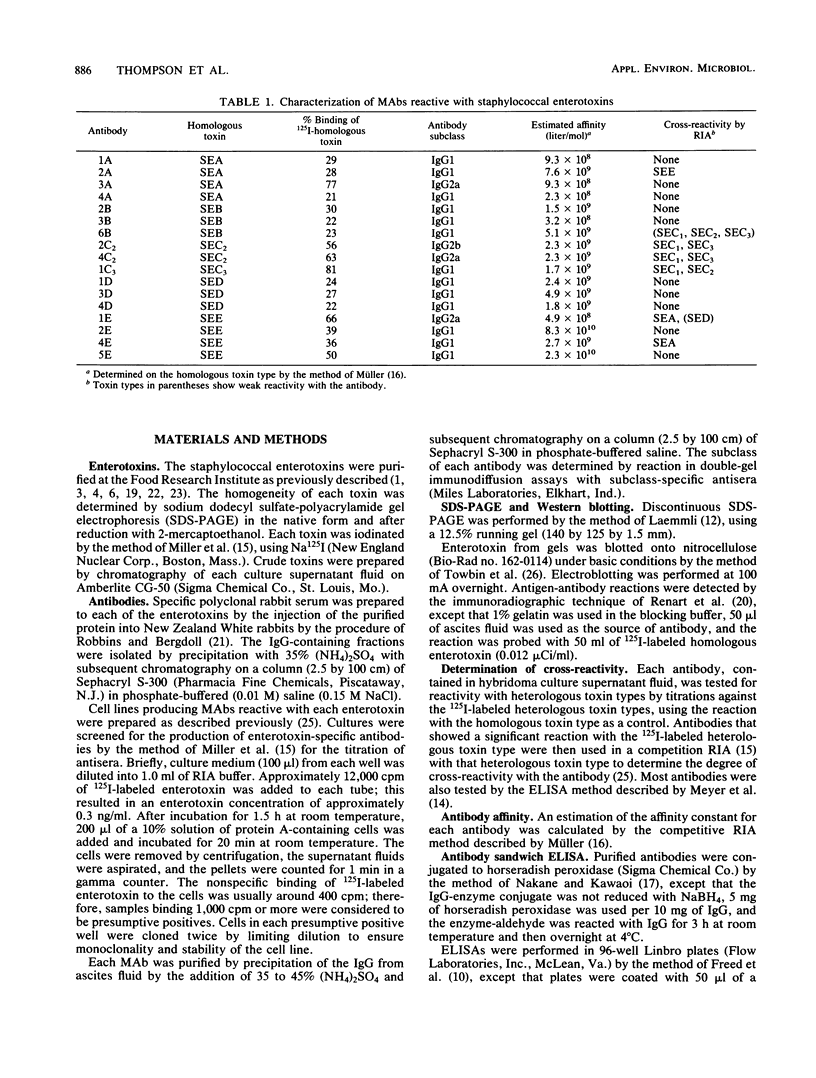
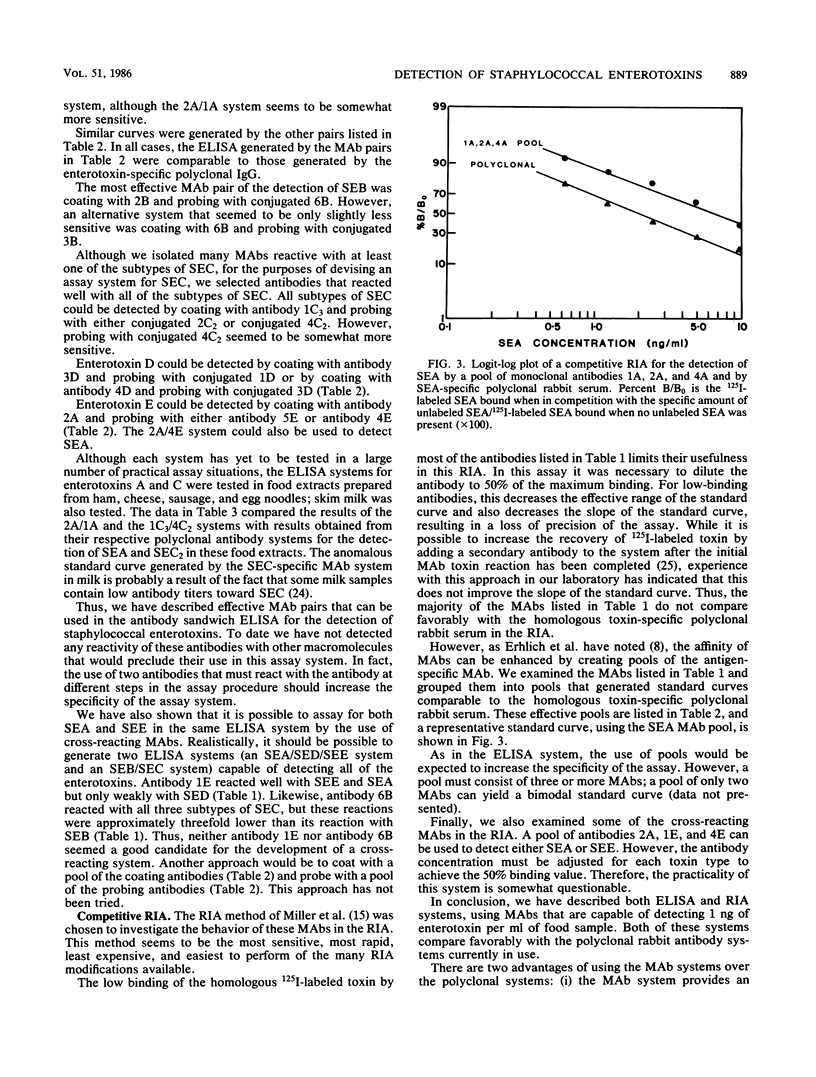
Abstract
Murine monoclonal antibodies reactive with at least one of the serological types of staphylococcal enterotoxin were examined for use in assay systems for the detection of enterotoxin at the level of 1.0 ng of enterotoxin per ml. An antibody sandwich enzyme-linked immunosorbent assay was devised for each toxin type by identifying an effective antibody pair. One antibody (the coating antibody) was coated onto a polystyrene plate and removed the enterotoxin from the test solution; the second antibody (the probing antibody) was conjugated to horseradish peroxidase and detected the captured toxin. Enterotoxins A and E could be detected in the same system by the use of cross-reacting monoclonal antibodies. All subtypes of enterotoxin C could be detected in one assay system. Two effective systems were described for each of types B and D. Each of these systems, when compared with the homologous enterotoxin-specific polyclonal rabbit antibody systems, was found to compare favorably. The monoclonal enzyme-linked immunosorbent assay systems for the detection of enterotoxins A and C2 were examined for a variety of food extracts; no abnormal interference could be detected from these extracts. The monoclonal antibody systems were also compared with the homologous enterotoxin-specific polyclonal serum for the detection of enterotoxin by the competitive radioimmunoassay (RIA). Single monoclonal antibodies generally did not perform as well in the RIA as did the homologous toxin-specific polyclonal serum. However, pools of monoclonal antibodies were prepared that approached the sensitivity and precision of the polyclonal system for the detection of each toxin by the RIA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avena R. M., Bergdoll M. S. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochemistry. 1967 May;6(5):1474–1480. doi: 10.1021/bi00857a033. [DOI] [PubMed] [Google Scholar]
- Borja C. R., Bergdoll M. S. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967 May;6(5):1467–1473. doi: 10.1021/bi00857a032. [DOI] [PubMed] [Google Scholar]
- Borja C. R., Fanning E., Huang I. Y., Bergdoll M. S. Purification and some physicochemical properties of staphylococcal enterotoxin E. J Biol Chem. 1972 Apr 25;247(8):2456–2463. [PubMed] [Google Scholar]
- CASMAN E. P., BENNETT R. W. DETECTION OF STAPHYLOCOCCAL ENTEROTOXIN IN FOOD. Appl Microbiol. 1965 Mar;13:181–189. doi: 10.1128/am.13.2.181-189.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Bergdoll M. S. Purification and some physicochemical properties of staphylococcal enterotoxin D. Biochemistry. 1979 May 15;18(10):1937–1942. doi: 10.1021/bi00577a013. [DOI] [PubMed] [Google Scholar]
