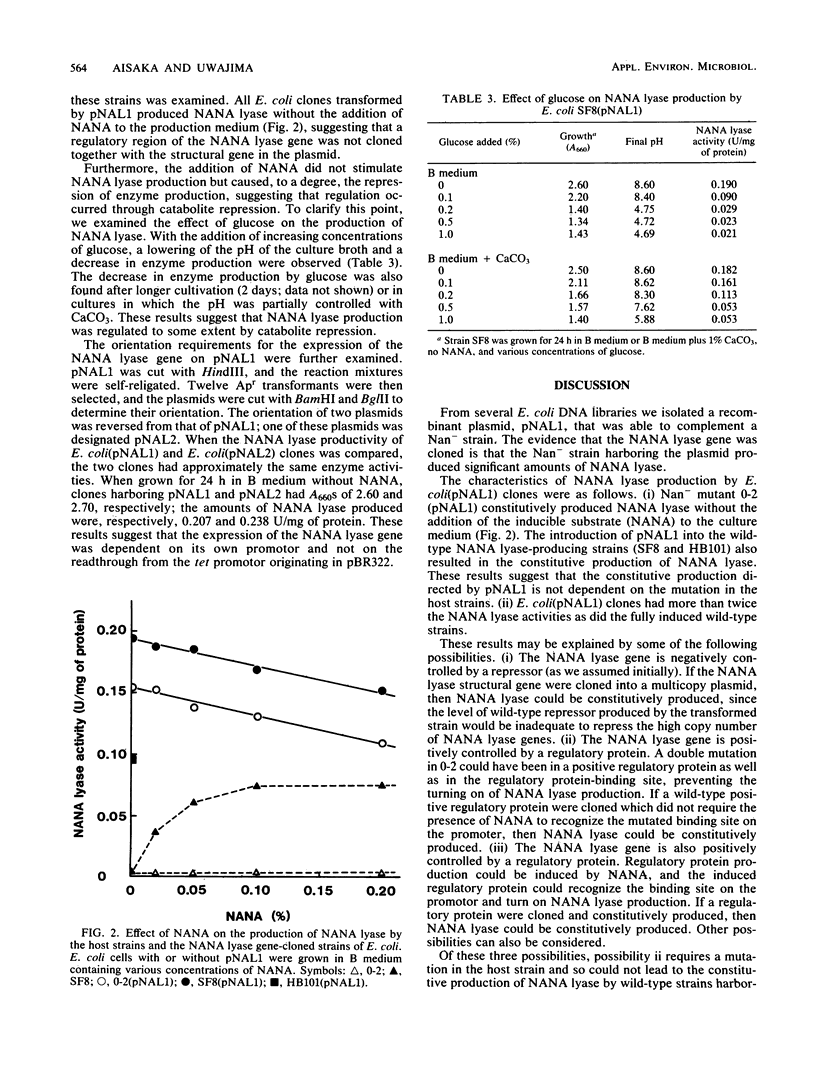
The N-acetylneuraminate (NANA) lyase (EC 4.1.3.3) gene from Escherichia coli was self-cloned in E. coli. Transformants were selected by complementation of a NANA lyase-deficient E. coli strain. One clone was found to produce NANA lyase, and it contained a recombinant plasmid, pNAL1, with a 9.0-kilobase HindIII insert. The cloning of the NANA lyase gene resulted in the change from inducible to constitutive production of the enzyme. The level of expression of the NANA lyase gene in E. coli(pNAL1) clones was two- to three-fold higher than that in the fully induced wild-type strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden S. B., Chang W. H., Barksdale L. Distribution of neuraminidase and n-acetylneuraminate lyase activities among corynebacteria, mycobacteria, and nocardias. J Bacteriol. 1972 Dec;112(3):1206–1212. doi: 10.1128/jb.112.3.1206-1212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960 Sep;235:2529–2537. [PubMed] [Google Scholar]
- Dnistrian A. M., Schwartz M. K. Lipid-bound sialic acid as a tumor marker. Ann Clin Lab Sci. 1983 Mar-Apr;13(2):137–142. [PubMed] [Google Scholar]
- Drzeniek R., Scharmann W., Balke E. Neuraminidase and N-acetylneuraminate pyruvate-lyase of Pasteurella multocida. J Gen Microbiol. 1972 Sep;72(2):357–368. doi: 10.1099/00221287-72-2-357. [DOI] [PubMed] [Google Scholar]
- Heimer R., Meyer K. STUDIES ON SIALIC ACID OF SUBMAXILLARY MUCOID. Proc Natl Acad Sci U S A. 1956 Oct;42(10):728–734. doi: 10.1073/pnas.42.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Molecular cloning of TOL genes xylB and xylE in Escherichia coli. J Bacteriol. 1981 Mar;145(3):1137–1143. doi: 10.1128/jb.145.3.1137-1143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Dahlbeck D., Staskawicz B., Belser W. Molecular cloning of pectate lyase genes from Erwinia chrysanthemi and their expression in Escherichia coli. J Bacteriol. 1984 Sep;159(3):825–831. doi: 10.1128/jb.159.3.825-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panbangred W., Kawaguchi O., Tomita T., Shinmyo A., Okada H. Isolation of two beta-xylosidase genes of Bacillus pumilus and comparison of their gene products. Eur J Biochem. 1984 Jan 16;138(2):267–273. doi: 10.1111/j.1432-1033.1984.tb07911.x. [DOI] [PubMed] [Google Scholar]
- Panbangred W., Kondo T., Negoro S., Shinmyo A., Okada H. Molecular cloning of the genes for xylan degradation of Bacillus pumilus and their expression in Escherichia coli. Mol Gen Genet. 1983;192(3):335–341. doi: 10.1007/BF00392172. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shamberger R. J. Serum sialic acid in normals and in cancer patients. J Clin Chem Clin Biochem. 1984 Oct;22(10):647–651. doi: 10.1515/cclm.1984.22.10.647. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Sugimoto K., Nomura O., Usui T. Enzymatic assay of serum sialic acid. Clin Chim Acta. 1980 Dec 22;108(3):493–498. doi: 10.1016/0009-8981(80)90360-5. [DOI] [PubMed] [Google Scholar]
- Takizawa N., Murooka Y. Cloning of the pullulanase gene and overproduction of pullulanase in Escherichia coli and Klebsiella aerogenes. Appl Environ Microbiol. 1985 Feb;49(2):294–298. doi: 10.1128/aem.49.2.294-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi K., Chifu K., Hayashi N., Nakamachi Y., Yamaguchi N., Miyamoto Y., Doi K., Baba S., Uchida Y., Tsukada Y. A new enzymatic method for the determination of sialic acid in serum and its application for a marker of acute phase reactants. Kobe J Med Sci. 1981 Jun;27(3):91–102. [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Purification and properties of N-acetylneuraminate lyase from Escherichia coli. J Biochem. 1984 Aug;96(2):507–522. doi: 10.1093/oxfordjournals.jbchem.a134863. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]



