Abstract
Accumulation of unfolded proteins within the endoplasmic reticulum (ER) of eukaryotic cells triggers the unfolded protein response (UPR), which activates transcription of several genes encoding ER chaperones and folding enzymes. This study reports that conversion of dolichol-linked Man2–5GlcNAc2 intermediates into mature Glc3Man9GlcNAc2 oligosaccharides in primary human adult dermal fibroblasts is also stimulated by the UPR. This stimulation was not evident in several immortal cell lines and did not require a cytoplasmic stress response. Inhibition of dolichol-linked Glc3Man9GlcNAc2 synthesis by glucose deprivation could be counteracted by the UPR, improving the transfer of Glc3Man9GlcNAc2 to asparagine residues on nascent polypeptides. Glycosidic processing of asparagine-linked Glc3Man9GlcNAc2 in the ER leads to the production of monoglucosylated oligosaccharides that promote interaction with the lectin chaperones calreticulin and calnexin. Thus, control of the dolichol-linked Glc3Man9GlcNAc2 supply gives the UPR the potential to maintain efficient protein folding in the ER without new synthesis of chaperones or folding enzymes.
Cells possess complex mechanisms to deal with stress caused by changes in the extracellular environment or in the cell’s own physiology. In eukaryotes, the unfolded protein response (UPR) of the endoplasmic reticulum (ER) has become a model for specific signaling and transcriptional events that respond to stress (1). The UPR is distinct from cytoplasmic stress responses and can be triggered by treatments that cause unfolding of proteins in the lumen of the ER. By a remarkable ER-to-nucleus signaling pathway involving the transmembrane kinase Ire1p, the UPR results in de novo synthesis of ER proteins (such as the “glucose-regulated proteins” GRP78 and GRP94) that aid protein folding (1). It has been suggested that the UPR may be activated in response to rapid increases in the production of secreted and cell-surface proteins (1) because such increases might exceed the folding capacity of the ER.
Since the late 1970s, there has been a clear link between sugar metabolism and the UPR. The UPR can be induced by extended glucose starvation for periods of 24 hr or more (2–5) or by inhibitors of asparagine-linked protein glycosylation, such as tunicamycin (TN) (1), or glycoprotein processing, such as castanospermine (CSN) (6, 7). It is likely that UPR induction by these conditions is caused in part by interference with the ER chaperones calnexin and calreticulin. Both are lectins involved in protein folding and oligomerization, requiring specific monoglucosylated asparagine-linked oligosaccharides on target proteins to initiate binding (8–12). TN (13) inhibits synthesis of the dolichol-linked, i.e., lipid-linked oligosaccharide (LLO) glucose3mannose9N-acetylglucosamine2 (Glc3Man9GlcNAc2) (14, 15), and therefore prevents asparagine-linked glycosylation catalyzed by oligosaccharyltransferase. CSN inhibits deglucosylation of oligosaccharides after transfer to protein (16), preventing direct trimming to the monoglucosylated form (9, 10) as well as later reglucosylation (17), and thereby blocking interaction with calnexin and calreticulin. Glucose starvation can cause accumulation of truncated LLO intermediates, such as Man5GlcNAc2 (18–21) that are transferred to protein less efficiently than Glc3Man9GlcNAc2 (22, 23) and are not readily converted into monoglucosylated structures (17).
Thus, proper sugar metabolism and LLO formation are essential for normal protein folding in the ER. Because the UPR had been shown to control the concentrations of a number of ER chaperones and folding enzymes, experiments were designed to determine whether the UPR might also regulate LLO synthesis.
Materials and Methods
Cell Cultures.
The following primary cultures of normal human adult dermal fibroblasts were tested: CRL 1987 (used for all experiments presented), CRL 1904, and CRL 1892 [American Type Culture Collection (ATCC)]; F1-7, F1-8, and F24-4 (National Psoriasis Tissue Bank, Dallas); and PN1.1 (University of Texas Southwestern Skin Disease Research Core, Dallas). Chinese hamster ovary (CHO)-K1 cells were as described (24), A375 melanoma cells were from the ATCC, HeLa cells were from David Russell (University of Texas Southwestern Medical Center, Dallas, TX), and RAW 264.7 cells were from Richard Kitchens (University of Texas Southwestern Medical Center).
UPR-Inducing Treatments.
CSN (either purchased from Matreya, Pleasant Gap, PA or obtained as a gift from Alan Elbein, University of Arkansas for Medical Sciences, Little Rock, AR) blocks processing glucosidases I and II and hence the calnexin/calreticulin cycle (16). DTT (Sigma) was used to reduce disulfide bonds (25). Azetidine-2-carboxylic acid (Sigma) was used to replace proline in nascent polypeptides and prevent isomerization (26). Geldanamycin (Calbiochem), which blocks heat shock protein (HSP)90-type chaperones, was used to inhibit GRP94 function (27). Treatments at 42°C (for 2 hr, unless stated otherwise) were used to thermally denature proteins.
