Abstract
In the high-rate anaerobic reactors studied (ca. 10 g of chemical oxygen demand [COD] removed per liter of reactor per day), the sulfate-reducing bacteria (SRB) were poor competitors of methane-producing bacteria (MPB), scavenging only on the order of 10 to 20% of the total electron flow. The relatively noncompetitive nature of the SRB in this type of reactor is in sharp contrast to the tendency of the SRB to dominate in natural environments and in other types of anaerobic digesters. Various factors such as the feedback inhibition of H2S on the SRB, iron limitation, the origin of the SRB inocula, biokinetics, and thermodynamics were investigated. The outcome of the SRB-MPB competition under the reactor conditions studied appeared to be particularly determined by two factors. The SRB, as predicted by the Vmax-Km kinetics, competed most effectively at low substrate levels (<0.5 g of COD per liter). The MPB, however, appeared to colonize and adhere much more effectively to the polyurethane carrier matrix present in the reactor, thus compensating for the apparent lower growth rates. Even if the reactor was initially allowed to be predominantly colonized by SRB, the MPB could regain dominance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Hoban D. J., van den Berg L. Effect of iron on conversion of acetic acid to methane during methanogenic fermentations. J Appl Bacteriol. 1979 Aug;47(1):153–159. doi: 10.1111/j.1365-2672.1979.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Ingvorsen K., Zehnder A. J., Jørgensen B. B. Kinetics of Sulfate and Acetate Uptake by Desulfobacter postgatei. Appl Environ Microbiol. 1984 Feb;47(2):403–408. doi: 10.1128/aem.47.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
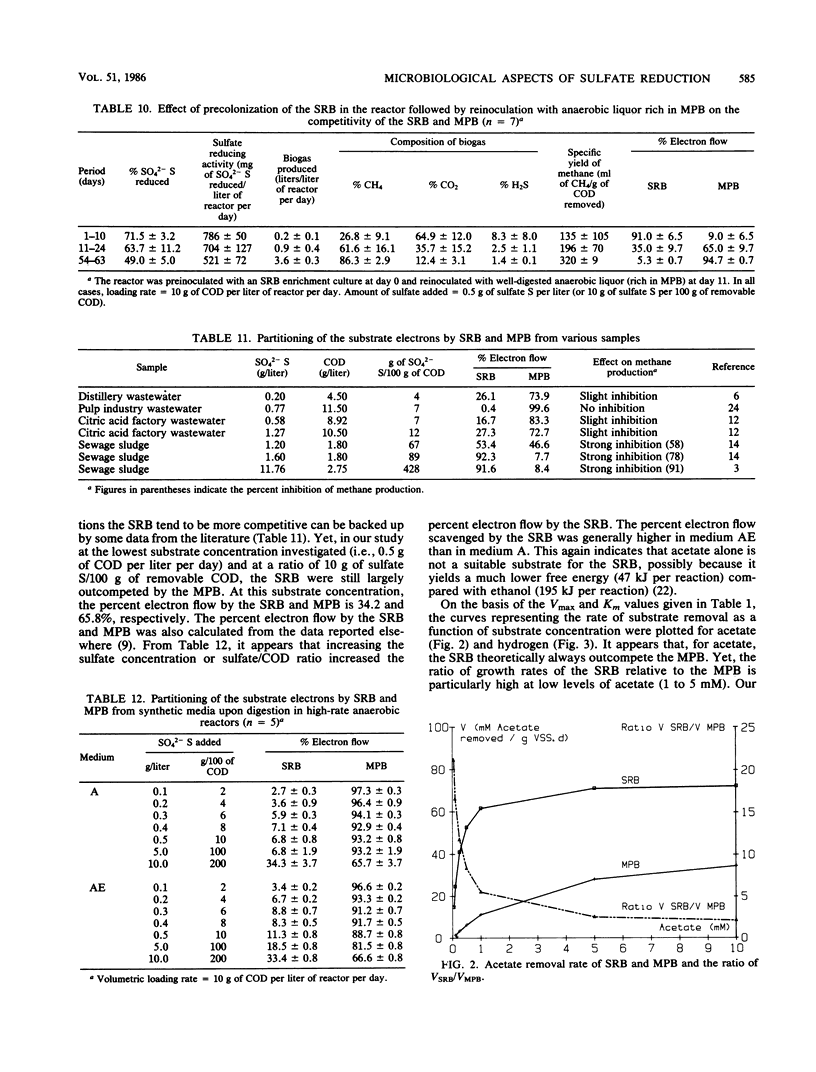
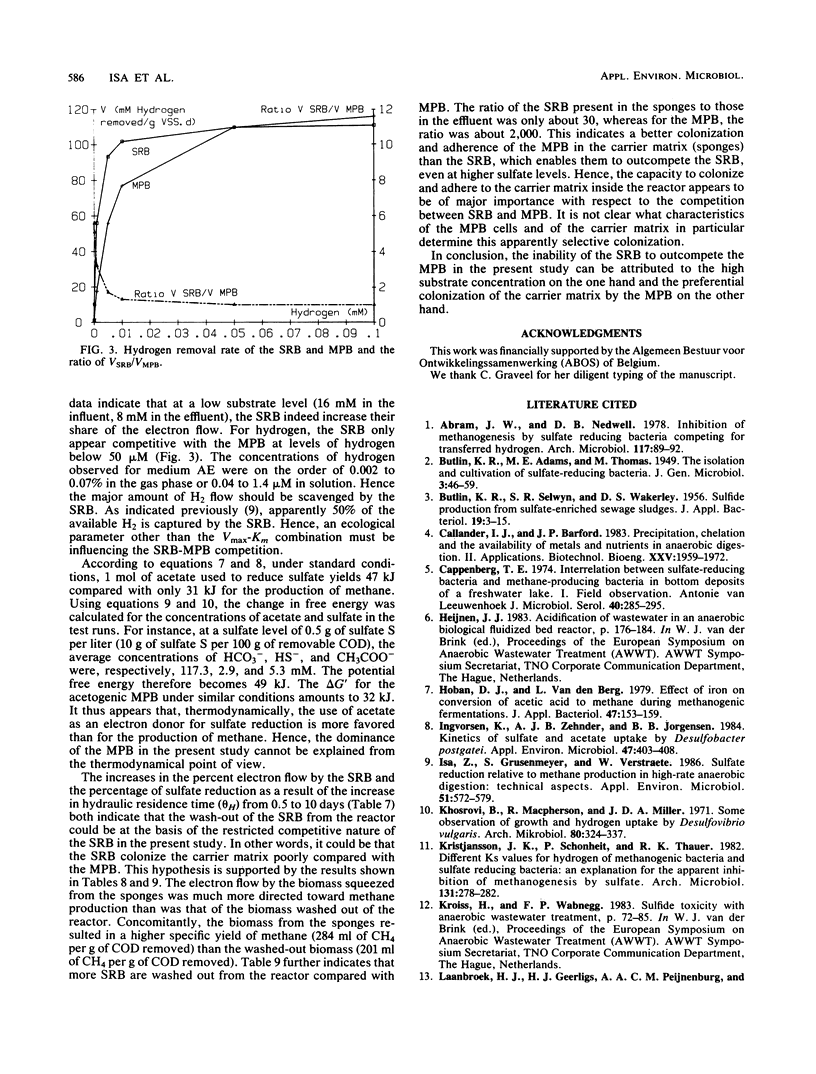
- Isa Z., Grusenmeyer S., Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol. 1986 Mar;51(3):572–579. doi: 10.1128/aem.51.3.572-579.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosrovi B., Macpherson R., Miller J. D. Some observations on growth and hydrogen uptake by Desulfovibrio vulgaris. Arch Mikrobiol. 1971;80(4):324–337. doi: 10.1007/BF00406220. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