- Edwin C., Tatini S. R., Strobel R. S., Maheswaran S. K. Production of monoclonal antibodies to staphylococcal enterotoxin A. Appl Environ Microbiol. 1984 Dec;48(6):1171–1175. doi: 10.1128/aem.48.6.1171-1175.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. H., Moyle W. R., Moustafa Z. A., Canfield R. E. Mixing two monoclonal antibodies yields enhanced affinity for antigen. J Immunol. 1982 Jun;128(6):2709–2713. [PubMed] [Google Scholar]
- Fey H., Pfister H., Rüegg O. Comparative evaluation of different enzyme-linked immunosorbent assay systems for the detection of staphylococcal enterotoxins A, B, C, and D. J Clin Microbiol. 1984 Jan;19(1):34–38. doi: 10.1128/jcm.19.1.34-38.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed R. C., Evenson M. L., Reiser R. F., Bergdoll M. S. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982 Dec;44(6):1349–1355. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Grey H. M., Williams R. C., Jr Protein A reactivity with mouse immunoglobulins. Structural relationship between some mouse and human immunoglobulins. J Immunol. 1970 Nov;105(5):1116–1123. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Meyer R. F., Miller L., Bennett R. W., MacMillan J. D. Development of a monoclonal antibody capable of interacting with five serotypes of Staphylococcus aureus enterotoxin. Appl Environ Microbiol. 1984 Feb;47(2):283–287. doi: 10.1128/aem.47.2.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. A., Reiser R. F., Bergdoll M. S. Detection of staphylococcal enterotoxins A, B, C, D, and E in foods by radioimnunoassay, using staphyloccal cells containing protein A as immunoadsorbent. Appl Environ Microbiol. 1978 Sep;36(3):421–426. doi: 10.1128/aem.36.3.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Noleto A. L., Khoe G. P., Bergdoll M. S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984 Sep;45(3):625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R., Conaway D., Bergdoll M. S. Detection of staphylococcal enterotoxin in foods. Appl Microbiol. 1974 Jan;27(1):83–85. doi: 10.1128/am.27.1.83-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Woodburn M. J., Lynch J. M., Jacoby H. M., Silverman S. J., Gorman J. C., Spero L. Purification and some chemical and physical properties of staphylococcal enterotoxin A. Biochemistry. 1972 Feb 1;11(3):360–366. doi: 10.1021/bi00753a009. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Gomez-Lucia E., Bergdoll M. S. Incidence of antibodies reactive with toxic shock syndrome toxin 1 in bovine milk. Appl Environ Microbiol. 1986 Apr;51(4):865–867. doi: 10.1128/aem.51.4.865-867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S. Monoclonal antibodies to staphylococcal enterotoxins B and C: cross-reactivity and localization of epitopes on tryptic fragments. Infect Immun. 1984 Jul;45(1):281–285. doi: 10.1128/iai.45.1.281-285.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]