Preparation of LLO.
A total of 100,000 human adult dermal fibroblast (HADF) cells were seeded in untreated 100-mm Pyrex dishes and grown at 37°C in a humidified 5% carbon dioxide atmosphere for 2–4 days in 10 ml of RPMI 1640 medium supplemented with 10% FBS. Cells were refed growth medium within 24 hr prior to metabolic labeling. Longer refeedings gave variable results, possibly the result of associated stresses such as nutrient deprivation (data not shown). In some experiments, the UPR was deliberately induced prior to metabolic labeling. For labeling, the growth medium was replaced with 1 ml of RPMI 1640 medium containing 0.5 mM d-glucose and 2.3 μM d-[2-3H]mannose (15–20 Ci/mmol; Amersham Pharmacia). Care was taken not to exceed this amount as the mass of the [3H]mannose itself contributes significantly to the LLO mass. Because serum contains both glucose and mannose, dialyzed FBS (10%) was used during labeling. After labeling for 20 min at 37°C, dishes were placed on ice, and the medium was removed. After rinsing with ice-cold PBS, cells were scraped directly into 10 ml of chloroform/methanol/water (10:10:3). This was followed by centrifugation (2,000 × g for 15 min; the pellet was used for glycopeptides as described below), evaporation of the supernatant to dryness, cleavage of the pyrophosphate bonds of the LLO with 0.1 M HCl, partitioning between butanol and water, reduction with sodium borohydride, desalting with a mixed-bed ion exchange resin, analysis of 3H-labeled oligosaccharides by silica HPLC, and comparison to known standards (28).
Preparation of Glycopeptides and Analysis by Endoglycosidase H (endo H) Digestion.
After extraction of cell samples, glycopeptides (obtained by trypsin digestion of the insoluble residues) were treated in the absence or presence of 5,000 units of endo H (New England Biolabs) and fractionated with a 1 × 45 cm column of Bio-Gel P-4 (superfine) in PBS at 0.1 ml/min to separate 3H-labeled glycopeptides and released 3H-labeled oligosaccharides (29). Only LLO-type oligosaccharides with six or more mannose residues can be cleaved by endo H.
2-Deoxyglucose Uptake.
Triplicate cell cultures were labeled for 10 min at 37°C with glucose-free RPMI 1640 containing 10% dialyzed FCS, 2 μCi/ml 2-deoxy-d-[3H]glucose (10 Ci/mmol; Sigma), and 2 mM 2-deoxy-d-glucose. Cells were washed three times with ice-cold PBS and solubilized with 2 ml of 1% SDS, and the associated radioactivity was determined by liquid scintillation spectrometry.
Results
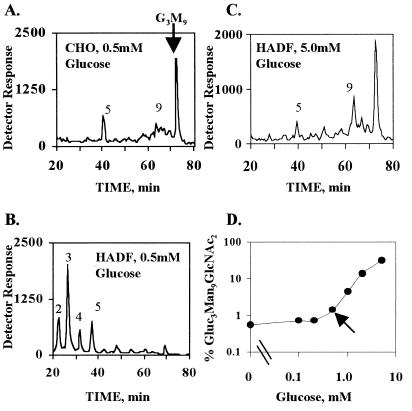
Studies were initiated to determine whether the UPR might have a positive regulatory effect on LLO synthesis in addition to its role in the maintenance of chaperones and folding enzymes. Mammalian cells are typically cultured in medium containing 5–10 mM d-glucose, but to enhance labeling of LLO with d-[2-3H]-mannose, it is common to reduce the glucose concentration to 0.5–1.0 mM. As shown previously by many laboratories, with essentially all immortal cell lines, LLO synthesis is unaffected by such changes, and mature Glc3Man9GlcNAc2 is the prominent LLO (CHO-K1, Fig. 1A; RAW 264.7, HeLa, and A375 melanoma cells, data not shown). Unexpectedly, primary HADF grown with 5 mM glucose and then labeled briefly for 20 min in 0.5 mM glucose with 2.3 μM [3H]mannose displayed extensive differences in their LLO profiles (Fig. 1B). The intermediates Man2–5GlcNAc2 became the major species, although there was little change in the relative glucosylation of the remaining Man9GlcNAc2. Glc3Man9GlcNAc2 represented an average of 56.5 ± 3.1% (n = 5) of all 9-mannose oligosaccharides labeled in 5 mM glucose and 53.1 ± 2.9% (n = 5) when labeled in 0.5 mM glucose. These effects were dependent on glucose concentration (Fig. 1D), and if 5 mM glucose was maintained during labeling, the HADF pattern resembled that of CHO-K1 cells labeled with 0.5 mM glucose (Fig. 1C). The same results were obtained whether cells were labeled in 0.5 mM glucose for 20 or 60 min, and there was no 3H-labeled LLO detectable after a 5-min chase with unlabeled mannose. This demonstrates that the results of the 20-min labeling period represent a steady state. Further, the LLO pool in HADF cells is short-lived, and a brief fluctuation of the extracellular glucose concentration can dramatically alter LLO synthesis. Thus, HADF were chosen for further study because the LLO pathway was potentially more susceptible to regulation than the pathway in immortalized lines.
Figure 1.
LLO synthesis in CHO-K1 and HADF cells. (A–C) LLO were metabolically labeled with [3H]mannose, and released oligosaccharides were analyzed by HPLC. Arbitrary units from an in-line tritium detector are given on the y-axis. The position of Glc3Man9GlcNAc2 (G3M9) is indicated by the vertical arrow. The positions of unglucosylated intermediates are indicated by numbers over the respective peaks that refer to the mannose content of each intermediate. CHO-K1 cells were labeled in 0.5 mM glucose (A). HADF cells were labeled in either 0.5 mM glucose (B) or 5 mM glucose (C). (D) The percentage of mature Glc3Man9GlcNAc2 LLO compared with Man2–5GlcNAc2 LLO intermediates in HADF is indicated as a function of extracellular glucose. The calculation was performed after determining the radioactivity of each oligosaccharide by HPLC and then normalizing by mannose content. The apparent plateau below 0.2 mM glucose was because of the mass of the [3H]mannose label (2.3 μM) itself. The arrow indicates 0.5 mM glucose, used throughout the remaining experiments.
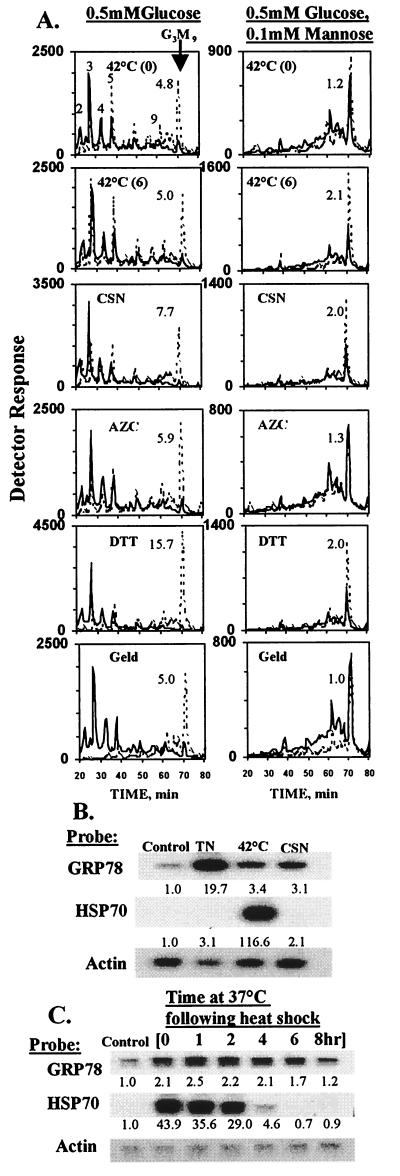
HADF were then incubated briefly (20 min) with [3H]mannose and 0.5 mM glucose to accumulate truncated LLO and tested for effects of the UPR (Fig. 2A, Left). Any of five treatments able to induce the UPR applied either during (disulfide disruption by DTT) or prior to labeling (processing glucosidase inhibition by CSN; inhibition of proline isomerization by azetidine-2-carboxylic acid; inhibition of GRP94 with geldanamycin; and 42°C heat shock with either 0 or 6 hr of recovery) consistently counteracted truncation of LLO, increasing Glc3Man9GlcNAc2 by 3- to 15-fold depending on the specific stress. While each treatment also had effects on other cellular processes, inhibition of protein folding in the ER was the only effect in common. In addition, glucosidase inhibition and disulfide disruption would be expected to affect protein folding in the ER, not the cytoplasm. Swainsonine, an inhibitor of Golgi mannosidase II that was not expected to affect ER function, had no effect on GRP78 mRNA or LLO synthesis (data not shown). The effects of TN and thapsigargin, two other well known inducers of the ER UPR, were not evaluated because the former eliminates LLO synthesis (13) and the latter, for unknown reasons, inhibited labeling of the LLO pool (data not shown).
Figure 2.
The UPR stimulates synthesis of mature (Glc3Man9GlcNAc2) LLO in HADF cells labeled in the presence of 0.5 mM glucose. (A) LLOs were analyzed from HADF cells labeled with 2.3 μM [3H]mannose and 0.5 mM glucose in the absence (Left) or presence (Right) of unlabeled 0.1 mM d-mannose. The graphs show oligosaccharides from cells subjected to various stresses (dashed lines) as well as from an equal number of cells from paired unstressed controls (solid lines). Panel labels: “42°C (0)” and “42°C (6),” heat shock with either 0 or 6 hr of recovery at 37°C; “CSN,” 200 μg/ml for 24 hr; “AZC,” 5 mM for 2 hr (AZC, azetidine-2-carboxylic acid); “DTT,” 200 μM for 20 min; and “Geld,” 2 μM for 24 hr (Geld, geldanamycin). The fold increase of Glc3Man9GlcNAc2 is indicated by the number next to the peak. Because of a delay in detector activation, time values for the dashed tracing in the heat shock/no recovery/0.1 mM mannose experiment were compensated by 4 min. (B) Northern blot [performed according to standard procedures (24)] showing GRP78 (BiP) (GenBank AA205990) and HSP70 (GenBank T74240) mRNA in control HADF, and after TN treatment (5 μg/ml, 6 hr), 2 hr 42°C heat shock (1 hr recovery), or CSN treatment (200 μg/ml, 24 hr). RNA signals were measured with a Fuji phosphorimager and normalized to actin. The fold increases calculated for GRP78 and HSP70 mRNAs relative to unstressed controls are presented below the bands. Longer exposures demonstrated that all of the increased HSP70 signal in the CSN and TN lanes resulted from spillover of signal from the adjacent heat shock lane. (C) Time course of GRP78 and HSP70 induction after 2 hr, 42°C heat shock. Results are expressed as for B.
While heat shock is not considered a classical inducer of the UPR, it has been reported to stimulate synthesis of GRPs in mammalian cells under certain conditions (30, 31). As shown in Fig. 2 B and C, compared with TN and CSN, 42°C shock of HADF caused a reproducible induction of GRP78 mRNA. By comparison, 42°C shock, but neither TN nor CSN, induced HSP70 mRNA. Thus, the cytoplasmic stress response was not involved in stimulation by CSN. Seven different normal HADF cultures (listed in Materials and Methods) responded to a 2-hr 42°C shock followed by a 6-hr recovery period at 37°C, increasing the relative abundance of Glc3Man9GlcNAc2 by an average factor of 3.4 ± 0.3 (SEM). No direct dependence on cell passage number was identified, although the effect of the UPR on LLO synthesis became attenuated as these primary cells became senescent (data not shown).
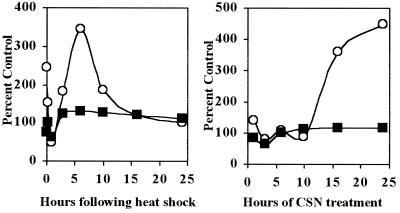
The increase of Glc3Man9GlcNAc2 was not because of decreased transfer from the LLO pool to nascent polypeptides, as might be expected if acceptors in the ER became aggregated or complexed with chaperones after stress, or if the oligosaccharyltransferase itself lost activity. First, when labeling reactions were supplemented with unlabeled 0.1 mM mannose (Fig. 2A, Right) or 5 mM glucose (data not shown), Glc3Man9GlcNAc2 was the predominant LLO in unstressed controls, and UPR effects were greatly attenuated. Second, time courses (Fig. 3) with heat shock and CSN showed that net transfer to protein was not affected when LLO synthesis was stimulated. Interestingly, the time course for heat shock was biphasic and not coincident with the time course for GRP78 mRNA stimulation (compare with GRP78 mRNA in Fig. 2C), suggesting differences in the respective regulatory mechanisms. Third, CHO-K1 cells and A375 melanoma cells labeled with 0.5 mM glucose produced mostly Glc3Man9GlcNAc2 LLO and exhibited little or no increase of Glc3Man9GlcNAc2 after treatment with CSN, DTT, or 42°C shock. The A375 results also ruled out a contribution by previously reported stress effects on LLO synthesis (ref. 32; data not shown). Taken together, these results demonstrate that the UPR has little effect if LLO synthesis is already optimal, and that the increase of Glc3Man9GlcNAc2 is not the result of accumulation of untransferred LLO.
Figure 3.
Time course of stimulation of LLO synthesis in HADF. (Left) HADF cells were incubated at 42°C for 2 hr and allowed to recover at 37°C for 0–24 hr, all in 5 mM glucose. (Right) HADF were incubated with 200 μg/ml CSN for the indicated periods in 5 mM glucose. In both cases, cells were then labeled for 20 min with [3H]mannose in 0.5 mM glucose, and LLO were analyzed by HPLC. Data were normalized to paired unstressed controls on the y-axis. ○, Radioactivity for Glc3Man9GlcNAc2 LLO. ■, The mannose radioactivity remaining after exhaustive organic extraction and solubilization with 1% SDS—i.e., protein-associated oligosaccharides. As shown in Fig. 4, the majority of this material must represent N-linked oligosaccharides because essentially all of the resulting glycopeptides can be digested by endo H when the synthesis of mature LLO is maximal.
Similarly, the increase of Glc3Man9GlcNAc2 was not the result of increased specific radioactivity of the intracellular mannose label. If it was, radioactivity incorporated into protein and LLO intermediates should have increased proportionally. However, radioactivity in LLO intermediates was either unchanged or diminished (Fig. 2A), depending on the specific stress, and radioactivity in the protein fraction was not affected (Fig. 3). Further, the total radioactivity in the chloroform/methanol/water (10:10:3) LLO fraction did not increase.
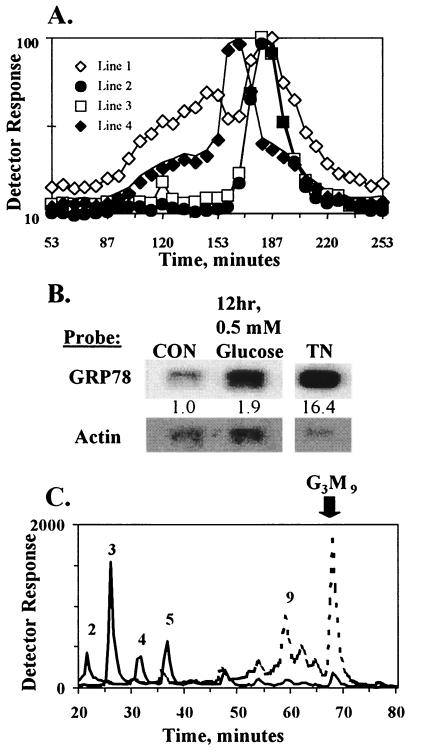
Glycopeptides obtained by proteolysis of the newly synthesized glycoproteins are sensitive to subsequent endo H treatment if the attached LLO-type oligosaccharides contain six or more mannose residues. Cleavage is detectable by retarded elution of 3H-labeled compounds on gel filtration. Glycopeptides from a CHO-K1 Lec35 mutant unable to extend LLO beyond the five-mannose stage were completely resistant to endo H (29), while glycopeptides from HADF maintained in 5 mM glucose during growth and labeling were fully sensitive to endo H (Fig. 4A, Line 2). In contrast, after a 20-min incubation in 0.5 mM glucose, much of the HADF glycopeptide fraction was resistant to endo H, indicating transfer of Man2–5GlcNAc2 intermediates (Fig. 4A, Line 1). Protein-bound Glc3Man9GlcNAc2 can be processed into Glc1Man9GlcNAc2 recognition markers for calreticulin and calnexin (9–12), but Man2–5GlcNAc2 are poorly glucosylated (17) and only the glucosylated form of Man5GlcNAc2 is a good ligand for calnexin and calreticulin in vitro (33, 34). Therefore, an extended incubation in 0.5 mM glucose should result in accumulation of unfolded protein, induction of the UPR, and an increase in Glc3Man9GlcNAc2 LLO. As shown in Fig. 4, 0.5 mM glucose incubation for 12 hr caused an increase of both GRP78 mRNA (Fig. 4B) and Glc3Man9GlcNAc2 LLO (Fig. 4C). Thus, incubation in 0.5 mM glucose for only 20 min is not sufficient to accumulate enough unfolded nascent polypeptide for the UPR, but the UPR is induced after 12 hr, in which case the dolichol pathway undergoes a form of self-correction. Twelve hours of glucose deprivation also enhanced glucose uptake; transport of 2-deoxy[3H]glucose was 234.5 ± 1.0% (SD) of control. Directly increasing the glucose supply by this amount enhanced Glc3Man9GlcNAc2 synthesis by 3-fold (Fig. 1D), while Glc3Man9GlcNAc2 LLO was increased 11-fold by glucose deprivation. Thus, the effect of glucose deprivation on LLO synthesis is the result of the combination of UPR activation and enhanced glucose transport.
Figure 4.
Extended glucose deprivation induces the UPR and reverses truncation of lipid-linked and protein-bound oligosaccharides. (A) Prior to glycopeptide analysis, HADF were either grown continuously in medium with 5 mM glucose (Lines 1 and 2), with 0.5 mM glucose during the final 12 hr (Line 3), or with 5 mM glucose plus 200 μg/ml CSN during the final 24 hr (Line 4). To begin glycopeptide analysis, cells were incubated in 0.5 mM (Lines 1, 3, and 4) or 5 mM (Line 2) glucose for 20 min without radiolabel, followed by labeling with [3H]mannose for 20 min in the same glucose concentration. Thus, the first 20-min period ensured complete equilibration with the glucose concentration prior to radiolabeling. Tryptic glycopeptides were isolated and incubated in the absence (data not shown) or presence of endo H and fractionated on Bio-Gel P-4. Undigested glycopeptides eluted as broad peaks between 90 and 160 min (data not shown), while oligosaccharides released by endo H eluted later. To facilitate direct comparisons of the amount of endo H-resistant material, the tracings were normalized to peak heights of the released oligosaccharides. Because CSN inhibits glucose removal, the released oligosaccharides from the CSN sample eluted earlier than for other samples. (B) mRNA for GRP78 was determined as in Fig. 2B. (C) LLO from cells grown in 5 mM (solid line) or 0.5 mM (dashed line) glucose during the 12 hr prior to labeling, then labeled for 20 min with [3H]mannose and 0.5 mM glucose, were analyzed as in Fig. 2A.
The Man2–5GlcNAc2 content on nascent proteins was reduced by UPR induction with CSN and nearly eliminated by extended glucose deprivation (Fig. 4A, Lines 4 and 3, respectively). This was consistent with the greater fold-increase of Glc3Man9GlcNAc2 LLO and the greater decrease of LLO intermediates after glucose deprivation (compare Figs. 2 and 4). Similar results were obtained with Pronase glycopeptides, or with tryptic peptides from cells grown continuously with swainsonine to block Golgi-type processing (a potential cause of endo H resistance). Thus, prior activation of the UPR by CSN can reduce accumulation of oligosaccharide intermediates, and subsequent UPR activation by glucose deprivation can reverse it. In both cases, the compositions of dolichol-linked and protein-bound oligosaccharides are changed.
Discussion
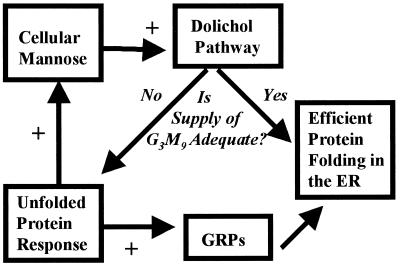
These results establish an unexpected link between the UPR and the dolichol pathway (Fig. 5). It is well known that the UPR activates transcription of chaperones, such as the GRPs, and folding enzymes, such as protein disulfide isomerase (1). The data presented here suggest that UPR regulation can also affect oligosaccharide “cofactors” in the cases of calnexin and calreticulin, and raise the possibility that other folding cofactors (such as ATP and oxidized glutathione) might also be regulated. It is not known whether the effects described here are mediated by a member of the Ire1p family (1) or a separate molecule, such as PKR-like ER kinase (35). It is also not known whether these effects involve transcriptional, translational, or posttranslational regulation. Because inhibitors of RNA synthesis and protein synthesis themselves have complex effects on the LLO pathway (36), this could not be tested directly.
Figure 5.
The relationship between the ER UPR and dolichol-linked oligosaccharide synthesis. When extracellular hexose levels fall or when one or more metabolic steps are inhibited, truncated LLO intermediates accumulate and are transferred to protein. Because such truncated intermediates do not yield effective ligands for lectin chaperones, they cause inefficient protein folding in the ER and trigger the UPR. In addition to activating the transcription of ER chaperones such as the GRPs, the UPR also compensates directly for the hexose deficiency, possibly by raising the intracellular mannose concentration. This reduces the accumulation of LLO intermediates, increases the amount of mature LLO transferred to protein, and facilitates protein folding.
Similarly, the target of the UPR remains to be established. In preliminary experiments, no differences between control and UPR-induced HADF were observed for UDP-GlcNAc:dolichol-P GlcNAc-1-P transferase activity in vitro, mannose-P-dolichol synthase activity in vitro, incorporation of [3H]mannose into mannose phosphates or GDP-mannose in vivo, synthesis of Man1–9GlcNAc2-P-P-dolichol from GDP-[3H]mannose in vitro, or microsomal dolichol-P assessed by reactivity with mannose-P-dolichol synthase (data not shown). Thus, a plausible explanation is an increase in the intracellular concentration of mannose or a mannose precursor, because small amounts of extracellular mannose (0.1 mM; Fig. 2A) fully compensated for much larger amounts of extracellular glucose (5 mM), in agreement with earlier experiments on HADF (37). It is unlikely that this could be due primarily to enhanced uptake of mannose or glucose; after induction of the UPR by either CSN or 42°C treatment, incorporation of extracellular [3H]mannose into total water-soluble plus insoluble metabolites was never increased by more than 30%, and, in some experiments, was unchanged (data not shown). In contrast, control experiments testing various extracellular mannose concentrations showed that increases of approximately 10-fold would be necessary to account for the UPR-dependent LLO changes. Heat shock increased uptake of 2-deoxy[3H]glucose by only 30%, and no increases were observed with CSN or TN (data not shown). Unfortunately, all attempts to determine the actual intracellular mannose were unsuccessful.
Conceivably, the UPR could mobilize an intracellular store of mannose, perhaps by degradation of cellular mannosyl conjugates or by converting other nucleotide sugars into GDP-mannose. Alternatively, an enzyme involved in the normal pathway leading to GDP-mannose, the immediate donor for the first five mannose residues of the LLO as well as for mannose-P-dolichol (itself the donor for the last four mannose residues), might be regulated. Obvious candidates include phosphomannomutase and phosphomannose isomerase, as these could increase the flux of mannose and glucose into oligosaccharides. Another possibility is phosphofructokinase, a highly regulated enzyme responsible for the committed step in glycolysis. Inhibition would increase the steady-state concentrations of fructose 6-phosphate and all downstream mannosyl phosphates.
To accumulate LLO intermediates in HADF so that UPR-dependent stimulation could be studied, it was necessary to lower glucose in the culture medium approximately 10-fold below the physiological range of concentrations in the blood. What is the relevance of such a condition? It is difficult to know what concentration of glucose would be truly appropriate for dermal fibroblasts or other nonendothelial tissue cells because little is understood about the glucose concentrations in the interstitial fluids surrounding such cells. The results presented here suggest that the LLO composition might be an indirect indicator, but information on compositions of LLO extracted directly from solid tissues is highly limited. One exception is the pancreas; careful compositional analyses of LLO revealed that Glc3Man9GlcNAc2 was no more abundant than truncated intermediates (38, 39). Further, studies with glucose-sensing microprobes implanted into canine skin suggest that the glucose concentrations at sites of inflammation are approximately 4-fold lower than the circulatory concentration (40). Thus, the studies reported here may be relevant for both healthy and damaged tissue.
LLO profiles in CHO-K1 and other rapidly growing immortal lines were not affected by 0.5 mM glucose, and there were no appreciable UPR effects. More drastic reduction of glucose in the medium (0.1 mM) did result in accumulation of truncated LLO in CHO-K1 cells, but their relative amounts were unaffected by 42°C or CSN treatment (J. Shang and M.A.L., unpublished data). This suggests that the step regulated by the UPR may be activated in immortal lines under basal conditions.
The calreticulin gene is responsive to the UPR (41), although calnexin is not enhanced by TN, a strong UPR inducer (42, 43). Because each is also a calcium-binding protein and calreticulin has multiple proposed functions (44), alterations of their concentrations in a UPR might have a multiplicity of effects. Thus, for these lectin chaperones, a more desirable first line of defense might be to regulate synthesis of their oligosaccharide ligands.
Carbohydrate-deficient glycoprotein syndrome (CDGS) is often caused by defective LLO synthesis (45). These data may therefore be relevant to the diagnosis of CDGS, which often involves metabolic labeling of LLO in CDGS dermal fibroblasts with low-glucose medium and can yield highly variable LLO profiles that are sometimes misleadingly similar to normal profiles (46–49). In our hands, variable results were obtained with normal HADF if care was not taken to avoid spurious stresses, such as nutrient deprivation, and to refeed cells within 24 hr of labeling. Also, incubations with [3H]mannose concentrations above 2.3 μM increased the relative amounts of mature LLO. Surprisingly, extended glucose (0.5 mM) deprivation of cultured CDGS type I-A cells for 12 hr reversed defective LLO synthesis (50). The data presented here suggest that this result may have been because of the combined effects of UPR stimulation and enhanced glucose transport, and raise the possibility that the UPR may be chronically altered in CDGS cells.
Acknowledgments
We thank Hudson Freeze, Jeffrey Rush, Salvatore Turco, Charles Waechter, and David Williams for their insightful comments on this work. This study was supported by Grants R01-GM38545 and P30-AR41940 from the National Institutes of Health, and Grant I-1168 from the Robert Welch Foundation.
Abbreviations
- UPR
unfolded protein response
- ER
endoplasmic reticulum
- TN
tunicamycin
- CSN
castanospermine
- LLO
lipid-linked oligosaccharide
- GRP
glucose-regulated protein
- HSP
heat shock protein
- endo H
endoglycosidase H
- CHO
Chinese hamster ovary
- HADF
human adult dermal fibroblast
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chapman R, Sidrauski C, Walter P. Annu Rev Cell Dev Biol. 1998;14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- 2.Pouyssegur J, Shui R P C, Pastan I. Cell. 1977;11:941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- 3.Shui R P C, Pouyssegur J, Pastan I. Proc Natl Acad Sci USA. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peluso R W, Lamb R A, Choppin P W. Proc Natl Acad Sci USA. 1978;75:6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gething M-J, Sambrook J. Nature (London) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 6.Pahl H L, Baeuerle P A. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watowich S S, Morimoto R I. Mol Cell Biol. 1988;8:393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 9.Hammond C, Braakman I, Helenius A. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware F E, Vassilakos A, Peterson P A, Jackson M R, Lehrman M A, Williams D B. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- 11.Vassilakos A, Cohen-Doyle M F, Peterson P A, Jackson M R, Williams D B. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond C, Helenius A. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 13.Elbein A D. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- 14.Waechter C J, Lennarz W J. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard S C, Ivatt R J. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- 16.Elbein A D. FASEB J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 17.Sousa M C, Ferrero-Garcia M A, Parodi A J. Biochemistry. 1992;31:97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- 18.Turco S J. Arch Biochem Biophys. 1980;205:330–339. doi: 10.1016/0003-9861(80)90115-0. [DOI] [PubMed] [Google Scholar]
- 19.Rearick J I, Chapman A, Kornfeld S. J Biol Chem. 1981;256:6255–6261. [PubMed] [Google Scholar]
- 20.Gershman H, Robbins P W. J Biol Chem. 1981;256:7774–7780. [PubMed] [Google Scholar]
- 21.Chapman A E, Calhoun J C. Arch Biochem Biophys. 1988;260:320–333. doi: 10.1016/0003-9861(88)90456-0. [DOI] [PubMed] [Google Scholar]
- 22.Turco S J, Stetson B, Robbins P W. Proc Natl Acad Sci USA. 1977;74:4411–4414. doi: 10.1073/pnas.74.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornfeld S, Gregory W, Chapman A. J Biol Chem. 1979;254:11649–11654. [PubMed] [Google Scholar]
- 24.Lehrman M A, Zhu X, Khounlo S. J Biol Chem. 1988;263:9796–19803. [PubMed] [Google Scholar]
- 25.Huppa J B, Ploegh H L. Cell. 1998;92:145–148. doi: 10.1016/s0092-8674(00)80907-1. [DOI] [PubMed] [Google Scholar]
- 26.Ou W-J, Cameron P H, Thomas D Y, Bergeron J J M. Nature (London) 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 27.Lawson B, Brewer J W, Hendershot L M. J Cell Physiol. 1998;174:170–178. doi: 10.1002/(SICI)1097-4652(199802)174:2<170::AID-JCP4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Lehrman M A. Anal Biochem. 1991;193:266–271. doi: 10.1016/0003-2697(91)90020-t. [DOI] [PubMed] [Google Scholar]
- 29.Lehrman M A, Zeng Y. J Biol Chem. 1989;264:1584–1593. [PubMed] [Google Scholar]
- 30.Shiu A L. J Cell Physiol. 1981;106:119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- 31.Welch W J, Garrels J I, Thomas G P, Lin J J-C, Feramisco J R. J Biol Chem. 1983;258:7102–7111. [PubMed] [Google Scholar]
- 32.Niewiarowska A, Caltabiano M M, Bailey D S, Poste G, Greig R G. J Biol Chem. 1987;262:14815–14820. [PubMed] [Google Scholar]
- 33.Spiro R G, Zhu Q, Bhoyroo V, Soling H-D. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- 34.Vassilakos A, Michalak M, Lehrman M A, Williams D B. Biochemistry. 1998;37:3480–3490. doi: 10.1021/bi972465g. [DOI] [PubMed] [Google Scholar]
- 35.Harding H P, Zhang Y, Ron D. Nature (London) 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 36.Lehrman M A. Glycobiology. 1991;1:553–562. doi: 10.1093/glycob/1.6.553. [DOI] [PubMed] [Google Scholar]
- 37.Panneerselvam K, Etchison J R, Freeze H H. J Biol Chem. 1997;272:23123–23129. doi: 10.1074/jbc.272.37.23123. [DOI] [PubMed] [Google Scholar]
- 38.Badet J, Jeanloz R W. Carbohydr Res. 1988;178:49–65. doi: 10.1016/0008-6215(88)80101-0. [DOI] [PubMed] [Google Scholar]
- 39.Gibbs B S, Coward J K. Bioorg Med Chem. 1999;7:441–447. doi: 10.1016/s0968-0896(98)00268-5. [DOI] [PubMed] [Google Scholar]
- 40.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. J Biomed Eng. 1992;14:33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 42.Parlati F, Dignard D, Bergeron J J M, Thomas D Y. EMBO J. 1995;14:3064–3072. doi: 10.1002/j.1460-2075.1995.tb07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jannatipour M, Rokeach L A. J Biol Chem. 1995;270:4845–4853. doi: 10.1074/jbc.270.9.4845. [DOI] [PubMed] [Google Scholar]
- 44.Nash P D, Opas M, Michalak M. Mol Cell Biochem. 1994;135:71–78. doi: 10.1007/BF00925962. [DOI] [PubMed] [Google Scholar]
- 45.Kornfeld S. J Clin Invest. 1998;101:1293–1295. doi: 10.1172/JCI3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell L D, Panneerselvam K, Vij R, Diaz S, Manzi A, Buist N, Freeze H, Varki A. J Clin Invest. 1994;94:1901–1909. doi: 10.1172/JCI117540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krasnewich D M, Holt G D, Brantly M, Skovby F, Redwine J, Gahl W A. Glycobiology. 1995;5:503–510. doi: 10.1093/glycob/5.5.503. [DOI] [PubMed] [Google Scholar]
- 48.Panneerselvam K, Freeze H H. J Clin Invest. 1996;97:1478–1487. doi: 10.1172/JCI118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korner C, Lehle L, von Figura K. Glycobiology. 1998;8:165–171. doi: 10.1093/glycob/8.2.165. [DOI] [PubMed] [Google Scholar]
- 50.Korner C, Lehle L, von Figura K. Glycoconjugate J. 1998;15:499–505.7. doi: 10.1023/a:1006939104442. [DOI] [PubMed] [Google Scholar]